Abstract
Background:
The aim of this study was to investigate the incidence and predictors (which have not previously been fully elucidated) of late-phase thromboembolism (TE) after catheter ablation (CA) for paroxysmal atrial fibrillation (AF).
Methods and Results:
We studied 1,156 consecutive patients (61±10 years; 891 men; CHADS2
score, 0.8±1.0) after CA for symptomatic paroxysmal AF and examined the details of late-phase TE. During a follow-up of 49.5±21.9 months (median, 47 months; range, 6–113 months) after CA, 9 patients (0.78%) developed late-phase TE, all of which were ischemic stroke. Of these, 5 patients with AF recurrence experienced cardioembolism; the AF was asymptomatic at recurrence. The remaining 4 without AF recurrence experienced cardioembolism (n=1), small-vessel occlusion (n=1), large-artery atherosclerosis (n=1), and stroke of other determined etiology (n=1). On Kaplan-Meier analysis patients with structural heart disease (P=0.003), AF recurrence after the final CA (P=0.01), prior stroke (P=0.002), CHADS2
score ≥2 (P=0.0002), left ventricular ejection fraction <50% (P<0.0001), and spontaneous echo contrast on transesophageal echocardiogram (P=0.0004) had a significantly higher risk of late-phase TE. Multivariate analysis indicated that CHADS2
score ≥2 (HR, 4.49; 95% CI: 1.08–22.56; P=0.04) independently predicted late-phase TE.
Conclusions:
The incidence of TE was low after CA for paroxysmal AF, but CHADS2
score ≥2 independently increased the risk of late-phase TE.
Catheter ablation (CA) has recently become standard therapy for treating patients with atrial fibrillation (AF).1–3
Studies on the incidence of thromboembolism (TE) after CA of AF, however, are scarce.4–7
Therefore, the present study investigated the incidence and predictors of late-phase TE after CA for paroxysmal AF.
Methods
Subjects
A total of 1,156 consecutive patients (mean age, 61±10 years; men, n=891) were referred to Yokosuka Kyosai Hospital between April 2003 and August 2009 for treatment with CA for symptomatic paroxysmal AF refractory to anti-arrhythmic drugs (AAD;
Table 1). AF was defined as paroxysmal when it terminated spontaneously within 7 days.8
All patients provided written informed consent before the CA procedures, and the institutional review board approved the study protocol.
Table 1.
Patient Characteristics (n=1,156)
| Characteristics |
|
| Patient age (years) |
61±10 |
| Age ≥75 years |
70 (6.1) |
| Female |
265 (22.9) |
| BMI |
23.6±3.0 |
| Obesity (BMI ≥25) |
346 (29.9) |
| SHD |
216 (18.6) |
| Hyperlipidemia |
329 (28.5) |
| CHF |
82 (7.1) |
| Hypertension |
513 (44.4) |
| Diabetes |
123 (10.6) |
| Stroke |
89 (7.7) |
| CHADS2 score |
0.8±1.0 |
| CHADS2 score ≥2 |
223 (19.3) |
| Echocardiography |
| LAD (mm) |
37.8±5.1 |
| LAD >40 mm |
406 (35.1) |
| LVEF (%) |
66.2±1.2 |
| LVEF <50% |
38 (3.3) |
| SEC |
61 (5.3) |
| AF recurrence after final CA |
188 (16.3) |
| Anticoagulation therapy after final CA |
159 (13.8) |
| Antiplatelet therapy after final CA |
144 (12.5) |
Data given as n (%) or mean±SD. The CHADS2
score is the sum of 1 point each for congestive heart failure, hypertension, age ≥75 years and diabetes mellitus, and 2 points for prior stroke or transient ischemic attack.
AAD, anti-arrhythmic drug; AF, atrial fibrillation; BMI, body mass index; CA, catheter ablation; CHF, congestive heart failure; CHADS2, congestive heart failure, hypertension, age ≥75 years, diabetes mellitus, and prior stroke or transient ischemic attack; LAD, left atrial dimension of end-systole; LVEF, left ventricular ejection fraction; SEC, spontaneous echo contrast; SHD, structural heart disease.
All AAD were discontinued for ≥7 days (amiodarone was discontinued for ≥1 month) before ablation. After at least 1-month oral anticoagulation therapy (OAT),9
the absence of any thrombus was confirmed on transesophageal echocardiography the day before the procedure. A 7-Fr, 20- or 14-pole, 2-site mapping catheter (Irvine Biomedical, Irvine, CA, USA) was inserted through the right jugular vein and positioned in the coronary sinus for pacing, recording, and internal cardioversion. These manipulations were conducted under sedation with i.v. propofol or dexmedetomidine, in the fasting state.
CA
The strategy of extensive pulmonary vein isolation (EPVI) has been described previously.10,11
Briefly, after a transseptal puncture, pulmonary venography and contrast esophagography were performed to determine the anatomical relationships of the PV ostia, left atrium (LA), and esophagus. An activated clotting time of 250–350 s was maintained with continuous infusion of heparin during the procedure.12
Two circular mapping catheters were placed in the superior and inferior PV, and the left and right ipsilateral PV were circumferentially and extensively ablated under fluoroscopic and electrophysiological guidance. Radiofrequency current was delivered using an 8-mm tip ablation catheter (Japan Lifeline, Tokyo, Japan) in the temperature control mode, with a target temperature of 55°C (maximum power, 35 W on the LA posterior wall and 40 W at the anterior aspect of the PV). The esophageal temperature was measured during current application to avoid esophagus-related complications,10,13
and the endpoint was the elimination of all PV potentials. After completing EPVI, adenosine triphosphate (20–40 mg) was injected to identify any dormant conductions, which were then disconnected.14
Isoproterenol (5–20 μg/min) was injected i.v. before the procedure was completed. If sustained or non-sustained AF were reproducibly initiated from non-PV foci, they were focally ablated.15
When non-PV foci were located at the superior vena cava (SVC), the SVC was electrically isolated.16,17
Rapid atrial pacing was performed to induce AF, if it did not occur spontaneously. After an episode of pacing-induced AF was sustained, internal cardioversion was attempted to convert the AF to sinus rhythm (SR), and AF foci were focally ablated if spontaneous re-initiation of AF occurred. Linear ablation was also conducted, if required, only when AF from undetermined origins or macroreentrant atrial tachycardia occurred spontaneously, with an endpoint of a bidirectional conduction block. At the end of the procedure, the endpoints of all procedures performed were re-confirmed.
Follow-up
All patients were prospectively followed up at 2, 6, 10, 14, 24, 36, and 48 weeks after the procedure and underwent 12-lead electrocardiography (ECG) at each visit and Holter monitoring every 3 months. Thereafter, the patients were followed up every 1–3 months at Yokosuka Kyosai Hospital or examined by a general physician. An annual telephone interview to patients and their families was performed to confirm patient history of hospitalization each year. Patients with symptoms received 1-month event recorder. At physician discretion, anticoagulation therapy was usually discontinued after 3–6 months in patients without AF recurrence and low TE risk. AAD were not prescribed after the procedure and successful ablation was defined as the absence of any atrial tachyarrhythmias for >30 s, in the absence of AAD, after a blanking period of 1 month, during which atrial tachyarrhythmias were considered a transient phenomenon. Repeat CA was usually recommended in patients who experienced atrial tachyarrhythmias after this time point. Re-initiation of AAD was considered, if necessary, when patients with AF recurrence did not undergo repeat CA for some reason.
Patients suspected of symptomatic TE were usually brought to Yokosuka Kyosai Hospital by ambulance. If ischemic stroke was suspected, neurologists analyzed the detailed medical history, laboratory findings, ECG and echocardiography, as well as imaging (magnetic resonance imaging and computed tomography) to precisely classify the type of stroke according to the TOAST classification: large artery atherosclerosis, cardioembolism, small vessel occlusion, stroke of other determined etiology, and stroke of undetermined etiology.18
When patients were admitted for TE at other institutions, their precise medical history and examination results were collected from the neurologists who treated them during the hospitalization. Late-phase TE was defined as that occurring >1 month after CA.
Statistical Analysis
Data are expressed as mean±SD for continuous variables and as frequency and percentage for categorical variables. Comparisons between 2 groups were performed using chi-squared analysis or Fisher’s exact test for categorical variables and unpaired t-test or Wilcoxon analysis for continuous variables. All categorical variables that were significant on univariate Cox proportional analysis were included in the multivariate analysis to identify significant risk factors and to calculate hazard ratios (HR) and 95% confidence intervals (95% CI). The follow-up period was calculated from the date of the final procedure to that of the event (TE) or censoring. TE-free survival rate was calculated using Kaplan-Meier survival analysis, and log-rank statistics were used for group comparisons. P<0.05 was considered statistically significant.
Results
Patient Characteristics and AF Recurrence
Table 1
lists the characteristics of the 1,156 enrolled patients (mean age, 61±10 years; mean CHADS2
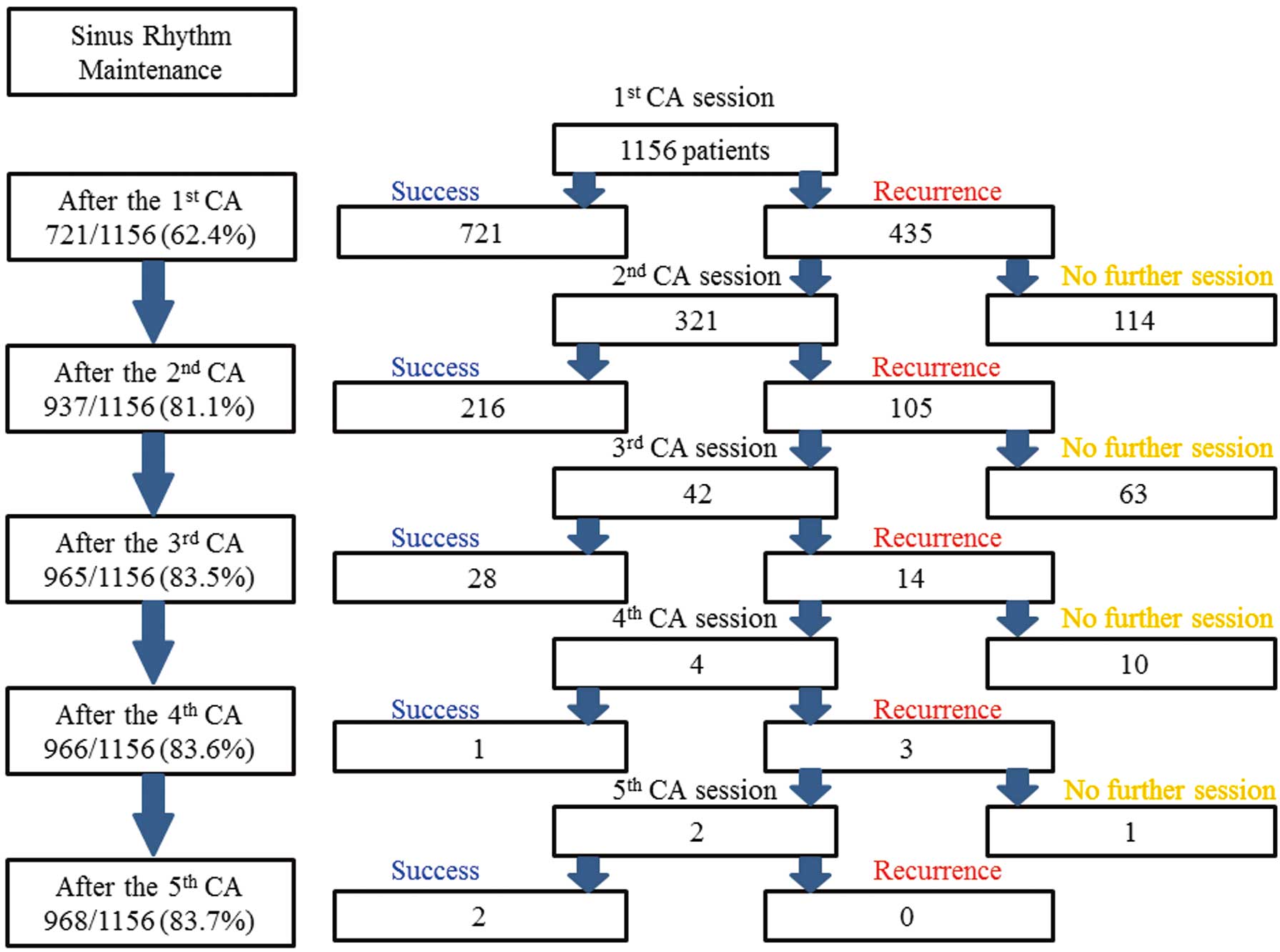
score, 0.8±1.0). In total, 1,525 CA sessions were carried out (mean, 1.3±0.6 CA sessions per patient;
Figure 1). Over the study duration, 835 patients (72.2%) had 1 CA session, 279 (24.1%) had 2 CA sessions, 38 (3.3%) had 3 CA sessions, 2 (0.2%) had 4 CA sessions, and 2 (0.2%) had 5 CA sessions. During the entire procedure, 289 patients (25.0%) had non-PV AF foci. Ultimately, SR was maintained in 968 patients (83.7%).
Late-Phase TE
During a mean follow-up period of 49.5±21.9 months after the final CA, 9 patients (0.78%), including 1 woman, developed late-phase TE (0.19%/year;
Table 2); all TE were ischemic strokes, which occurred after the final ablation, and no other systemic emboli occurred. Four of them were admitted to Yokosuka Kyosai Hospital, and the other 5 to other medical emergency centers. The mean interval between the date of the final CA session and the occurrence of late-phase TE was 43.4±21.1 months. The incidence of late-phase TE was higher among patients with AF recurrence than among those without (5/188, 2.66% for AF recurrence vs. 4/968, 0.41% for non-recurrence, P=0.001). As shown in
Table 2, when stroke patients with (patients 1–5) or without (patients 4–7) AF recurrence were compared, the latter tended to have a higher incidence of hypertension (2/5, 40% for AF recurrence vs. 4/4, 100% for non-recurrence, P=0.06) and structural heart disease (2/5, 40% for AF recurrence vs. 4/4, 100% for non-recurrence, P=0.06) and a lower left ventricular ejection fraction (LVEF; 65.9%±11.3% for AF recurrence vs. 46.4%±14.9% for non-recurrence, P=0.05).
Table 2.
Summary of Patients With Late-Phase TE
| Patient no. |
1 |
2 |
3 |
4 |
5 |
6 |
7 |
8 |
9 |
| AF recurrence |
+ |
+ |
+ |
+ |
+ |
– |
– |
– |
– |
| AF symptoms |
Asymptomatic |
Asymptomatic |
Asymptomatic |
Asymptomatic |
Asymptomatic |
No AF |
No AF |
No AF |
No AF |
| Age (years) |
59 |
59 |
60 |
72 |
75 |
71 |
66 |
71 |
72 |
| Age at the time of stroke (years) |
60 |
63 |
63 |
76 |
77 |
77 |
70 |
72 |
77 |
| Gender |
M |
M |
M |
F |
M |
M |
M |
M |
M |
| Interval to stroke (months) |
18.4 |
51.1 |
34.3 |
42.5 |
30.9 |
76.3 |
54.2 |
14.9 |
68.3 |
| Anticoagulation therapy |
– |
– |
– |
+ |
– |
– |
– |
– |
– |
| Antiplatelet therapy |
– |
– |
– |
– |
+ |
– |
+ |
– |
+ |
| SHD |
DCM |
VHD |
– |
– |
– |
DCM |
OMI |
HCM |
OMI |
| CHF |
– |
– |
– |
– |
– |
+ |
– |
– |
+ |
| Hypertension |
– |
– |
+ |
– |
+ |
+ |
+ |
+ |
+ |
| Age >75 years |
– |
– |
– |
– |
+ |
– |
– |
– |
– |
| Diabetes |
+ |
– |
– |
– |
– |
– |
– |
– |
– |
| Stroke |
+ |
– |
+ |
– |
– |
– |
+ |
– |
– |
| CHADS2 score |
3 |
0 |
3 |
0 |
2 |
2 |
3 |
1 |
2 |
CHADS2 score at time of
stroke |
3 |
0 |
3 |
1 |
2 |
3 |
3 |
1 |
3 |
| LAD (mm) |
41 |
38.5 |
31 |
33.5 |
42 |
50.2 |
39 |
35 |
41 |
| LVEF (%) |
46 |
70.4 |
73 |
68.1 |
72 |
28.2 |
51 |
63.8 |
42.5 |
| SEC |
+ |
– |
– |
– |
+ |
+ |
– |
– |
– |
| TOAST classification |
CE |
CE |
CE |
CE |
CE |
Other
etiology |
CE |
SVO |
LAA |
| Cerebral lesion |
Left MCA |
Left PCA |
Right PCA |
Right MCA |
Right ICA,
Right MCA |
Left ICA,
VBA |
Right
MCA |
Left
thalami |
Boundary
of right
MCA and
PCA |
CE, cardioembolism; DCM, dilated cardiomyopathy; F, female; HCM, hypertrophic cardiomyopathy; ICA, internal carotid artery; LAA, large-artery atherosclerosis; M, male; MCA, middle cerebral artery; OMI, old myocardial infarction; PCA posterior cerebral artery; SVO, small-vessel occlusion; TE, thromboembolism; TOAST, Trial of Org 10172 in Acute Stroke Treatment [The TOAST classification system includes five categories: 1) large-artery atherosclerosis, 2) cardioembolism, 3) small-vessel occlusion (lacune), 4) stroke of other determined etiology, and 5) stroke of undetermined etiology]; VBA, vertebrobasilar artery; VHD, valvular heart disease. Other abbreviations as in Table 1.
In the 5 patients with AF recurrence (patients 1–5), AF was asymptomatic, and these patients were all considered to have cardioembolism according to the TOAST classification. In 4 individuals (patients 1–4), AF recurrence was not detected until they were hospitalized for TE because of the asymptomatic nature of the recurrence. Furthermore, AF was not detected on continuous ECG monitoring during hospitalization or frequent Holter monitoring after discharge. The remaining 1 patient (patient 5) was incidentally diagnosed with asymptomatic AF recurrence during routine follow-up ECG, and anticoagulation therapy was re-initiated. That patient, however, eventually developed TE. Among the 4 patients without AF recurrence (patients 6–9), only 1 (patient 7) was diagnosed with cardioembolism. This patient had received an implantable cardiac defibrillator for the treatment of LV aneurysm-related ventricular tachycardia due to a previous myocardial infarction, and intracardiac ECG did not show evidence of AF in this patient. Finally, LV thrombus at the aneurysm site was considered the cause of the stroke. Patient 6 was diagnosed with a stroke of other determined etiology according to the TOAST classification; that patient was affected by multiple TE due to LV microthrombi associated with marantic endocarditis resulting from terminal gastric signet ring cell carcinoma. The patient died because of repeated cerebral infarctions.
CHADS2
Score
Table 3
lists the relationships between CHADS2
score, AF recurrence rate, and OAT. Overall, the incidence of TE tended to be higher in patients with CHADS2
score ≥2 compared to those with CHADS2
score <2 in both the total group and the subset of patients not receiving OAT, regardless of AF recurrence. This tendency, however, does not appear to be remarkable among patients receiving OAT.
Table 3.
Incidence of TE vs. CHADS
2 Score and Presence of OAT
| CHADS2 score |
Total |
0 |
1 |
P-value† |
≥2 |
| All patients |
| Total (%) |
9/1,156 (0.78) |
2/518 (0.39) |
1/415 (0.24) |
0.002 |
6/223 (2.69) |
| OAT (+) (%) |
1/158 (1.09) |
0/54 (0) |
0/57 (0) |
0.29 |
1/47 (2.13) |
| OAT (–) (%) |
8/998 (0.80) |
2/464 (0.43) |
1/358 (0.28) |
0.006 |
5/176 (2.84) |
| Patients with AF recurrence |
| Total (%) |
5/188 (2.66) |
2/87 (2.30) |
0/56 (0) |
0.09 |
3/45 (6.67) |
| OAT (+) (%) |
1/92 (1.09) |
0/41 (0) |
0/30 (0) |
0.23 |
1/21 (4.76) |
| OAT (–) (%) |
4/96 (4.17) |
2/46 (4.35) |
0/26 (0) |
0.26 |
2/24 (8.33) |
| Patients without AF recurrence |
| Total (%) |
4/968 (0.41) |
0/431 (0) |
1/359 (0.28) |
0.02 |
3/178 (1.69) |
| OAT (+) (%) |
0/66 (0) |
0/13 (0) |
0/27 (0) |
0.99 |
0/26 (0) |
| OAT (–) (%) |
4/902 (0.44) |
0/418 (0) |
1/332 (0.30) |
0.02 |
3/152 (1.97) |
†Comparison between patients with CHADS2
score <2 and ≥2.
OAT, oral anticoagulation therapy. Other abbreviations as in Tables 1,2.
SR was maintained in the 968 patients after the final CA, and anticoagulation therapy was discontinued in 902 (93.2%) of these patients, among whom 110 (12.2%) received aspirin instead. The incidence of TE was 4/902 (0.44%) among the patients not receiving anticoagulation therapy, and 0/66 (0%) among those receiving anticoagulation therapy (P=0.99). In contrast, SR was not maintained in 188 patients after the final CA, and 96 (51.1%) of them did not receive any anticoagulation therapy. The reasons for this were low CHADS2
score (≤1), successful AAD therapy for preventing frequent AF burden, contraindications for or intolerance to anticoagulation therapy, patient refusal, and unawareness of asymptomatic AF recurrence. In the AF recurrence group, the incidence of TE was 4/96 (4.17%) among those who did not receive anticoagulation therapy, whereas it was 1/92 (1.09%) among those who received anticoagulation therapy (P=0.37). The 4 patients with TE who did not receive anticoagulation therapy were unaware of AF recurrence until they were admitted for TE because the AF had recurred asymptomatically. In contrast, the remaining 1 patient developed TE despite re-initiation of anticoagulation therapy for AF recurrence, which had been incidentally detected on routine follow-up ECG.
Clinical Predictors of Late-Phase TE
On univariate Cox proportional analysis, structural heart disease, prior stroke, AF recurrence after the final CA, CHADS2
score ≥2, LVEF <50%, and spontaneous echo contrast on transesophageal echocardiogram were found to be significantly associated with late-phase TE (Table 4).
Figure 2
shows the incidence of TE among patients with and without the clinical parameters compared using log-rank analysis.
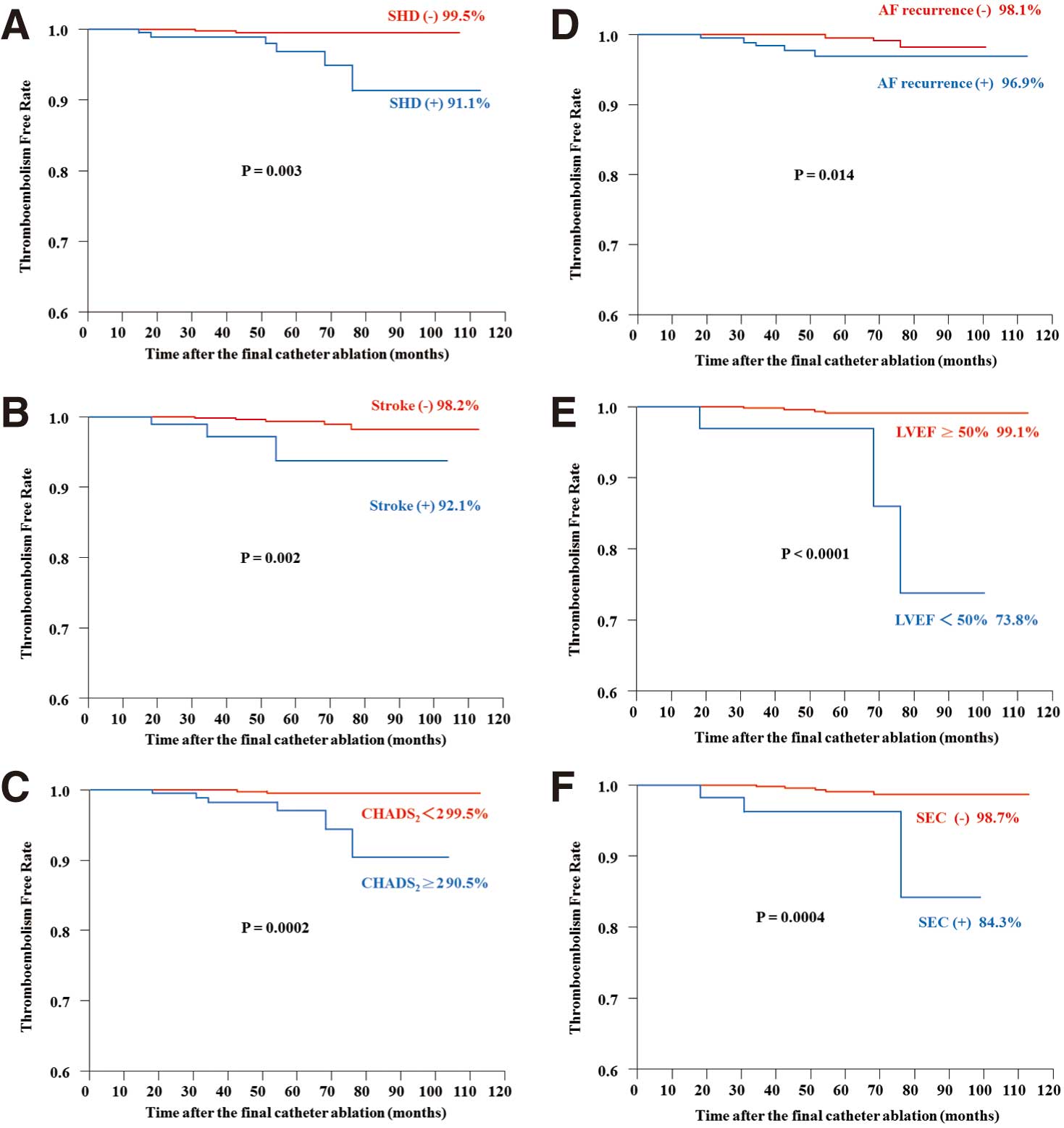
Table 4.
Significant Predictors of Late-Phase TE
| |
P-value |
HR |
95% CI |
| Univariate analysis |
| Age ≥75 years |
0.49 |
2.28 |
0.12–12.5 |
| Female |
0.34 |
0.41 |
0.02–2.23 |
| Obesity |
0.51 |
0.60 |
0.09–2.49 |
| SHD |
0.002 |
8.45 |
2.23–40.1 |
| Hyperlipidemia |
0.77 |
0.79 |
0.12–3.29 |
| CHF |
0.15 |
3.76 |
0.56–15.58 |
| Hypertension |
0.17 |
2.55 |
0.67–12.11 |
| Diabetes |
0.91 |
1.13 |
0.06–6.15 |
| Stroke |
0.02 |
6.77 |
1.43–25.74 |
| CHADS2 score ≥2 |
0.001 |
9.03 |
2.38–42.83 |
| Echocardiography |
| LAD >40 mm |
0.51 |
1.57 |
0.39–5.93 |
| LVEF <50% |
0.003 |
13.49 |
2.83–51.4 |
| SEC |
0.01 |
8.26 |
1.74–31.31 |
| AF recurrence after final CA |
0.028 |
4.53 |
1.19–18.39 |
| Anticoagulation therapy |
0.53 |
1.71 |
0.25–7.10 |
| Antiplatelet therapy |
0.16 |
2.96 |
0.62–11.26 |
| Multivariate analysis |
| SHD |
0.18 |
3.08 |
0.57–17.17 |
| CHADS2 ≥2† |
0.04 |
4.49 |
1.08–22.56 |
| AF recurrence after final CA |
0.06 |
3.79 |
0.99–15.47 |
| Echocardiography |
| LVEF <50% |
0.21 |
2.91 |
0.52–15.67 |
| SEC |
0.15 |
3.16 |
0.62–13.20 |
†CHADS2 ≥2 entirely covered prior stroke, and the correlation between these 2 parameters was so strong (r2=0.52) that only CHADS2 ≥2 was included in multivariate analysis.
CI, confidence interval; HR, hazard ratio. Other abbreviations as in Tables 1,2.
On multivariate analysis as well, CHADS2
score ≥2 (HR, 4.49; 95% CI: 1.08–22.56; P=0.04) was found to be a significant predictor of late-phase TE, and AF recurrence after the final CA (HR, 3.79; 95% CI: 0.99–15.47; P=0.06) was weakly associated with late-phase TE (Table 4).
Discussion
Major Findings
In the present study, the incidence of late-phase TE was low (0.19% per year) in patients with paroxysmal AF undergoing CA, and CHADS2
score ≥2 was an independent predictor of late-phase TE.
Incidence and Features of Late-Phase TE
The incidence of late-phase TE was low (9 patients during a mean follow-up period of 49.5±21.9 months, 0.19% per year). AF resulting in cardioembolic stroke recurred in 5 of these patients. Importantly, none of these patients had AF symptoms; in fact, in 4 patients, recurrent AF was first observed on admission for TE. Other studies have also described the frequent incidence of asymptomatic AF recurrence after CA,19,20
and in these studies it was speculated that continuation or discontinuation of AAD or AV nodal blockers after CA and detailed procedures during CA might be related to the symptoms. Although the definite factor for the absence of symptoms in this study could not be determined, β-blocker use for the treatment of heart failure and hypertension in patients 1–3 and the advanced age in patients 4 and 5 might be related to the lack of symptoms.
Although Verma et al reported that the maximum duration of asymptomatic atrial tachyarrhythmias was only 18±12 min, the present findings support the potential risk of TE in patients with asymptomatic AF recurrence after CA.19
In addition, oral anticoagulant therapy might decrease the risk of TE in patients with CHADS2
score ≥2 irrespective of rhythm status (Table 3). Cardioembolic stroke even occurred in late-phase TE patients without documented AF recurrence. The strokes in those patients, however, were not LA related or LA appendage related but LV related.
A few reports have shown that the middle- and long-term incidence of TE after CA for AF ranges from 0% to 2.7%.4–7
The largest reported series was published by Themistoclakis et al in a multicenter retrospective analysis of 3,355 patients, wherein 18% had CHADS2
score ≥2, and the mean follow-up period was 28±13 months.5
Anticoagulation therapy was terminated and replaced with aspirin in 80% of the patients after successful CA. Among the total patient group, only 5 (0.15%) had TE. Saad et al examined 327 drug-refractory AF patients with a relatively higher CHADS2
score of 1.89 and found no symptomatic ischemic cerebrovascular events during a follow-up period of 46±19 months after CA, despite termination of anticoagulation (298 patients, 91%) and AAD (293 patients, 89%). Antiplatelet therapy was used indefinitely in 308 patients (94%) and was discontinued mostly in low-risk patients (CHADS2
score =0 or 1).6
In the present study, the incidence of TE was higher than in those 2 studies. Although the discrepancy in the results may be partially attributed to the differences in the subjects and the follow-up period, the prevalence of antiplatelet therapy after the cessation of anticoagulation therapy may have potentially influenced the outcome. A higher risk of hemorrhagic stroke21
and the ineffectiveness of aspirin in the prevention of stroke in Japanese patients, however,22
may be related to the lower rate of alternative preventative antiplatelet therapy prescribed by Japanese physicians. Chao et al provided a detailed description on the incidence of TE in 565 patients with AF after CA, during a follow-up period of 39.2±22.6 months in Taiwan; 167 patients (29.6%) had AF recurrence, and 18 (3.2%) developed TE during the follow-up, including 9 with cerebral infarction, 6 with transient cerebral ischemia, 2 with pulmonary embolism, and 1 with peripheral embolism.7
Although we targeted a similar Asian patient group, a simple comparison between the present study and the previous Taiwanese study is difficult because of several differences in the study methodologies.
Clinical Predictors of Late-Phase TE
On Kaplan-Meier analysis the present patients with structural heart disease, prior stroke, CHADS2
score ≥2, AF recurrence after the final CA, LVEF <50%, and spontaneous echo contrast on transesophageal echocardiogram had a higher risk of late-phase TE. Multivariate analysis showed that CHADS2
score ≥2 was an independent predictor of late-phase TE, and that AF recurrence was weakly associated with late-phase TE. Chao et al found that AF recurrence after the final CA, and CHADS2
score were significant predictors of adverse events after CA, including TE and death.7
Hunter et al also described the significance of AF recurrence after CA as a predictor of late-phase stroke.4
Although AF recurrence may be associated with late-phase TE, more precise modalities, such as implantable loop recorders, may be required to detect asymptomatic AF recurrence and thereby determine the actual impact of AF recurrence. In the present study, no significant difference was found in the incidence of late-phase TE between patients with and without high CHADS2
score ≥2 on oral anticoagulants, regardless of rhythm status, suggesting a potential effect of OAT in higher CHADS2
score.
Clinical Implications
To the best of our knowledge, this is the first report to focus on late-phase TE after CA for paroxysmal AF and to individually examine this in detail. Although the incidence of late-phase TE after CA of paroxysmal AF was low, CHADS2
score ≥2 was independently associated with the risk of TE. Anticoagulation might be able to be recommended for patients with CHADS2
score ≥2 to minimize late-phase TE, regardless of rhythm status even after CA.
Study Limitations
This study was subject to all of the limitations inherent to a retrospective study design, but these limitations were offset by the study design: the prospective collection of clinical and demographic characteristics, ablation data, and follow-up outcome; and the large sample size. Additionally, although an irrigated ablation catheter may have improved the success rate of CA, an 8-mm tip non-irrigated catheter was used, because irrigated catheter was not available in Japan during the study period. Further, despite our efforts to perform 1-month event recording in any patient who reported symptoms, completely asymptomatic AF episodes could have been missed in these patients, and the actual recurrence rate of AF could have been higher. Although we must have obtained the precise incidence of symptomatic late-phase TE given that we had conducted a thorough annual telephone interview of patients and their families, we may have missed patients with silent ischemic stroke. Finally, the low stroke incidence in this study may not be generalized to all patients with AF and further examinations are required to elucidate the efficacy of CA for patients with persistent and permanent AF or with higher CHADS2
score.
Conclusions
The incidence of TE was low after CA for paroxysmal AF, but CHADS2
score ≥2 independently increased the risk of late-phase TE.
Disclosures
Funding Sources: None. Declarations: None. Financial Support: None.
References
- 1.
Haïssaguerre M, Jaïs P, Shah DC, Takahashi A, Hocini M, Quiniou G, et al. Spontaneous initiation of atrial fibrillation by ectopic beats originating in the pulmonary veins. N Engl J Med 1998; 339: 659–666.
- 2.
Bhargava M, Di Biase L, Mohanty P, Prasad S, Martin DO, Williams-Andrews M, et al. Impact of type of atrial fibrillation and repeat catheter ablation on long-term freedom from atrial fibrillation: Results from a multicenter study. Heart Rhythm 2009; 6: 1403–1412.
- 3.
Ouyang F, Bänsch D, Ernst S, Schaumann A, Hachiya H, Chen M, et al. Complete isolation of left atrium surrounding the pulmonary veins: New insights from the double-Lasso technique in paroxysmal atrial fibrillation. Circulation 2004; 110: 2090–2096.
- 4.
Hunter RJ, McCready J, Diab I, Page SP, Finlay M, Richmond L, et al. Maintenance of SR with an ablation strategy in patients with atrial fibrillation is associated with a lower risk of stroke and death. Heart 2012; 98: 48–53.
- 5.
Themistoclakis S, Corrado A, Marchlinski FE, Jais P, Zado E, Rossillo A, et al. The risk of thromboembolism and need for oral anticoagulation after successful atrial fibrillation ablation. J Am Coll Cardiol 2010; 55: 735–743.
- 6.
Saad EB, d’Avila A, Costa IP, Aryana A, Slater C, Costa RE, et al. Very low risk of thromboembolic events in patients undergoing successful catheter ablation of atrial fibrillation with a CHADS2 score ≤3: A long-term outcome study. Circ Arrhythm Electrophysiol 2011; 4: 615–621.
- 7.
Chao TF, Lin YJ, Tsao HM, Tsai CF, Lin WS, Chang SL, et al. CHADS(2) and CHA(2)DS(2)-VASc scores in the prediction of clinical outcomes in patients with atrial fibrillation after catheter ablation. J Am Coll Cardiol 2011; 58: 2380–2385.
- 8.
European Heart Rhythm Association (EHRA); European Cardiac Arrhythmia Society (ECAS); American College of Cardiology (ACC); American Heart Association (AHA); Society of Thoracic Surgeons (STS),
Calkins H, Brugada J, Packer DL, Cappato R, Chen SA, Crijns HJ, et al. HRS/EHRA/ECAS expert consensus statement on catheter and surgical ablation of atrial fibrillation: Recommendations for personnel, policy, procedures and follow-up. A report of the Heart Rhythm Society (HRS) Task Force on Catheter and Surgical Ablation of Atrial Fibrillation. Heart Rhythm 2007; 4: 816–861.
- 9.
Inoue H, Okumura K, Atarashi H, Yamashita T, Origasa H, Kumagai N, et al. Target international normalized ratio values for preventing thromboembolic and hemorrhagic events in Japanese patients with non-valvular atrial fibrillation: Results of the J-RHYTHM Registry. Circ J 2013; 77: 2264–2270.
- 10.
Kuwahara T, Takahashi A, Kobori A, Miyazaki S, Takahashi Y, Takei A, et al. Safe and effective ablation of atrial fibrillation: Importance of esophageal temperature monitoring to avoid periesophageal nerve injury as a complication of pulmonary vein isolation. J Cardiovasc Electrophysiol 2009; 20: 1–6.
- 11.
Miyazaki S, Kuwahara T, Kobori A, Takahashi Y, Takei A, Sato A, et al. Preprocedural predictors of atrial fibrillation recurrence following pulmonary vein antrum isolation in patients with paroxysmal atrial fibrillation: Long-term follow-up results. J Cardiovasc Electrophysiol 2011; 22: 621–625.
- 12.
Kuwahara T, Takahashi A, Takahashi Y, Kobori A, Miyazaki S, Takei A, et al. Prevention of periprocedural TE and management of hemorrhagic complications in atrial fibrillation ablation under continuous warfarin administration. J Cardiovasc Electrophysiol 2013; 24: 510–515.
- 13.
Redfearn DP, Trim GM, Skanes AC, Petrellis B, Krahn AD, Yee R, et al. Esophageal temperature monitoring during radiofrequency ablation of atrial fibrillation. J Cardiovasc Electrophysiol 2005; 16: 589–593.
- 14.
Hachiya H, Hirao K, Takahashi A, Nagata Y, Suzuki K, Maeda S, et al. Clinical implications of reconnection between the left atrium and isolated pulmonary veins provoked by adenosine triphosphate after extensive encircling pulmonary vein isolation. J Cardiovasc Electrophysiol 2007; 18: 1–7.
- 15.
Lin WS, Tai CT, Hsieh MH, Tsai CF, Lin YK, Tsao HM, et al. Catheter ablation of paroxysmal atrial fibrillation initiated by non-pulmonary vein ectopy. Circulation 2003; 107: 3176–3183.
- 16.
Tsai CF, Tai CT, Hsieh MH, Lin WS, Yu WC, Ueng KC, et al. Initiation of atrial fibrillation by ectopic beats originating from the superior vena cava: Electrophysiological characteristics and results of radiofrequency ablation. Circulation 2000; 102: 67–74.
- 17.
Miyazaki S, Taniguchi H, Kusa S, Uchiyama T, Hirao K, Iesaka Y. Conduction recovery after electrical isolation of superior vena cava: Prevalence and electrophysiological properties. Circ J 2013; 77: 352–358.
- 18.
Adams HP Jr, Bendixen BH, Kappelle LJ, Biller J, Love BB, Gordon DL, et al. Definitions for use in a multicenter clinical trial. TOAST: Trial of Org 10172 in Acute Stroke Treatment. Stroke 1993; 24: 35–41.
- 19.
Verma A, Minor S, Kilicaslan F, Patel D, Hao S, Beheiry S, et al. Incidence of atrial arrhythmias detected by permanent pacemakers (PPM) post-pulmonary vein antrum isolation (PVAI) for atrial fibrillation (AF): Correlation with symptomatic recurrence. J Cardiovasc Electrophysiol 2007; 18: 601–606.
- 20.
Hindricks G, Piorkowski C, Tanner H, Kobza R, Gerds-Li JH, Carbucicchio C, et al. Perception of atrial fibrillation before and after radiofrequency catheter ablation: Relevance of asymptomatic arrhythmia recurrence. Circulation 2005; 112: 307–313.
- 21.
Suzuki K, Sarti C, Tuomilehto J, Kutsuzowa T, Narva EV, Sivenius J, et al. Stroke incidence and case fatality in Finland and in Akita, Japan: A comparative study. Neuroepidemiology 1994; 13: 236–244.
- 22.
Sato H, Ishikawa K, Kitabatake A, Ogawa S, Maruyama Y, Yokota Y, et al. Japan Atrial Fibrillation Stroke Trial Group: Low-dose aspirin for prevention of stroke in low-risk patients with atrial fibrillation: Japan Atrial Fibrillation Stroke Trial. Stroke 2006; 37: 447–451.