論文ID: CJ-14-0933
論文ID: CJ-14-0933
Background: Ascending aorta wrapping is rarely recommended for the management of dilated aorta, because of late complications. The aim of the present study was to analyze the early and late outcomes of the aortic wrapping technique at the time of aortic valve replacement (AVR) for bicuspid aortic stenosis (BAS).
Methods and Results: Among patients who underwent primary AVR for BAS between 2002 and 2011, 79 who underwent ascending aortic wrapping (wrapping group) were compared with 144 patients who underwent AVR alone. The preoperative ascending aortic diameters were larger in the wrapping group (40.9±4.2 mm vs. 48.6±4.0 mm, P<0.001). Operative technique was to wrap the ascending aorta transversely with a semi-elliptically resected Dacron graft. The follow-up for the wrapping group was 76.5±35.5 (median 71.1) months. There were no early deaths. Early and late morbidity did not differ between groups. The 24 late deaths, including 10 cardiac-related deaths, occurred in the entire group; 3 sudden deaths occurred only in the AVR group. The 10-year overall survival in the wrapping group was higher than the AVR group (88.1±6.8% vs. 80.0±4.6%, P=0.048). No late aortic complications were detected. The aortic diameter was reduced from 49.5±4.1 mm to 45.3±5.0 mm after wrapping (P<0.001).
Conclusions: The aortic wrapping technique may be an option for treating a moderately dilated ascending aorta in selected patients undergoing AVR for BAS. Longer follow-up, however, is necessary to verify later complications.
According to the 2014 AHA/ACC guideline,1 if aortic valve replacement (AVR) is scheduled in patients with a bicuspid aortic valve (BAV), concomitant ascending aorta replacement (AAR) is reasonable if the diameter of the aorta is greater than 4.5 cm. Although low rates of early death have been reported in several papers,2–4 simultaneous AVR and AAR may increase early morbidity and mortality because of the longer aorta cross-clamping time and the higher bleeding risk, especially in elderly patients with comorbidities.5 In contrast, some researchers showed that AVR alone had reasonable and acceptable late outcomes for patients with a mildly or moderately dilated aorta at the time of AVR.6–8 There is still great debate on the optimal management of this condition.
Aortic wrapping with or without reduction aortoplasty has been suggested as a less invasive alternative technique. Several wrapping techniques9–14 have been introduced since the first case in 1956.15 However, aortic wrapping is no longer recommended as an alternative treatment for patients with a moderately dilated aorta because late complications make the long-term safety of this technique questionable. In particular, distal migration of the wrap seems the most important complication, which is related to aortic kinking, intimal erosion or dilatation of the proximal aorta.5,16,17
The aims of this study were to analyze the early and late outcomes of the wrapping technique for patients with a moderately dilated ascending aorta at the time of AVR for bicuspid aortic stenosis (BAS).
This novel aortic wrapping technique has been performed at Samsung Medical Center since 2002 and the data of patients treated between January 2002 and December 2011 were analyzed. A total of 420 patients underwent primary AVR for degenerative aortic stenosis (AS). Among 245 patients who had BAS, 79 underwent concomitant ascending aorta wrapping (wrapping group), and 144 patients underwent AVR alone (AVR group). During the same period, 21 patients (20 with BAS) underwent AAR for a dilated ascending aorta together with AVR, and 15 patients (2 with BAS) underwent AAR under circulatory arrest because of severe aortic calcification precluding aorta cross-clamping.
The aortic wrapping was performed when the aorta was fusiform and moderately dilated (45–55 mm in diameter) without aortic root dilatation. During the study period, the preoperative ascending aortic diameter in the AVR group was less than 45 mm (40.9±4.2 (median 40.6) mm, Table 1). AAR was performed if the aorta was more than 50 mm in diameter and the patient was young. Patients who had rheumatic AS or dominant aortic regurgitation (AR) were excluded from this study, as were patients who underwent concomitant coronary artery bypass grafting, Bentall procedure or other valvular replacement.
| AVR alone (n=144) | Wrapping (n=79) | P value | |
|---|---|---|---|
| Female, n (%) | 41 (28.5) | 38 (48.1) | 0.003* |
| Age (mean±SD (median)) | 62.7±9.7 (65) | 63.6±8.5 (64) | 0.733† |
| Body surface area (m2) | 1.68±0.2 | 1.65±0.2 | 0.177‡ |
| Comorbidity, n (%) | |||
| Hypertension | 55 (38.2) | 40 (50.0) | 0.072* |
| Diabetes mellitus | 20 (13.9) | 6 (7.6) | 0.161* |
| Coronary artery disease | 8 (5.6) | 5 (6.3) | 0.775§ |
| Cerebrovascular accident | 10 (6.9) | 3 (3.8) | 0.390§ |
| CKD disease on dialysis | 0 (0) | 0 (0) | – |
| COPD | 9 (6.3) | 1 (1.3) | 0.102§ |
| Atrial fibrillation | 15 (10.4) | 10 (12.7) | 0.612* |
| Ascending aortic diameter# | 40.9±4.2 (40.6) | 48.6±4.0 (47.8) | <0.001† |
*Chi-square test; †Mann-Whitney test; ‡Student’s t-test; §Fisher’s exact test; #measured on CT scan. AVR, aortic valve replacement; CKD, chronic kidney disease; COPD, chronic obstructive pulmonary disease; SD, standard deviation.
There were more female patients (28.5 vs. 48.1%, P=0.003) and preoperative ascending aortic diameters were larger (40.9±4.2 vs. 48.6±4.0, P<0.001) in the wrapping group (Table 1).
The institutional review board approved this study and waived the requirement for individual consent from patients or their families (IRB file number; 2012-05-064).
Operative TechniqueThe ascending aorta was mobilized circumferentially by careful dissection before cardiopulmonary bypass. AVR was performed according to the previously described method.18 After aortic decannulation, the wrapping procedure was performed with a Hemashield woven vascular graft (Meadox Medicals Inc, Oakland, NJ, USA). The dilated ascending aorta was wrapped with the vascular graft opened transversely, so that the corrugation lines of the graft were positioned parallel to the ascending aorta. Before wrapping, the length of the greater curvature, the lesser curvature, and the maximal diameter of the ascending aorta were measured (Figure 1A). The diameter of the graft was selected according to the length of the greater curvature because the circumference of the graft should be the length of the greater curvature (Figure 1B). The most frequently used diameter of the graft was 28 mm. The maximally stretched graft should be long enough to encircle the dilated aorta (Figure 1B). After bilateral semielliptical resections of the graft, the remnant narrow width should be the length of the lesser curvature (Figure 1C). After encircling the ascending aorta with the graft, the edges of the graft were approximated with multiple interrupted 3-0 polypropylene mattress sutures (Figure 1D).
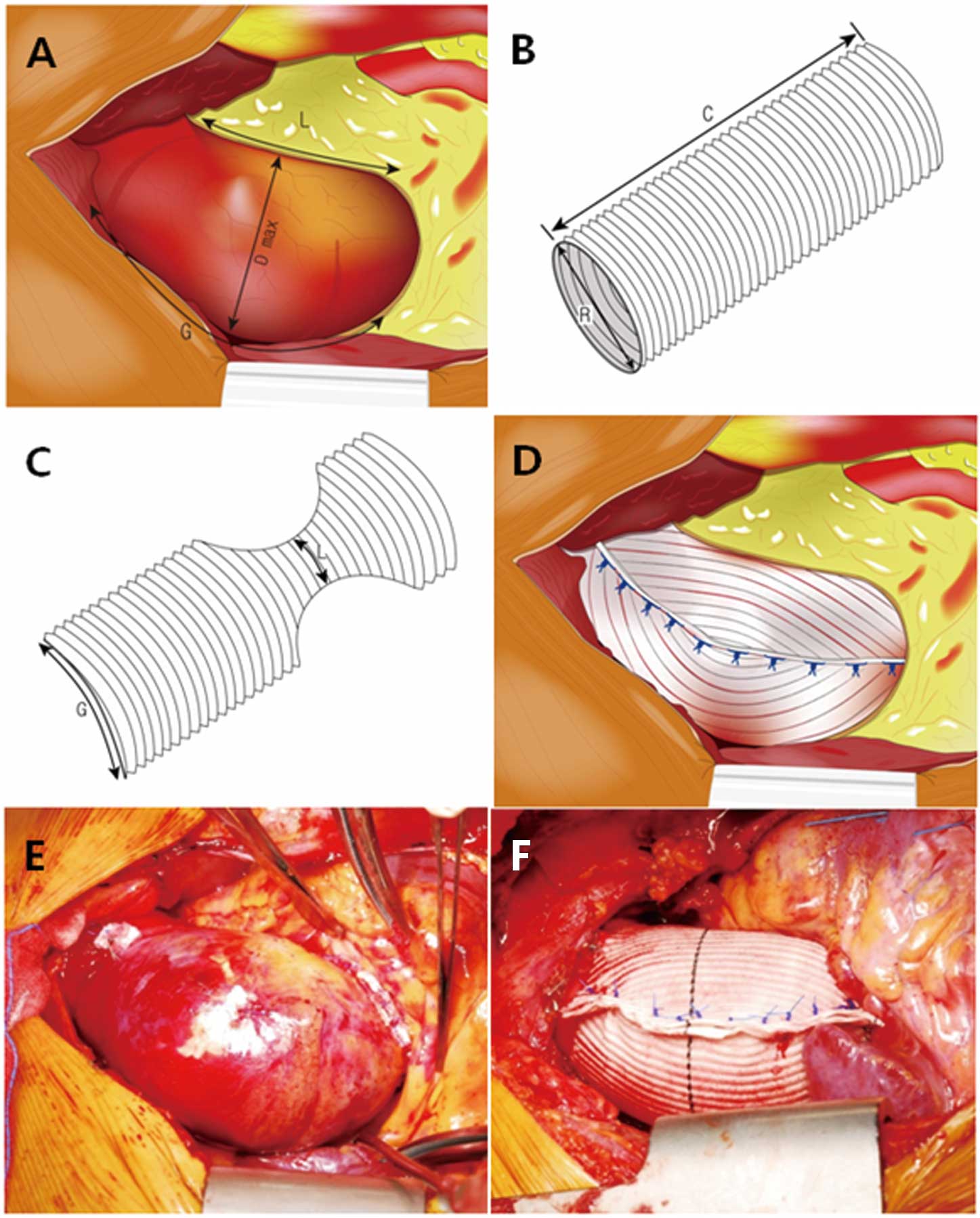
Sequential illustrations (A–D) of ascending aortic wrapping and photographs (E,F) before and after the procedure. (R=G/π, C>π×Dmax; C, length of the vascular graft; Dmax, maximal diameter of the ascending aorta; G, greater curvature of the ascending aorta; L, lesser curvature of the ascending aorta; R, diameter of the vascular graft.)
Postoperative outcome data were acquired from review of medical records, telephone interviews with patients or their families, and the national medical insurance registry. The patients in the AVR and wrapping groups were followed up for 74.3±31.8 (median, 72.4) and 76.5±35.5 (median, 71.1) months, respectively. Among 199 patients, excluding 24 patients who died, 178 patients (89.4%) were regularly followed up at Samsung Medical Center and 21 patients (10.6%) at other institutions.
Early death was defined as within 30 days of operation, and late death was defined as occurring during follow-up. Late morbidity included structural or nonstructural valve dysfunction, infective endocarditis, hemorrhagic or thromboembolic events, or aortic complications such as dissection, enlargement, or wrapping-related complications. The cause of cardiac-related death included heart failure, myocardial infarction, aortic complications such as dissection or rupture, sudden death or death of unknown cause. Transthoracic echocardiography was performed before discharge and at 1 year, and then biennially thereafter. During echocardiography, the diameter of the ascending aorta was measured at 1–2 cm distal to the sinotubular junction in the parasternal long-axis view during the early systolic period. However, it was difficult to measure the mid-ascending aorta where the vessel was maximally dilated, so computed tomography (CT) was used to overcome any underestimation by echocardiography.19 CT is also useful for diagnosing aortic complications.20,21 In the wrapping group, 44 patients had CT scans taken preoperatively and 46 had postoperative CT scans. Late follow-up CT scans were taken 57.7±35.0 months postoperatively. Contrast and 3D reconstructed CT scans were useful for verifying aortic configuration or detect wrapping-related complications such as dislocation of the wrap or aortic kinking. Changes in diameter were evaluated by comparing the preoperative and postoperative CT scans of the same patient.
Statistical AnalysisIBM SPSS Statistics (version 19, SPSS Inc, Chicago, IL, USA) was used. Data are presented as mean±standard deviation, median, or proportion. If the data did not show normal distribution, median is given in parentheses next to mean±standard deviation. Categorical variables were compared between the groups using the chi-square test or Fisher’s exact test according to the expected frequency. Continuous variables were compared between groups using Student’s t-test or the Mann-Whitney test according to the normal distribution of the variables. To determine whether there were differences in the diameter of the aorta after aortic wrapping, the paired t-test was used for confirmation following the Shapiro-Wilk test. The Kaplan-Meier method was used to estimate overall survival and freedom from cardiac-related death. Differences in survival between the groups were analyzed with the log rank or Breslow test. P<0.05 was considered statistically significant.
Early Outcomes No early deaths occurred in either group. There was no difference in the incidence of early morbidity between the groups (Table 2).
| AVR alone (n=144) | Wrapping (n=79) | P value | |
|---|---|---|---|
| Tissue/mechanical valve | 83/61 | 45/34 | 0.922* |
| Concomitant procedures | |||
| Mitral valve repair | 10 (6.9) | 6 (7.6) | 0.857* |
| Tricuspid valve repair | 3 (2.1) | 5 (6.3) | 0.135† |
| Maze procedure | 11 (7.6) | 4 (5.1) | 0.463* |
| LVOT muscle resection | 15 (10.4) | 10 (12.7) | 0.612* |
| Early morbidity | |||
| Sternal wound infection | 1 (0.7) | 0 (0.0) | 1.000† |
| Bleeding | 3 (2.1) | 0 (0.0) | 0.554† |
| Acute renal failure | 0 (0.0) | 0 (0.0) | – |
| Cerebrovascular accident | 2 (1.4) | 0 (0.0) | 0.540† |
| Low cardiac output syndrome | 1 (0.7) | 0 (0.0) | 1.000† |
| Early mortality | 0 (0.0) | 0 (0.0) | – |
| Late cardiac-related morbidity | |||
| Structural valve dysfunction | 0 (0.0) | 0 (0.0) | – |
| Nonstructural valve dysfunction | 1 (0.7)‡ | 0 (0.0) | – |
| Infective endocarditis | 2 (1.4) | 0 (0.0) | 0.540† |
| Hemorrhagic or thromboembolic event | 1 (0.7) | 3 (3.8) | 0.129† |
| Aortic dissection, enlargement or wrapping- related complication |
0 (0.0) | 0 (0.0) | – |
| Late mortality | 20 (13.9) | 4 (5.1) | 0.042* |
| Late cardiac-related death | 7 (4.9) | 3 (3.8) | 1.000† |
| Sudden death | 3 (2.1) | 0 (0.0) | – |
*Chi-square test; †Fisher’s exact test; ‡minimal paravalvular leak. AVR, aortic valve replacement; LVOT, left ventricular outflow tract.
Late Outcomes No significant differences between the groups were found for late morbidity (Table 2). Aortic complications such as dissection, enlargement, or wrapping-related complications were not found on CT or echocardiography. The 10-year survival rates in the AVR alone and wrapping groups were compared and although the 10-year freedom from cardiac-related death did not differ (91.7±3.2% vs. 89.3±6.8%, P=0.519), the 10-year overall survival rate was higher in the wrapping group (80.0±4.6% vs. 88.1±6.8%, P=0.048, Figure 2). A total of 3 sudden deaths were included in the 7 cardiac-related deaths in the AVR alone, and there were no sudden deaths among the 3 cardiac-related deaths in the wrapping group. The characteristics of the 3 patients who died suddenly were as follows. One patient had a stroke 7 months after AVR and died suddenly after 68 months, although the last follow-up echocardiographic scan showed normal cardiac function and aortic diameter (31 mm). Another patient had low left ventricular ejection fraction (LVEF <30%) and a dilated ascending aorta (42 mm) before AVR. The LVEF was 56% at 1 year later and the aortic diameter remained large (43 mm) until the last CT scan. The aortic diameter of the other patient increased from 32 mm to 39 mm in 5 years after AVR, but he died suddenly 2 years later.

Kaplan-Meier method of estimating overall survival (A) and freedom from cardiac-related death (B) between the AVR and wrapping groups. *Log rank test; †Breslow test.
CT Measurement When the postoperative CT scans of 46 patients were reviewed, neither aortic kinking nor progressive aortic disease such as progressive dilatation of aortic root or ascending aorta, or aortic dissection was found (Figure 3). When the preoperative and postoperative diameters were compared, the diameter had slightly reduced from 49.4±4.1 mm to 45.3±4.9 mm (difference: 4.1±2.7 mm, P<0.001, 95% confidence interval (CI) 3.0–5.2). The diameter divided by body surface area had reduced from 29.9±3.0 to 27.5±3.6 mm/m2 (difference: 2.5±1.7 mm, P<0.001, 95% CI 1.8–3.1).

Preoperative (A) and postoperative (B) CT scans of the same patient. Fusiform aorta is still preserved after wrapping, which might have a protective effect against distal migration of the wrap. Follow-up CT scans taken at 11 (C), 10 (D), and 8 (E,F) years after aortic wrapping show no wrapping-related complications. CT, computed tomography.
Echocardiographic Measurement In the AVR group, the diameter of the proximal ascending aorta changed from 32.5±4.8 to 32.8±4.7 cm (P=0.244) and in the wrapping group, from 33.7±4.7 to 34.4±4.5 cm (P=0.140) on late follow-up scans.
The present study had 3 main findings. First, the aortic wrapping procedure at the time of AVR for BAS did not increase early mortality and morbidity (Table 2). Second, the overall survival rate in the wrapping group, which had moderately dilated ascending aortas, was better than that in the AVR group but the late cardiac-related mortality rate was not different (Figure 2). During the follow-up period, no sudden deaths occurred in the wrapping group but there were 3 in the AVR group, 2 of which may have been aortic events. The aortic diameter of 1 of the patients had increased more than 20% during follow-up, while the other patient already had a dilated ascending aorta before AVR. Third, wrapping-related complications were not detected by CT or echocardiography for up to 12 years (Table 2). In particular, neither distal migration nor kinking of the wrapping graft was found on follow-up CT scans (Figure 3).
The standard technique to manage a dilated ascending aorta at the time of AVR is AAR, but the possible operative risk can lead surgeons to consider a less invasive procedure or no aortic procedure in high-risk patients.
Borger et al22 and Svensson et al3 reported that the AAR should be considered during BAV surgery if the aorta is larger than 4.5 cm or the aortic cross-sectional area/height ratio is greater than 8–10. Matsuyama et al suggested preventive aortic surgery even in a diameter of 40 mm at the time of AVR.23 AAR can be performed with low rates of mortality and morbidity if patients do not have high-risk factors such as older age, left ventricular dysfunction, emergency operation, coronary artery disease, reoperation or chronic renal insufficiency.24 It has been often reported that mortality rates did not increase even after concomitant AAR during AVR,2,25 However, less experienced surgeons may feel uncomfortable performing concomitant AAR because of the longer cross-clamping time and the higher bleeding risk, especially in elderly patients with comorbidities.5,26 McKeller et al advised against aggressive prophylactic aortic surgery during AVR.27 The 2010 ACCF/AHA/AATS/STS guidelines recommended consideration of concomitant AAR if AVR is scheduled and the ascending aortic diameter is ≥4.5 cm and they also suggest ascending aortic aortoplasty as an alternative if the aortic diameter is <5.0 cm and the patient is elderly.28
Another option is AVR alone without the aortic procedure. In a review of previous reports of AVR alone, numerous variables influenced the outcomes, such as aortic valve pathology, aortic valve function, aortic diameter, or aortic root enlargement. Girdauskas et al8 reported no aortic dissection after AVR alone for BAS with concomitant ascending aortic dilatation (40–50 mm) during 11.5-year follow-up; the 10-year freedom from adverse aortic events was 95%. Goland et al found that the initial aortic dilation (40–50 mm) at the time of AVR alone for BAV was not a significant predictor of late death during a 9-year follow-up.7 On the other hand, Borger et al reported significant differences in 15-year freedom from aortic events according to the initial aortic diameter.22 McKellar et al studied risk of aortic events following AVR in patients with BAV during 12 year follow-up and reported that the 15-year freedom from aortic events was lower in patients with initial aortic diameter >40 mm (85% vs. 92%).27 Thus, AVR alone in patients with a moderately dilated ascending aorta may not to be a safe choice. In our experience, we could not exclude the possibility of late aortic complications because the 3 sudden deaths occurred in the AVR group. Therefore, we suggest concomitant aortic wrapping for patients with a moderately dilated ascending aorta at the time of AVR.
Although we performed aortic wrapping in patients with mildly dilated (40–45 mm) aortas at the beginning of the study, now we do not perform prophylactic wrapping if the ascending aorta is mildly dilated, because of the low complication rates as previously reported.6,29 However, if the ascending aorta is moderately dilated either aortic wrapping or AAR is considered according to age, LVEF, bleeding risk, emergency operation, infective endocarditis, previous cardiac surgery, or complex combined cardiac surgery.24,30–32 Aortic wrapping can be used for patients who are more than 70 years old or who have limited life expectancy. If the ascending aorta is severely dilated, it should be replaced. Contraindications of aortic wrapping, in our opinion, are pure AR and dilated aortic root. Lima et al reported that BAV patients with predominantly AR had more frequent root dilatation than those with AS.33 Progressive root dilatation can cause distal migration of the wrap or erosion of the aortic intima during follow-up.16 So we do not perform aortic wrapping in patients who have aortic root dilatation or AR.
Another important consideration is how the wrapping technique is applied in cases of tricuspid aortic valve (TAV). During the study period, we performed concomitant aortic wrapping in 12 patients, and AAR in 1 patient with TAV without any late aortic complications. Gaudino et al6 reported their 14.7-year follow-up results of AVR alone in patients with TAV and a 50–59 mm ascending aorta at the time of AVR. There were no aortic events or significant increases in aortic diameter. We used aortic wrapping rather more conservatively in TAV patients than in those with BAV. When the patient had an ascending aorta >50 cm in diameter, we performed wrapping or replacement of the ascending aorta according to age, comorbidity and body surface area.
Our technique has several characteristics that may protect the wrapping graft from distal migration or kinking: transverse positioning, bilateral semielliptical resections of the wrapping graft and no reduction aortoplasty. Transverse wrapping refers to parallel, not vertical, positioning of the corrugation lines of the Dacron graft to the ascending aorta (Figure 1D). In most of the wrapping techniques, the corrugation lines are usually positioned vertical to the ascending aorta (longitudinal wrapping).12 In longitudinal wrapping, the corrugation lines can act as baffle to aortic blood flow and then the wrapping graft may kink, and intimal erosion can occur. Transverse wrapping has been rarely reported except by Buxton et al14 and Ogus et al.11 Our wrapping method is a modification of such previous techniques. Another characteristic is bilateral semielliptical resections of the wrapping graft (Figure 1C). During longitudinal wrapping, preparation of the Dacron graft is somewhat difficult because of the curvature of the ascending aorta and thus the shorter length of the lesser curvature. Milgalter et al used fine Dacron mesh to overcome this problem.9 Bilateral semielliptical resections also allow the graft to fit compactly along the lesser curvature of the ascending aorta. We did not perform reduction aortoplasty because it makes the ascending aorta tubular. In the analysis of previous reports of dislocation of the wrap, reduction aortoplasty was a common factor.5,17 Reduction aortoplasty changes the fusiform aorta to a tubular shape, which might be a risk factor for distal migration of the wrap. If the ascending aorta preserves its fusiform shape, the maximal diameter of the wrap at the mid-ascending aorta is fixed between the smaller diameters of the proximal and distal ascending aorta (Figures 1D,F). In the previous reports, secure anchoring of the wrap was emphasized10,17 or a plicating suture of the aorta and the wrapping graft was suggested to prevent it.34 Hwang et al reported reduction aortoplasty without aortic wrapping and acceptable long-term outcomes.35
Study LimitationsFirst, the study was not performed in a prospective manner, although the 2 study groups were established during same study period and the patients’ characteristics did not differ except for the sex ratio (Table 1). Second, the number of enrolled patients in the wrapping group was relatively small for reaching a definite conclusion. Third, unknown causes of death in the cardiac-related mortality. Despite no sudden deaths in the wrapping group, 3 cardiac-related deaths included 2 unknown causes of death. Fourth, follow-up duration may not have been long enough to compare the late clinical outcomes between groups and thus discover late wrapping-related complications. Longer follow-up, more than 15 years, seems necessary for more accurate results. Fifth, although no clinical aortic complications occurred in the wrapping group, complete follow-up CT scanning should be undertaken to evaluate even tiny complications.
In conclusion, our aortic wrapping technique at the time of AVR for BAS was a simple, safe, and durable procedure for up to 12 years of follow-up. Compared with the AVR group, the wrapping group, which had a larger initial aortic diameter, achieved comparable clinical outcomes. The aortic wrapping technique may be an alternative treatment for a moderately dilated ascending aorta in selected, risky patients undergoing AVR for BAS. But further study is still necessary to determine the long-term durability of the wrapping procedure.
The authors thank Joo Min Hwang for her contribution to data collection. We also thank Sook-Young Woo, Seonwoo Kim, PhD, and Shinyi Jang, PhD, in the Division of Biostatistics in Samsung Medical Center for statistical support.
The authors have no relationships or conflicts to disclose.