論文ID: CJ-14-0266
論文ID: CJ-14-0266
Background: Prasugrel is being developed in Japan as an antiplatelet therapy for use during percutaneous coronary intervention (PCI). Up to 70% of Japanese patients with coronary artery disease undergo elective PCI. The PRASugrel For Japanese PatIenTs with Coronary Artery Diseases Undergoing Elective PCI (PRASFIT-Elective) study investigated the efficacy and safety of different prasugrel dosing regimens in Japanese patients undergoing elective PCI.
Methods and Results: A total of 742 patients scheduled for elective coronary artery stenting were enrolled. Patients were randomized to receive either prasugrel (20/3.75 mg, loading/maintenance dose) or clopidogrel (300/75 mg) in a double-blind manner. Endpoints, including cardiovascular events and bleeding, were assessed at weeks 24–48. The incidence rate of major cardiovascular events (cardiovascular death, non-fatal myocardial infarction, or non-fatal ischemic stroke) up to week 24 was 4.1% (15/370) and 6.7% (25/372) in the prasugrel and clopidogrel groups, respectively. Other incidence rates were: non-coronary artery bypass graft-related major bleeding, 0% and 2.2%; major/minor bleeding, 1.6% and 3.0%; and all bleeding events, 38.1% and 34.4% in the prasugrel and clopidogrel groups, respectively. The incidence rate of bleeding-related adverse events was similar in both groups, being 40.8% and 35.8% in the prasugrel and clopidogrel groups, respectively.
Conclusions: These results support the risk-benefit profile of an adjusted dosing regimen of prasugrel in Japanese patients undergoing PCI. Larger studies are required to confirm these findings.
Coronary artery disease (CAD) is the second leading cause of death in Japan and 250,000 patients undergo percutaneous coronary intervention (PCI) annually. To prevent re-infarction after PCI, patients are routinely treated with aspirin in combination with a thienopyridine antiplatelet drug,1,2 especially clopidogrel, based on its established safety and efficacy. Ischemic events, however, are still observed after PCI in patients treated with clopidogrel. One of the reasons for this is that the pharmacodynamic effects of clopidogrel are affected by polymorphisms in the CYP2C19 gene, which is found in approximately 20% of the Japanese population, such that inhibition of platelet aggregation is decreased in poor metabolizers (PM), increasing the risk of subsequent cardiovascular events.3–5
Editorial p ????
Prasugrel is a third-generation thienopyridine antiplatelet drug that rapidly and potently inhibits platelet aggregation, with less pharmacological variability among patients with similar tolerability to other thienopyridines.
The Trial to Assess Improvement in Therapeutic Outcomes by Optimizing Platelet Inhibition with Prasugrel-Thrombolysis in Myocardial Infarction (TRITON-TIMI 38 study) examined the efficacy and safety of prasugrel (loading dose [LD]/maintenance dose [MD], 60/10 mg) and clopidogrel (300/75 mg) in 13,608 patients with acute coronary syndrome (ACS) undergoing PCI.6 That study found that prasugrel significantly reduced the incidence of ischemic cardiovascular events compared with clopidogrel. The incidence rate of major bleeding events not associated with coronary artery bypass grafting (CABG), however, was significantly higher in the prasugrel group than in the clopidogrel group.6
A phase II dose-finding study was performed in Japanese patients treated with prasugrel to determine the incidence of bleeding events and inhibition of platelet aggregation, together with CYP2C19 gene polymorphisms (Kimura et al, unpublished data, 2014). Based on the results of that study, the optimal LD/MD for prasugrel for Japanese patients was established as 20/3.75 mg, which was used in the subsequent phase III Prasugrel Compared with Clopidogrel for Japanese Patients With ACS Undergoing PCI (PRASFIT-ACS) study.7 Notably, the prasugrel dosing regimen adjusted for Japanese patients in PRASFIT-ACS had a similar efficacy profile to the regimen used in TRITON-TIMI 38, and the incidence of bleeding events in the prasugrel group was similar to that in the clopidogrel group in PRASFIT-ACS.7
The Clopidogrel Trial in Patients with Elective Percutaneous Coronary Intervention for Stable Angina and Old Myocardial Infarction (CLEAN) study compared the efficacy of clopidogrel and ticlopidine following elective PCI in patients with stable CAD in Japan. The incidence of myocardial infarction (MI) at week 12 was 7.7% in the clopidogrel group vs. 9.2% in the ticlopidine group.8 This suggested that Japanese patients with stable CAD had a moderate risk of ischemic cardiovascular events.
To evaluate the efficacy and safety of prasugrel in elective PCI patients with CAD, we conducted the PRASugrel For Japanese PatIenTs with Coronary Artery Diseases Undergoing Elective PCI (PRASFIT-Elective) study using the prasugrel dose regimen adjusted for Japanese patients (ie, 20/3.75 mg). We also determined the effects of CYP2C19 gene polymorphisms on inhibition of platelet aggregation, as evaluated in the PRASFIT-ACS study.7 In addition, we included a reference group in which patients were randomized to clopidogrel to help identify possible efficacy and safety concerns regarding the use of prasugrel in these high-risk patients; the objective was not to demonstrate the non-inferiority or superiority of prasugrel to clopidogrel.
Patients aged ≥20 years who were scheduled for elective PCI to treat CAD such as stable angina or prior MI with stenosis confirmed on coronary computed tomography were eligible for this study. Patients with any of the following were excluded: (1) acute MI, ischemic symptoms (at rest), or unstable angina within 72 h after the onset of these conditions; (2) left main artery disease, chronic complete obstruction, scheduled for stenting to treat venous graft diseases, or scheduled for rotablator treatment; (3) current or history of intracranial bleeding; (4) current or history of cerebral infarction (plus one of the following: needing anticoagulation therapy, age ≥75 years, or within 6 months after the onset of cerebral infarction); (5) blood disorder, such as hemophilia; (6) tendency for bleeding; (7) uncontrolled hypertension (systolic/diastolic pressure: ≥160/≥100 mmHg); (8) severe heart failure (New York Heart Association class III–IV); (9) severe arrhythmia; (10) current or history of thrombotic thrombocytopenic purpura or agranulocytosis; and (11) severe blood, liver, or renal disorder.
Study Design and TreatmentThe current study was designed as a multicenter, active-referenced, randomized, double-blind, double-dummy, parallel-group study, and was performed between August 2011 (date of receiving first patient consent) and December 2012 (last observation date). The study design is summarized in Figure S1. The study consisted of a 24–48-week treatment period followed by a 2-week untreated observation period. After providing informed consent and preoperative examination, patients were randomized to receive either prasugrel (20/3.75 mg) or clopidogrel (300/75 mg). The allocated LD of the study drug (20 mg prasugrel or 300 mg clopidogrel) was given on day 1, 6–96 h before PCI. The mean duration of LD pre-treatment (from the start of LD treatment to PCI) was 2.3±0.9 days and 2.3±1.1 days in the prasugrel and clopidogrel arms, respectively. Thereafter, the patients took the MD of the allocated drug (ie, 3.75 mg prasugrel or 75 mg clopidogrel) orally, once daily after breakfast for 24–48 weeks. In addition, patients could be started on the MD of either drug, without the LD. In this case, the MD was continued for 14–21 days before the patient underwent PCI. The decision to give the LD was made by the clinician in accordance with patient condition and the medical institution’s policy; patients were randomized after the decision to give the LD had been made.
Aspirin (81–100 mg/day) was used from the time of the initial dosing of the study drug to the last dose. Patients were prohibited from using any of the following types of drugs: (1) those known to reduce cardiovascular events by inhibition of platelet aggregation; (2) those that may increase the risk of bleeding by inhibition of platelet aggregation or anticoagulant effects; (3) thrombolytic drugs; and (4) acidic non-steroidal anti-inflammatory drugs.
EndpointsPrimary Endpoint The primary endpoint was determined as described in the PRASFIT-ACS study.7 Patients who had at least 1 dose of the study drugs were followed up for 24–48 weeks to evaluate efficacy events. The primary efficacy endpoint was the incidence of major adverse cardiovascular events (MACE) at 24 weeks, which was defined as a composite of cardiovascular death, non-fatal MI, and non-fatal ischemic stroke. Individual endpoints were also defined as described in the PRASFIT-ACS study,7 and included cardiovascular death (any cardiovascular-related death), stroke (presence of neurological symptoms or signs consistent with stroke and confirmation on magnetic resonance imaging), and non-fatal MI. Stroke was classified as either ischemic (cerebral infarction) or non-ischemic stroke. Non-fatal MI was defined as any of the following 3 events.7
1. In patients with normal creatine kinase (CK)-MB before PCI/CABG, CK-MB had to be (1) ≥3-fold the upper limit of normal (ULN) in 2 samples or ≥5-fold the ULN in 1 sample obtained <48 h after PCI, or (2) ≥10-fold the ULN in 1 sample obtained <48 h after CABG. Patients whose CK-MB exceeded ULN before PCI/CABG had to show a transient decrease with a subsequent increase ≥1.5-fold the previous value and satisfy (1) or (2).
2. More than 48 h after PCI, CK-MB or troponin had to be ≥2-fold the ULN, accompanied by 1 or more of the following: new or recurrent sustained ischemic chest pain, hemodynamic decompensation, or new or recurrent ST elevation/depression ≥0.1 mV.
3. Abnormal Q waves had to persist for ≥0.04 s.
Safety As in the PRASFIT-ACS study,7 the safety endpoint was the incidence of non-CABG-related bleeding events that occurred up until 2 weeks after the last dose. Five categories of bleeding events were evaluated: (1) non-CABG-related TIMI major bleeding (major bleeding): intracranial or clinically significant bleeding with a decrease in hemoglobin ≥5 g/dl; (2) non-CABG-related TIMI minor bleeding (minor bleeding): clinically significant bleeding with a decrease in hemoglobin 3–<5 g/dl; (3) clinically relevant non-major or minor bleeding: bleeding from critical sites (eg, retroperitoneal, intrapericardial, intravitreous/retinal, intraspinal, and intra-articular hemorrhage); gastrointestinal bleeding accompanied by decreased hemoglobin; gross hematuria not attributed to external factors; epistaxis requiring otolaryngology; gingival bleeding requiring dental treatment; and bleeding requiring discontinuation of the study treatment at the investigator’s discretion; these bleeding events were accompanied by a decrease in hemoglobin <3 g/dl; (4) other bleeding: bleeding events not satisfying criteria (1–3); and (5) life-threatening bleeding: a composite of fatal bleeding, bleeding requiring i.v. inotropic medication, and bleeding requiring the transfusion of ≥4 units of red blood cells.
We also examined the incidence of treatment-emergent adverse events that occurred up to 14–35 days after the last dose.
Pharmacodynamics Platelet function was assessed using the VerifyNow® assay (Accumetrics, San Diego, CA, USA). The VerifyNow® assay measures adenosine diphosphate-induced platelet function in terms of the increase in light transmittance and reports values in P2Y12 reaction units (PRU).9 The assay was performed before the LD or MD treatment, and at 4 and 12 weeks after treatment.
Genetic Analysis A QIAamp Blood Kit (Qiagen, Hilden, Germany) was used to isolate genomic DNA. Genotyping of CYP2C19 single-nucleotide polymorphisms (SNP; 681G>A and 636G>A) was performed using the Invader DNA assay method (Third Wave Technologies, Madison, WI, USA). SNP genotype was translated into a star-allele genotype and the patients were classified into 3 phenotypes, as follows: extensive metabolizer (EM; *1/*1), intermediate metabolizer (IM; *1/*2, *1/*3), and PM (*2/*2, *2/*3, *3/*3).
Statistical AnalysisSample Size We conducted a descriptive study analyzing the efficacy and safety of prasugrel in Japanese patients undergoing elective PCI. In prior stent studies, the incidence of cardiac death/MI ranged from 2.9 to 5.9% after 9 months of treatment with a thienopyridine together with aspirin.10–14 With an estimated incidence of events of 3.0–3.5%, a 95% confidence interval (95% CI) of the incidence ranging from 1.0 to 2.1 in 300 subjects, and an upper limit of the 95% CI of <5.9%, 300 needed to be enrolled in the prasugrel group. To provide reference data, we also planned to enroll a similar number of patients in the clopidogrel group. The sample size required to determine either non-inferiority or superiority of prasugrel to clopidogrel was not calculated.
Efficacy Endpoints Intergroup analysis comparing the prasugrel and clopidogrel groups was not planned for certain efficacy endpoints. There were 2 reasons for this. First, the study did not have a statistically adequate sample size even though an extremely low incidence of events was predicted. Second, clopidogrel was not indicated for patients with stable CAD undergoing PCI in any country at the time this study was planned and started.
Efficacy endpoints were analyzed in the full analysis set, which included all patients who received one or more doses of the study drug. The incidence rate and 95% CI were calculated for MACE and each secondary efficacy endpoint occurring between day 1 and week 24. The cumulative incidence of MACE in each group was estimated using the Kaplan-Meier method.
Safety The incidence rates and 95% CI of each class of bleeding events were calculated for events occurring between day 1 and the final dose, and up to 14 days after discontinuation of the study drug.
Genetic and Pharmacodynamic Variables Genetic and pharmacodynamic variables were evaluated descriptively and are presented as mean±SD. Platelet aggregation (ie, PRU) was compared among the 3 subgroups of metabolizers (ie, EM, IM, and PM) within each treatment group using Kruskal-Wallis and Steel-Dwass tests. Genetic and pharmacodynamic variables were not statistically compared between the 2 treatment groups.
Figure S2 presents the flow of patients through the study. Overall, 751 patients were randomized: 377 to prasugrel and 374 to clopidogrel. Of these, 742 were included in the efficacy and safety evaluations (prasugrel, n=370; clopidogrel, n=372). The mean duration of exposure was similar in both groups and all patients were followed up for 24 weeks. One hundred and seventy-nine subjects were followed for up to 48 weeks (24–48 weeks); among these, the rate of completion of follow-up was 4.6% (17/370) in the prasugrel group and 7.5% (28/372) in the clopidogrel group.
As shown in Table 1, the main characteristics of the 2 groups were similar, with no marked differences. Overall, 30% of the patients were women, the mean age and weight were 67 years and 64 kg, respectively, and 30% of patients weighed <60 kg. Approximately 80% of patients had stable angina. Complications included hypertension (81%), dyslipidemia (81%), and diabetes (38%). Prior cerebral infarction, prior transient ischemic attack (TIA), and current asymptomatic cerebral infarction were found in 10% of patients. The primary revascularization procedure was similar between the 2 groups, with drug-eluting stents used in ≥90% of patients in each group.
| Prasugrel (n=370) | Clopidogrel (n=372) | |
|---|---|---|
| Female | 96 (25.9) | 109 (29.3) |
| Age (years) | 67.5±7.5 | 67.4±7.4 |
| ≥75 | 86 (23.2) | 93 (25.0) |
| Body weight (kg) | 64.0±10.87 | 63.7±11.54 |
| ≤50 | 34 (9.2) | 40 (10.8) |
| ≤60 | 131 (35.4) | 146 (39.2) |
| BMI (kg/m2) | 24.49±3.14 | 24.64±3.42 |
| Current smoker | 67 (18.1) | 63 (16.9) |
| Underlying disease | ||
| Stable angina | 277 (74.9) | 284 (76.3) |
| Prior MI | 21 (5.7) | 16 (4.3) |
| Unstable angina | 30 (8.1) | 35 (9.4) |
| Silent myocardial ischemia | 41 (11.1) | 33 (8.9) |
| Medical history | ||
| Hypertension | 295 (79.7) | 304 (81.7) |
| Dyslipidemia | 296 (80.0) | 305 (82.0) |
| Diabetes | 150 (40.5) | 132 (35.5) |
| Prior ischemic stroke | 14 (3.8) | 9 (2.4) |
| Prior transient ischemic attack | 3 (0.8) | 2 (0.5) |
| Asymptomatic cerebral infarction | 20 (5.4) | 29 (7.8) |
| Revascularization vessel | ||
| Right coronary artery | 120 (32.4) | 109 (29.3) |
| Left main coronary trunk | 1 (0.3) | 1 (0.3) |
| LAD | 179 (48.4) | 182 (48.9) |
| Left circumflex coronary artery | 90 (24.3) | 83 (22.3) |
| Stent type (first PCI) | ||
| Bare metal stent | 37 (10.3) | 28 (8.2) |
| Drug-eluting stent | 324 (90.5) | 319 (93.0) |
Data given as n (%) or mean±SD. Differences between the 2 treatment groups were not tested for statistical significance. BMI, body mass index; LAD, left anterior descending coronary artery; MI, myocardial infarction; PCI, percutaneous coronary intervention.
Figure 1 shows the Kaplan-Meier cumulative incidence rate of MACE from day 1 to week 24. The incidence rate was 4.1% (15/370) in the prasugrel group and 6.7% (25/372) in the clopidogrel group. Figure 2 shows the cumulative incidence rate of MACE from day 1 to week 24 in patients who received the LD (LD+) and those who did not (LD−) according to treatment received. As shown in Figure 2, the cumulative MACE incidence rates for the 2 drugs were similar in both subsets of patients. The Kaplan-Meier cumulative MACE incidence rate at 48 weeks was 5% in the prasugrel group and 7.9% in the clopidogrel group.
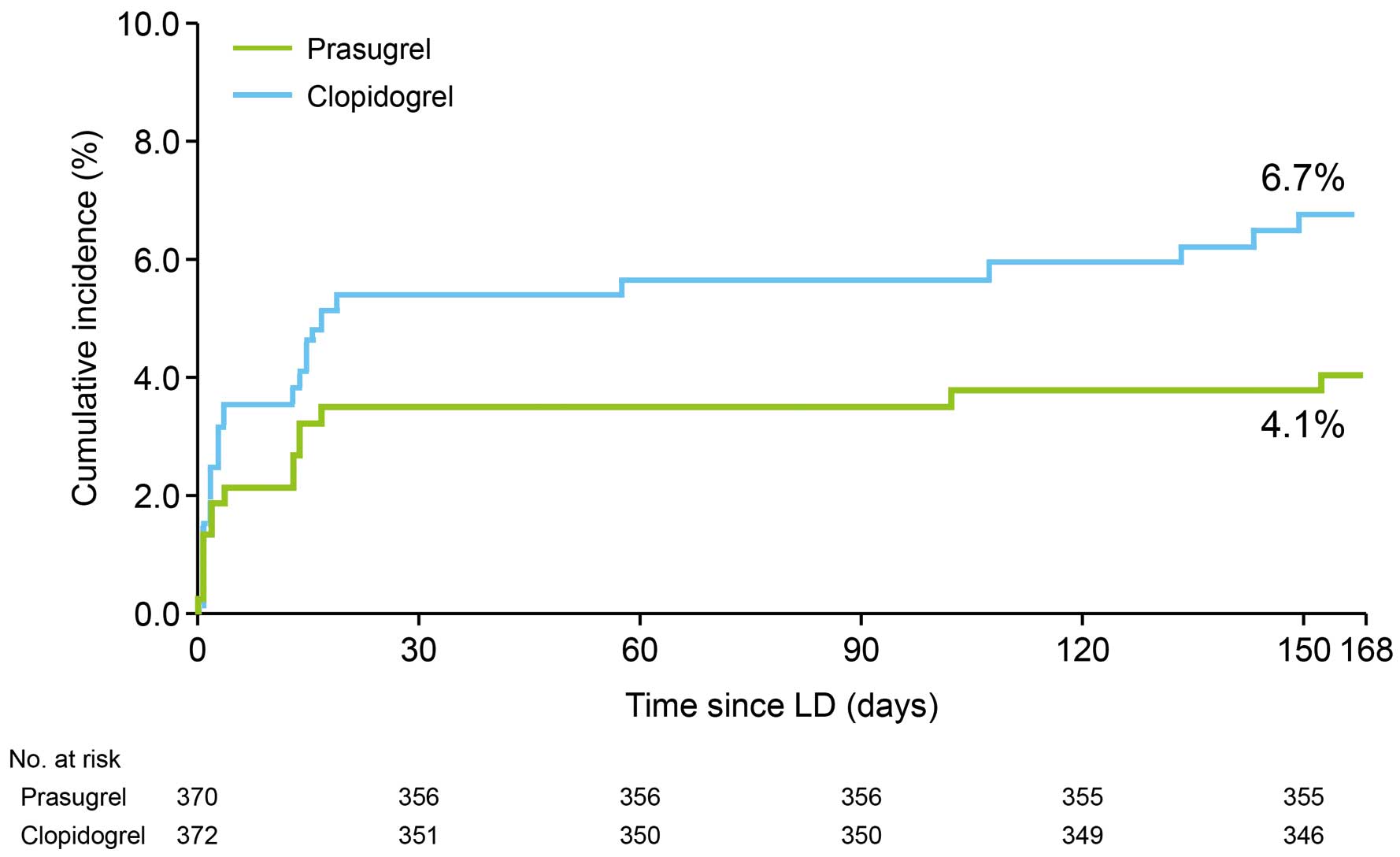
Kaplan-Meier cumulative incidence of major adverse cardiovascular events over 24 weeks of treatment (full analysis set). The difference between the 2 groups was not tested for statistical significance. LD, loading dose.

Kaplan-Meier cumulative incidence of major adverse cardiovascular events over 24 weeks of treatment in patients who did (LD+) or did not (LD−) receive the loading dose (full analysis set). Differences between the 2 treatment groups were not tested for statistical significance. LD, loading dose.
Table 2 lists the incidence rates of the MACE and the individual efficacy endpoints. The most frequent event was non-fatal MI, which occurred in 3.2% and in 6.5% of patients in the prasugrel and clopidogrel groups, respectively. There were no deaths (cardiovascular or non-cardiovascular), and the incidences of other events were similar in both groups.
| Endpoint | Prasugrel (n=370) | Clopidogrel (n=372) |
|---|---|---|
| MACE | 15 (4.1) [2.3, 6.6] | 25 (6.7) [4.4, 9.8] |
| CV death | 0 (0.0) [0.0, 1.0] | 0 (0.0) [0.0, 1.0] |
| Non-fatal MI | 12 (3.2) [1.7, 5.6] | 24 (6.5) [4.2, 9.4] |
| Non-fatal ischemic stroke | 3 (0.8) [0.2, 2.4] | 1 (0.3) [0.0, 1.5] |
| All-cause death | 0 (0.0) [0.0, 1.0] | 0 (0.0) [0.0, 1.0] |
| Non-fatal stroke | 3 (0.8) [0.2, 2.4] | 2 (0.5) [0.1, 1.9] |
| Hospitalization for angina | 0 (0.0) [0.0, 1.0] | 1 (0.3) [0.0, 1.5] |
| Revascularization | 8 (2.2) [0.9, 4.2] | 9 (2.4) [1.1, 4.5] |
| Stent thrombosis | 0 (0.0) [0.0, 1.0] | 1 (0.3) [0.0, 1.5] |
Data given as n (%) [95% confidence interval]. Differences between the 2 treatment groups were not tested for statistical significance. CV, cardiovascular; MI, myocardial infarction; MACE, major adverse cardiovascular event.
Figure 3 shows the incidence rates of non-CABG-related bleeding events. TIMI major bleeding occurred in 0% (0/370) of patients in the prasugrel group and in 2.2% (8/372) of patients in the clopidogrel group. The incidence rates of major/minor bleeding were 1.6% and 3.0%, and those for all bleeding events were 38.1% and 34.4% in the prasugrel and in the clopidogrel groups, respectively. Bleeding events as a complication of PCI (classified as TIMI major or minor) occurred in 0.8% of patients in the prasugrel group and in 0.5% of patients in the clopidogrel group. There were very few bleeding events classified as spontaneous bleeding or bleeding with an exogenous cause.

Incidence of bleeding events unrelated to coronary artery bypass grafting (safety analysis set). Differences between the 2 treatment groups were not tested for statistical significance. PCI, percutaneous coronary intervention; TIMI, Thrombolysis in Myocardial Infarction.
Adverse events occurred in 82.2% (304/370) of patients treated with prasugrel and in 78.5% (292/372) of patients treated with clopidogrel (Table 3). Serious adverse events occurred in 20.8% (77/370) and in 21.0% (78/372) of patients treated with prasugrel and clopidogrel, respectively. Adverse events requiring drug withdrawal occurred in 4.9% (18/370) and in 5.4% (20/372) of patients treated with prasugrel and clopidogrel, respectively.
| Prasugrel (n=370) | Clopidogrel (n=372) | |
|---|---|---|
| Adverse events (all) | 304 (82.2) | 292 (78.5) |
| Adverse events (drug-related) | 160 (43.2) | 148 (39.8) |
| Serious adverse events (all) | 77 (20.8) | 78 (21.0) |
| Deaths (all) | 0 (0.0) | 0 (0.0) |
| Serious adverse events (drug-related) | 14 (3.8) | 17 (4.6) |
| Adverse events leading to treatment withdrawal (all) | 18 (4.9) | 20 (5.4) |
| Adverse events considered related to the study drug in ≥5% of patients | ||
| Hemorrhage subcutaneous | 47 (12.7) | 34 (9.1) |
| Epistaxis | 21 (5.7) | 22 (5.9) |
Data given as n (%). Differences between the 2 treatment groups were not tested for statistical significance.
Figure 4 shows the changes in platelet aggregation (ie, PRU) over time in the LD+ and LD− groups. In the prasugrel-treated LD+ group, platelet aggregation was 324.7±54.83 PRU before the first dose and 135.8±94.49 PRU at 3–6 h after the LD. It then plateaued between weeks 4 and 48. In the prasugrel-treated LD− group, platelet aggregation was 283.4±79.44 PRU at baseline and 214.4±76.29 PRU shortly before PCI. It then plateaued until week 48.

Platelet aggregation measured using the VerifyNowTM assay (pharmacodynamic analysis set). Differences between the 2 treatment groups were not tested for statistical significance. LD, loading dose; MD, maintenance dose; PCI, percutaneous coronary intervention; PRU, P2Y12 reaction unit.
Gene Polymorphisms In terms of CYP2C19 polymorphisms, the frequency of star alleles was similar between the 2 groups. Overall, 31.8% (83/261) and 32.3% (84/260) of patients in the prasugrel and clopidogrel groups, respectively, were classified as EM, while 48.3% (126/261) and 48.8% (127/260), respectively, were classified as IM, and 19.9% (52/261) and 18.8% (49/260), respectively, were classified as PM.
Pharmacodynamics There was no apparent relationship between the number/presence of CYP2C19*2 or *3 alleles and platelet aggregation in prasugrel-treated LD+ or LD− patients. Figure 5 shows platelet aggregation at week 4 in the prasugrel and clopidogrel groups according to phenotype (EM, IM, and PM). In the prasugrel group at week 4, platelet aggregation was 181.2±68.63 PRU, 195.0±69.98 PRU, and 205.2±74.95 PRU in the EM, IM, and PM subgroups, respectively, and was not significantly different (P=0.2910). In contrast, in the clopidogrel group, platelet aggregation at week 4 was 212.7±79.41 PRU, 247.5±65.13 PRU, and 303.9±41.43 PRU, respectively, in the EM, IM, and PM groups, with significant differences between PM and IM subgroups (P<0.001) and between IM and EM subgroups (P<0.001).

Effect of CYP2C19 phenotype on platelet aggregation at week 4 (pharmacodynamic analysis set). Platelet aggregation was measured using the VerifyNowTM assay. EM, extensive metabolizer; IM, intermediate metabolizer; PM, poor metabolizer; PRU, P2Y12 reaction unit. *P<0.005.
In the current study, MACE generally occurred in the periprocedural period, followed by a slight increase that was maintained until week 24. The majority of events were non-fatal MI, often associated with the initial PCI, and the overall incidence rate of MACE was similar between the LD+ and LD− patients. Similarly, in the CLEAN study in which patients undergoing elective PCI received clopidogrel or ticlopidine, no cardiovascular death occurred during the first 12 weeks of treatment in the clopidogrel group, and the incidence rates of acute MI and ischemic stroke were 7.7% and 0.2%, respectively,8 similar to those observed in the present clopidogrel group (6.5% and 0.3%, respectively).
In the PRASFIT-ACS study involving Japanese ACS patients undergoing PCI, the incidence rates of MACE observed from day 1 to week 24 were 9.3% in the prasugrel group and 11.8% in the clopidogrel group.7 In TRITON-TIMI 38, which was performed in ACS-PCI patients in Western countries,6 it seemed that the difference in the event rate between clopidogrel and prasugrel was at least partly due to a difference in the efficacy of the LD. The present data suggest that prasugrel at an LD of 20 mg and an MD of 3.75 mg, or an MD of 3.75 mg alone achieved sufficient platelet inhibition and reduced the incidence of MACE in Japanese patients undergoing elective PCI.
In subgroup analysis, there was no appreciable effect of background factors, such as age, body weight, and the presence/absence of diabetes, on the incidence rates of efficacy events, similar to the PRASFIT-ACS results.7
With regard to safety, the incidence rates of non-CABG-related bleeding events that occurred between the initial dose and 14 days after discontinuation were similar between the prasugrel and clopidogrel groups. Therefore, consistent with the earlier phase II study (Kimura et al, unpublished data, 2014), prasugrel did not appear to increase the risk of bleeding in patients undergoing elective PCI. Furthermore, although TIMI major bleeding occurred in some patients in TRITON-TIMI 38,6 this class of events did not occur in the present prasugrel group and occurred only in 2.2% of patients in the clopidogrel group. These results are similar to those of the PRASFIT-ACS study7 and suggest that prasugrel adjusted for Japanese patients did not increase the risk of TIMI major bleeding in patients with ACS or those undergoing elective PCI in these studies as compared with clopidogrel.
In TRITON-TIMI 38, the incidence of bleeding events was greater in patients with prior stroke/TIA. Accordingly, in the present study prasugrel was given to Japanese patients at doses lower than those used in international studies to reduce the risk of bleeding events. Although patients with prior stroke/TIA together with asymptomatic cerebral infarction were included in the current study, there were no major or minor bleeding events in the prasugrel group, suggesting that the absence of these events was due to the lower LD and MD used in this study.
With regard to pharmacodynamics, in the prasugrel group, PRU was lower at 3–6 h after the LD than at baseline, and decreased to a level similar to that observed at 5–12 h after the LD in the PRASFIT-ACS study,7 confirming that prasugrel achieves rapid inhibition of platelet aggregation. In patients who did not receive the LD, PRU decreased between baseline and just before PCI, which was followed by a plateau until week 48.
Platelet aggregation (ie, PRU) measured at week 4 in the prasugrel group was not affected by CYP2C19 genotype. In contrast, there were significant differences in platelet aggregation according to CYP2C19 genotype in the clopidogrel group, suggesting that CYP2C19 polymorphisms have an adverse effect on inhibition of platelet aggregation. This is in line with a study in Japanese patients undergoing coronary stent implantation that showed that the CYP2C19 loss-of-function genotype was associated with more frequent high platelet reactivity, with a significant gene-dose effect observed in EM, IM and PM.15
In studies performed in Western countries, cardiovascular events mainly occurred in patients with decreased clopidogrel function.16,17 In the present study, although the clopidogrel regimen was the same as that used in Western studies, such as TRITON-TIMI 38,6 the prasugrel dosing regimen was approximately one-third of that used in Western studies (20/3.75 mg vs. 60/10 mg), and was the same as that used in PRASFIT-ACS.7 Notably, the present results and those of PRASFIT-ACS suggest that these lower doses of prasugrel achieved similar risk reduction rates, as seen in TRITON-TIMI 38. In particular, the present prasugrel regimen achieved consistent antiplatelet effects in a cohort of Japanese patients, even though 20% of these patients were considered poor metabolizers of clopidogrel owing to CYP2C19 polymorphisms.
Some limitations of this study warrant mention. First, clopidogrel was used as a reference drug and we did not assess whether the differences between the 2 groups were statistically significant. Second, relatively few patients aged ≥85 or <45 years were enrolled, and the number of elderly patients with low body weight was small, limiting the ability to assess the efficacy and safety of prasugrel in such patients. Therefore, further studies that specifically enroll these patients may be needed. Furthermore, the study was not designed to show non-inferiority or superiority of prasugrel relative to clopidogrel, partly because the expected rate of MACE in prasugrel-treated patients was unknown at the time of the study. The present results, however, can provide a basis for future larger studies examining the efficacy and safety of prasugrel.
The cumulative incidence rates of MACE from day 1 to week 24 in patients undergoing elective PCI were 4.1% in the prasugrel group and 6.7% in the clopidogrel group, with similar values in patients who did or did not receive a LD. TIMI major bleeding, major/minor bleeding, and all bleeding events occurred in 0%, 1.6%, and 38.1% of patients, respectively, with similar rates observed in the clopidogrel group (2.2%, 3.0%, and 34.4%, respectively). Larger studies are required to further explore these findings. The results of the PRASFIT-Elective study in CAD patients undergoing elective PCI were consistent with those reported in the PRASFIT-ACS study in ACS patients undergoing PCI, and highlight the risk-benefit profile of an adjusted dosing regimen of prasugrel in Japanese patients undergoing PCI.
The authors greatly appreciate the contributions of the investigators and other clinical/research staff involved in the present study. We thank Nicholas D. Smith, PhD, and Helen Roberton for providing assistance with the manuscript. We also acknowledge the support of the institutions that participated in or helped implement the PRASFIT-Elective study, as listed in the supplementary materials.
The authors declare the following interests: Takaaki Isshiki has received honoraria from AstraZeneca, Daiichi Sankyo, Otsuka Pharmaceutical, and Sanofi; Takeshi Kimura has received honoraria, clinical research funding, and other research funding from Daiichi Sankyo and Sanofi; Hisao Ogawa has received honoraria from AstraZeneca, Bayer Yakuhin, Boehringer Ingelheim Japan, Daiichi Sankyo, Dainippon Sumitomo Pharma, Eisai, Kyowa Hakko Kirin, Mitsubishi Tanabe Pharma Corporation, MSD, Pfizer Japan, Sanofi, and Takeda Pharmaceutical, clinical research funding from Daiichi Sankyo, and other research funding from Astellas Pharma, AstraZeneca, Boehringer Ingelheim Japan, Chugai Pharmaceutical, Daiichi Sankyo, Eisai, Kowa Company, Mitsubishi Tanabe Pharma Corporation, MSD, Novartis Pharma, Otsuka Pharmaceutical, Shionogi & Co, and Takeda Pharmaceutical; Hiroyoshi Yokoi has no conflicts of interest to declare; Shinsuke Nanto has received honoraria from Daiichi Sankyo, Medtronic, Otsuka Pharmaceutical, Sanofi, and Takeda Pharmaceutical, clinical research funding from Abbott Vascular Japan and Terumo, other research funding from Abbott Vascular Japan, Boston Scientific Japan, Daiichi Sankyo, Medtronic, Sanofi and St. Jude Medical Japan, and an endowment from Terumo; Morimasa Takayama is a clinical advisor for Abbott Vascular Japan and Kaneka Medics, and has received honoraria from Daiichi Sankyo; Kazuo Kitagawa has received honoraria from Sanofi, and other research funding from Boehringer Ingelheim Japan and Sanofi; Masakatsu Nishikawa is a medical advisor for and holds stock in D. Western Therapeutics, and has received honoraria from Daiichi Sankyo and Otsuka Pharmaceutical, and other research funding from Otsuka Pharmaceutical; Shunichi Miyazaki has received other research funding from MSD; Yasuo Ikeda has received research funding from Daiichi Sankyo and Sanofi; Masato Nakamura has received honoraria from Daiichi Sankyo, Sanofi, and AstraZeneca; Shigeru Saito is a medical advisor for Terumo and has received honoraria from Abbot Vascular Japan, Boston Scientific Japan, and Medtronic.
Financial Support: This study was sponsored by Daiichi Sankyo (Tokyo, Japan).
Supplementary File 1
Institutions that participated in or helped implement the PRASFITElective Study
Figure S1. Study design.
Figure S2. Flow of patients through the study.
Please find supplementary file(s);
http://dx.doi.org/10.1253/circj.CJ-14-0266