論文ID: CJ-15-0870
論文ID: CJ-15-0870
Background: In the setting of elective percutaneous coronary intervention (PCI), intravascular ultrasound (IVUS)-guided PCI is associated with a reduction in the incidence of target vessel revascularization (TVR), but the impact of IVUS on long-term clinical outcome in the setting of emergency PCI for ST-segment elevation acute myocardial infarction (STEMI) is still unclear.
Methods and Results: The subjects consisted of 3,028 STEMI patients who underwent primary PCI within 24 h of symptom onset in the CREDO-Kyoto acute myocardial infarction registry. Of these, 932 patients (31%) underwent IVUS-guided PCI. Compared with the angiography-guided PCI without IVUS, IVUS-guided PCI was associated with significantly lower incidences of TVR (primary outcome measure; 22% vs. 27%, log-rank P<0.001) and definite stent thrombosis (ST; 1.2% vs. 3.1%, log-rank P=0.003). The cumulative incidence of all-cause death was not significantly different between the 2 groups. After adjusting for confounders, however, there were no significant differences between the 2 groups in risk for TVR (adjusted HR, 1.14; 95% CI: 0.86–1.51, P=0.38) and definite ST (adjusted HR, 0.58; 95% CI: 0.19–1.72, P=0.33).
Conclusions: IVUS-guided PCI was not associated with a lower risk for TVR or ST in STEMI patients undergoing primary PCI.
Intravascular ultrasound (IVUS) can provide useful information about lesion characteristics such as severity of calcification and existence of lipid-rich plaque. Moreover, the ability of IVUS to detect coronary dissection and stent under-expansion could improve the safety and usefulness of percutaneous coronary intervention (PCI) as compared with that guided by angiography only.1,2 In the setting of elective PCI, randomized trials and meta-analyses indicated that IVUS-guided PCI was associated with a reduction in the incidence of target vessel revascularization (TVR), particularly after bare-metal stent (BMS) implantation,3–8 although the benefit of IVUS-guided PCI was less clear after drug-eluting stent (DES) implantation.9–12 In the setting of emergency PCI for ST-segment elevation myocardial infarction (STEMI), however, the impact of IVUS on long-term clinical outcome has not been adequately evaluated as yet.13,14 Therefore, the aim of this study was to investigate the impact of IVUS guidance on long-term clinical outcome in a large Japanese observational database of patients with STEMI undergoing primary PCI.
The Coronary REvascularization Demonstrating Outcome Study in Kyoto (CREDO-Kyoto) acute myocardial infarction (AMI) registry is a physician-initiated non-company-sponsored multicenter registry that enrolled consecutive AMI patients who underwent coronary revascularization within 7 days of symptom onset between January 2005 and December 2007 at 26 tertiary hospitals in Japan (Appendix S1). The relevant review boards or ethics committees at all 26 participating hospitals approved the study protocol. The protocol for the study was approved by the human research ethics committees of the Kyoto University Graduate School of Medicine. Obtaining written informed consent from the patients was waived because of the retrospective nature of the study, but we excluded those patients who refused participation in the study when contacted at follow-up. This strategy is in concordance with the guidelines of the Japanese Ministry of Health, Labor and Welfare.
The details of the design and patient enrollment of this registry have been described previously.15 Among 5,429 patients enrolled in this registry, we excluded 9 patients who refused to participate in the study, 195 patients treated with coronary artery bypass grafting (CABG) surgery, 721 patients with cardiogenic shock at presentation, 685 patients with non-STEMI, 436 patients who underwent PCI beyond 24 h after symptom onset, 21 patients whose symptom onset was unknown, and 334 patients whose culprit lesions were treated without stent implantation (Figure 1). Therefore, the subject group for the current analysis consisted of 3,028 patients with STEMI who underwent primary PCI within 24 h of symptom onset.
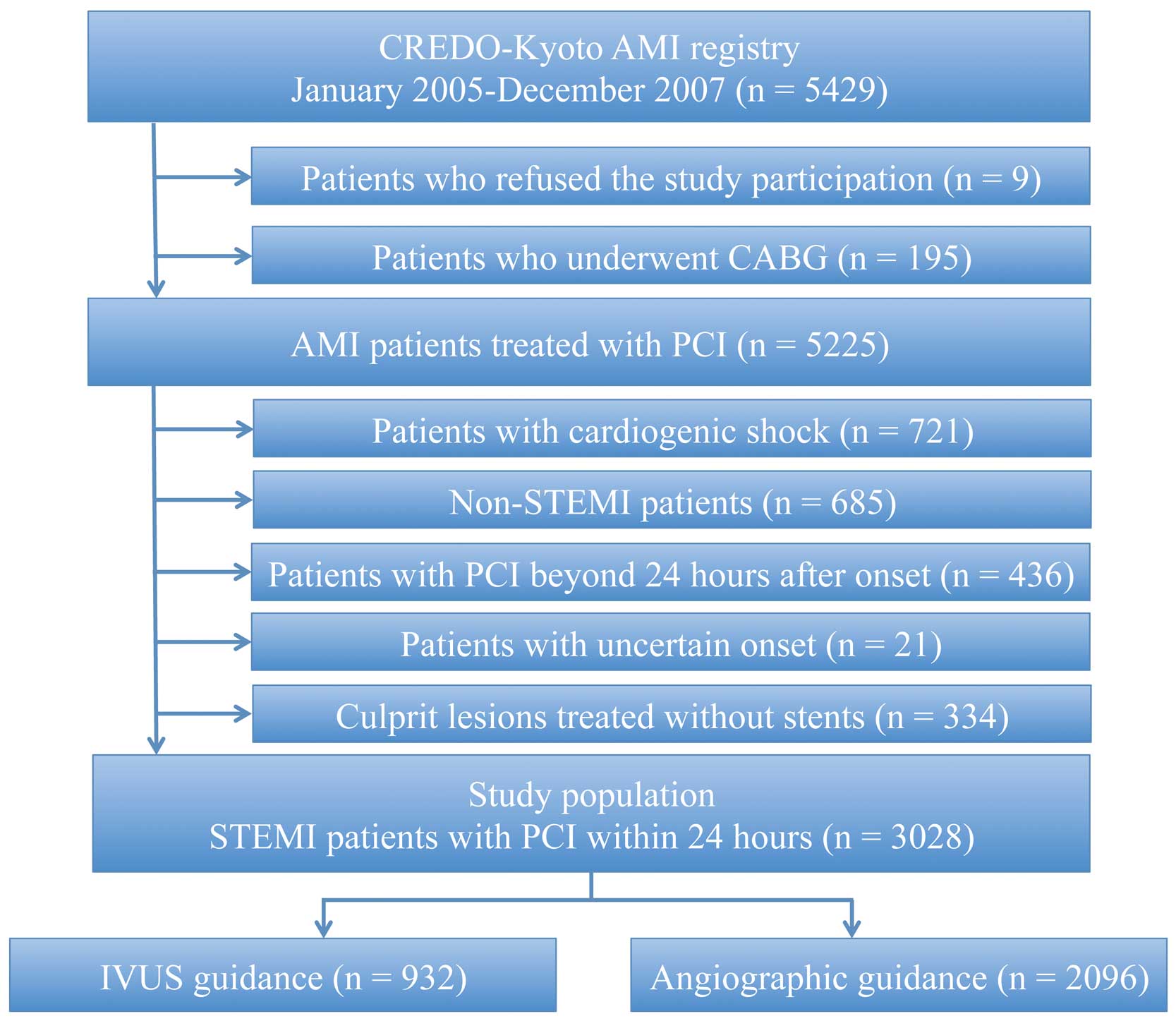
Flow chart for subject selection. AMI, acute myocardial infarction; CABG, coronary artery bypass grafting; IVUS, intravascular ultrasound; PCI, percutaneous coronary intervention; STEMI, ST-segment elevated myocardial infarction.
Experienced clinical research coordinators from the independent clinical research organization (Research Institute for Production Development, Kyoto, Japan; Appendix S2) collected baseline clinical, angiographic and procedural data from the hospital charts or hospital databases according to the pre-specified definitions. Collection of follow-up information was mainly conducted through review of the inpatient and outpatient hospital charts by the clinical research coordinators, and additional follow-up information was collected through contact with patients, relatives and/or referring physicians via mail with questions regarding vital status, subsequent hospitalizations, and status of antiplatelet therapy. Clinical events such as all-cause death, myocardial infarction (MI), TVR, and stent thrombosis (ST) were adjudicated by the clinical event committee in a blinded fashion to the clinical data including IVUS use (Appendix S3).
The IVUS guidance group was defined as patients with IVUS during PCI for the culprit lesions, regardless of the type of IVUS catheter or the timing of IVUS (either before or after intervention or both). The angiography guidance group was defined as patients who underwent PCI for the culprit lesions without IVUS. Detailed definitions of baseline clinical characteristics have been described previously.15,16
The primary outcome measure for the current analysis was any TVR. TVR was defined as any repeated PCI or CABG, owing to restenosis or thrombosis of the culprit vessel. The secondary outcome measures included definite ST, all-cause death, MI, and a composite of all-cause death, MI, or TVR (major adverse cardiac events; MACE). Definite ST was defined as thrombosis at culprit lesions, confirmed on angiography or pathology in accordance with the criteria of the Academic Research Consortium.17 Death was regarded as cardiac in origin unless obvious non-cardiac causes could be identified. MI was defined according to the definition in the Arterial Revascularization Therapy Study.18 Within 1 week of the index procedure, only Q-wave MI was adjudicated as MI.
Statistical AnalysisContinuous variables are given as mean±SD or median (IQR) and categorical variables as numbers and percentages. We compared continuous variables using Student’s t-test or the Wilcoxon rank-sum test on the basis of the distributions. Categorical variables were compared using the chi-squared test when appropriate; otherwise, Fisher’s exact test was used.
The Kaplan-Meier method was used to estimate cumulative incidences of clinical events, and the differences were assessed using log-rank test. Consistent with our previous reports, we used a multivariate Cox proportional hazards model stratified by participating centers to estimate the hazard ratio (HR) of IVUS-guided PCI for the primary and secondary outcome measures by incorporating IVUS-guided PCI together with 38 clinically relevant risk-adjusting variables (Table 1).15,16 Consistent with our previous reports, continuous variables were dichotomized using clinically meaningful reference values or medians. Adjusted HR and 95% CI were calculated. Because of the small number of events for ST and MI and reflecting our preference for parsimonious models to avoid over-fitting, we selected 11 more relevant risk-adjusting variables (Table 1) for the multivariate Cox proportional hazard models for ST and MI. A subgroup analysis according to stent type (BMS or DES) was conducted. Because of the small number of events for all-cause death and MI in the BMS subgroup, we again selected 10 relevant risk-adjusting variables (Table 1) for the multivariate Cox proportional hazard models. The low frequency of definite ST did not allow construction of a multivariate model in the BMS subgroup. In the DES subgroup, because of the small number of events for TVR, all-cause death and MACE, we used the same 10 relevant risk-adjusting variables (Table 1) for the multivariate Cox proportional hazard models. The low frequency of definite ST and MI did not allow construction of a multivariate model in the DES subgroup.
| Variables | IVUS guidance (n=932) |
Angiographic guidance (n=2,096) |
P-value |
|---|---|---|---|
| Baseline characteristics | |||
| Age (years) | 65.9±12.2 | 67.4±12.1 | 0.002 |
| Age ≥75 years†,‡,§,¶ | 224 (24) | 649 (31) | <0.001 |
| Male†,§ | 700 (75) | 1,568 (75) | 0.86 |
| Body mass index <25.0†,§ | 655 (70) | 1,480 (71) | 0.85 |
| Hypertension†,‡,§,¶ | 720 (77) | 1,704 (81) | 0.01 |
| DM | 274 (29) | 672 (32) | 0.14 |
| DM on insulin therapy†,‡,§,¶ | 26 (2.8) | 79 (3.8) | 0.17 |
| Current smoking†,§ | 404 (43) | 867 (41) | 0.31 |
| Heart failure†,§ | 181 (19) | 409 (20) | 0.95 |
| Multi-vessel disease†,§ | 474 (51) | 1,049 (50) | 0.68 |
| Mitral regurgitation grade 3/4†,§ | 12 (1.3) | 52 (2.5) | 0.04 |
| Ejection fraction ≤40% | 118 (16) | 239 (14) | 0.19 |
| Prior MI†,‡,§,¶ | 63 (6.8) | 162 (7.7) | 0.35 |
| Prior stroke (symptomatic)†,§ | 81 (8.7) | 163 (7.8) | 0.39 |
| Peripheral vascular disease†,§ | 20 (2.2) | 61 (2.9) | 0.23 |
| eGFR <30 ml/min/1.73 m2, without hemodialysis†,§ | 22 (2.4) | 56 (2.7) | 0.62 |
| Hemodialysis†,‡,§ | 8 (0.9) | 25 (1.2) | 0.41 |
| Atrial fibrillation†,§ | 65 (7.0) | 163 (7.8) | 0.44 |
| Anemia (hemoglobin <11.0 g/dl)†,§ | 54 (5.8) | 180 (8.6) | 0.008 |
| Thrombocytopenia (platelets <100,000)†,§ | 9 (1.0) | 26 (1.2) | 0.51 |
| COPD†,§ | 29 (3.1) | 71 (3.4) | 0.7 |
| Liver cirrhosis†,§ | 16 (1.7) | 51 (2.4) | 0.22 |
| Malignancy†,§ | 51 (5.4) | 181 (8.6) | 0.002 |
| Peak creatine phosphokinase (IU/L) | 3,180±2,960 | 2,892±2,974 | 0.01 |
| Presentation | |||
| Onset-to-presentation time (h) | 2.7 (1.3–5.1) | 2.6 (1.2–5.7) | 0.98 |
| ≤2 h | 367 (41) | 874 (43) | 0.37 |
| Door-to-balloon time (min) | 102 (78–144) | 84 (54–126) | <0.001 |
| ≤90 min | 310 (41) | 1,084 (57) | <0.001 |
| Killip class 1 | 834 (89) | 1,814 (87) | 0.08 |
| Killip class 2 | 77 (8.3) | 217 (10) | |
| Killip class 3 | 21 (2.3) | 65 (3.1) | |
| Killip class 4 | 0 (0) | 0 (0) | |
| Angiographic characteristics | |||
| Infarct-related artery | |||
| Left anterior descending artery | 457 (49) | 1,028 (49) | 0.58 |
| Left circumflex artery | 95 (10) | 192 (9.2) | |
| Right coronary artery | 369 (40) | 855 (41) | |
| Left main coronary artery | 10 (1.1) | 15 (0.7) | |
| Bypass graft | 1 (0.1) | 6 (0.3) | |
| Initial TIMI flow grade=0 | 407 (44) | 696 (33) | <0.001 |
| Procedural characteristics (culprit lesion) | |||
| Target of proximal left anterior descending artery†,‡,§,¶ | 444 (48) | 988 (59) | 0.8 |
| Target of unprotected left main coronary artery†,§ | 12 (1.3) | 16 (0.8) | 0.16 |
| Target of bifurcation†,‡,§,¶ | 186 (20) | 401 (19) | 0.6 |
| Side-branch stenting | 5 (0.5) | 40 (1.9) | 0.003 |
| Total stent length (mm) | 25.0±11.9 | 24.9±11.7 | 0.79 |
| Total stent length >28 mm†,‡,§,¶ | 216 (23) | 474 (23) | 0.74 |
| Minimum stent diameter (mm) | 3.2±0.5 | 3.1±0.5 | <0.001 |
| Minimum stent size <3.0 mm†,‡,§,¶ | 164 (18) | 520 (25) | <0.001 |
| Final balloon pressure (atm) | 15.6±4.0 | 14.5±3.6 | <0.001 |
| DES use†,‡ | 368 (39) | 237 (11) | <0.001 |
| Thrombectomy†,‡,§,¶ | 661 (71) | 1318 (63) | <0.001 |
| Distal protection†,§ | 86 (9.2) | 168 (8.0) | 0.27 |
| Discharge at medications | |||
| Antiplatelet therapy | |||
| Thienopyridine | 926 (99) | 2,075 (99) | 0.33 |
| Aspirin | 929 (99) | 2,083 (99) | 0.3 |
| Cilostazol†,§ | 478 (51) | 648 (31) | <0.001 |
| Other medications | |||
| Statins†,§ | 602 (65) | 1,119 (53) | <0.001 |
| β-blockers†,§ | 369 (40) | 921 (44) | 0.03 |
| ACEI/ARB†,§ | 722 (77) | 1,603 (76) | 0.55 |
| Nitrates†,§ | 197 (21) | 726 (35) | <0.001 |
| Calcium channel blockers†,§ | 187 (20) | 423 (20) | 0.94 |
| Nicorandil†,§ | 262 (28) | 591 (28) | 0.96 |
| Warfarin†,§ | 126 (14) | 156 (7.4) | <0.001 |
| Proton pump inhibitors†,§ | 348 (37) | 693 (33) | 0.02 |
| H2 blockers†,§ | 369 (40) | 681 (32) | <0.001 |
Data given as n (%), mean±SD or median (IQR). Potential independent variables selected for Cox proportional hazard models for †TVR, all-cause death, and MACE in the whole group; ‡definite ST and MI in the whole group; §TVR and MACE in the subgroup of BMS; ¶all-cause death and MI in the subgroup of BMS, and for TVR, all-cause death, and MACE in the subgroup of DES. ACEI, angiotensin-converting enzyme inhibitors; ARB, angiotensin receptor blockers; BMS, bare-metal stent; COPD, chronic obstructive pulmonary disease; DES, drug-eluting stent; DM, diabetes mellitus; eGFR, estimated glomerular filtration rate, calculated by the modification of diet in renal disease formula modified for Japanese patients; IVUS, intravascular ultrasound; MACE, major adverse cardiac events, a composite of all-cause death, MI, or TVR; MI, myocardial infarction; ST, stent thrombosis; TVR, target vessel revascularization.
Statistical analysis was conducted by a physician (K.N.) and by a statistician (T.M.) using JMP 10.0 (SAS Institute, Cary, NC, USA) and SAS 9.4 (SAS Institute). All the statistical analyses were 2-tailed. P<0.05 was regarded as statistically significant.
Among 3,028 patients eligible for the current analysis, 932 patients (31%) underwent IVUS-guided PCI (IVUS guidance group), while 2096 patients (69%) received primary PCI without IVUS (angiography guidance group). The prevalence of IVUS during primary PCI varied across the participating centers (Figure S1). Regarding baseline clinical characteristics, advanced age, hypertension, severe mitral regurgitation, anemia, and history of malignancy were more common in the angiography guidance group (Table 1). The IVUS guidance group had a significantly longer door-to-balloon time as compared with the angiography guidance group, whereas onset-to-presentation time was not significantly different between the 2 groups (Table 1). There was no significant difference in infarct-related artery between the 2 groups. Regarding the procedural characteristics, DES use and thrombectomy were more common in the IVUS guidance group, whereas side-branch stenting was more frequently performed in the angiography guidance group. Stent size was significantly larger and final balloon inflation pressure was significantly higher in the IVUS guidance group than in the angiography guidance group (Table 1).
Median follow-up duration for the surviving patients was 5.1 years (IQR, 4.3–6.0 years). Complete 1-, 3-, and 5-year follow-up information was obtained in 98.5%, 96.1%, and 68.9% of patients, respectively.
Compared with the angiography guidance group, the IVUS guidance group was associated with significantly lower 5-year incidences of TVR (22% vs. 27%, log-rank P<0.001), and MACE (34% vs. 40%, log-rank P=0.003). After adjustment for confounders, however, no significant difference was observed in the risks for TVR (adjusted HR, 1.14; 95% CI: 0.86–1.51, P=0.38) or MACE (adjusted HR, 1.12; 95% CI: 0.89–1.40, P=0.33) between the 2 groups (Figure 2; Table 2). Detailed data on the multivariate analysis for TVR both before and after stratification by center are given in Tables S1,S2. The crude and adjusted risks for all-cause death and MI were also not significantly different between the 2 groups (Table 2). The IVUS guidance group was associated with significantly lower cumulative 5-year incidence of definite ST (1.2% vs. 3.1%, log-rank P=0.003), although the difference between the 2 groups was no longer significant after adjusting for confounders (adjusted HR, 0.58; 95% CI: 0.19–1.72, P=0.33; Table 2). The cumulative incidences of early (0–30 days) and late (>30–365 days) definite ST were significantly lower in the IVUS guidance group compared with the angiography guidance group, but the cumulative incidence of very late (>365 days) definite ST was not significantly different between the 2 groups (Figure 3).
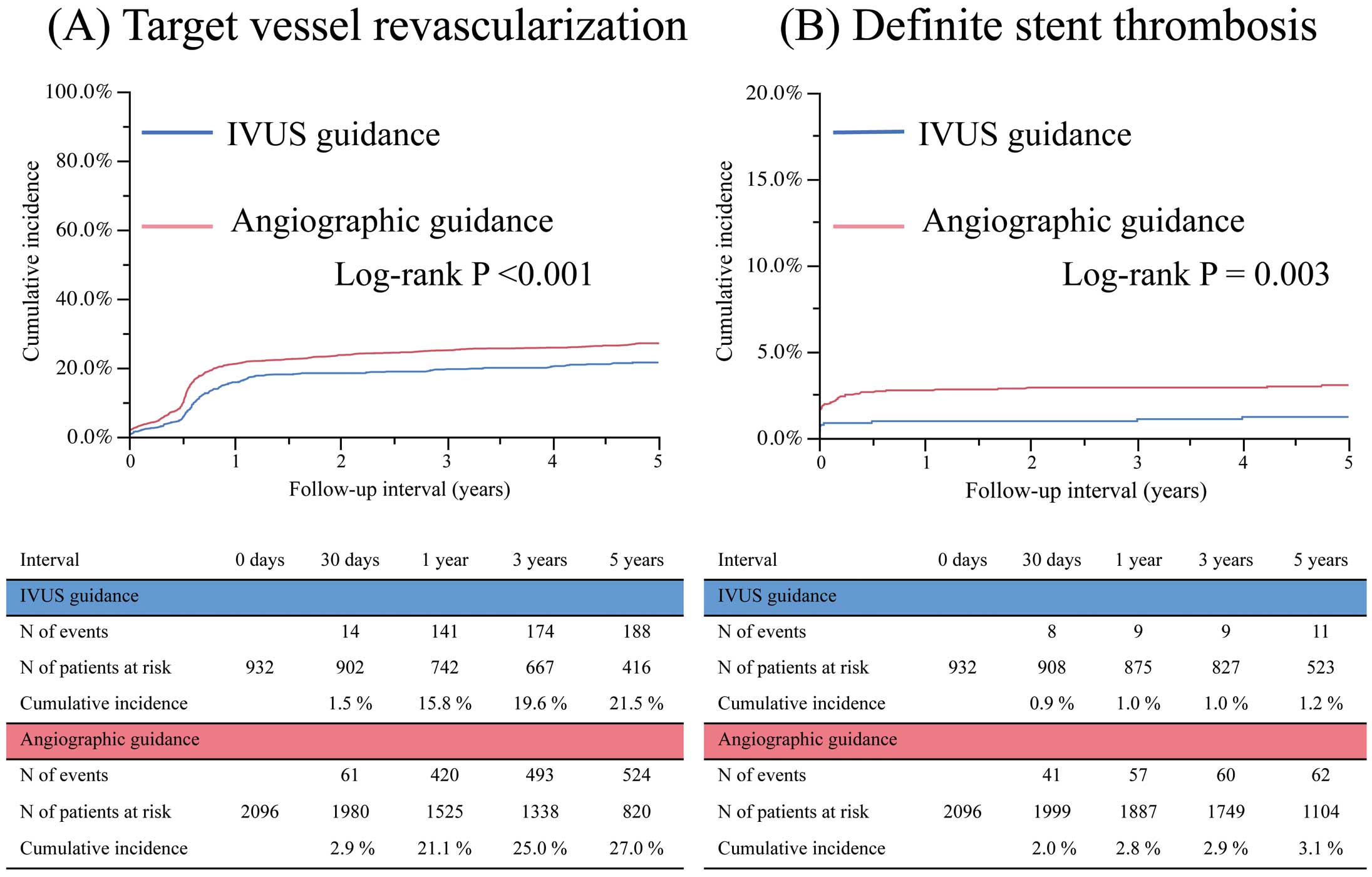
Cumulative incidences of (A) target vessel revascularization and (B) definite stent thrombosis compared between the intravascular ultrasound (IVUS) guidance group and angiographic guidance group.
| Variables | IVUS-guided PCI | Angiography- guided PCI |
IVUS guidance | |||||
|---|---|---|---|---|---|---|---|---|
| No. patients with events (Cumulative incidence) |
No. patients with events (Cumulative incidence) |
Unadjusted | P-value | Adjusted | P-value | |||
| HR | 95% CI | HR | 95% CI | |||||
| n=932 | n=2,096 | |||||||
| TVR | 188 (22) | 524 (27) | 0.76 | 0.64–0.89 | <0.001 | 1.14 | 0.86–1.51 | 0.38 |
| Definite ST | 11 (1.2) | 62 (3.1) | 0.39 | 0.20–0.71 | 0.003 | 0.58 | 0.19–1.72 | 0.33 |
| All-cause death | 112 (13) | 311 (16) | 0.87 | 0.71–1.05 | 0.15 | 0.82 | 0.57–1.19 | 0.31 |
| MI | 45 (5.2) | 123 (6.6) | 0.81 | 0.58–1.12 | 0.21 | 0.81 | 0.45–1.48 | 0.50 |
| MACE† | 313 (34) | 813 (40) | 0.83 | 0.73–0.94 | 0.003 | 1.12 | 0.89–1.40 | 0.33 |
Cumulative incidence was estimated using the Kaplan-Meier method. Unadjusted and adjusted HR and 95% CI were estimated using the Cox proportional hazard models. †All-cause death, MI, or TVR. MACE, major adverse cardiac events; PCI, percutaneous coronary intervention. Other abbreviations as in Table 1.
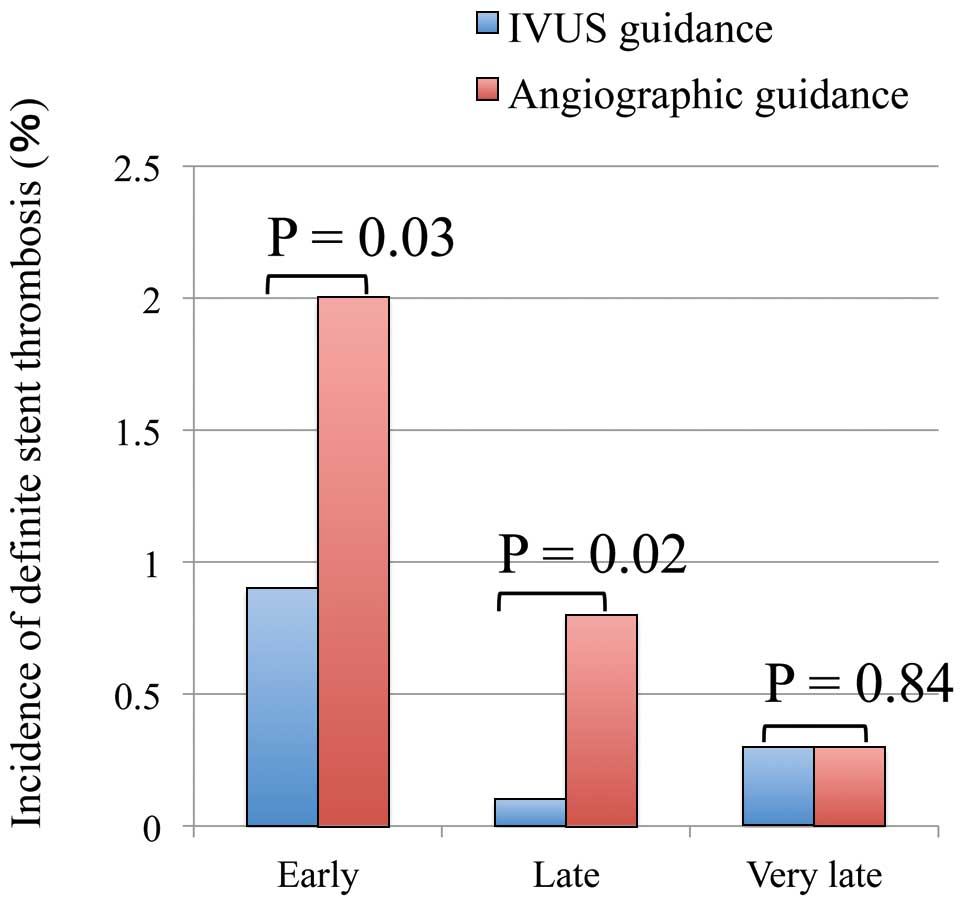
Cumulative incidences of early (0–30 days), late (>30 days–1 year), and very late (>1 year) definite stent thrombosis vs. guidance modality. IVUS, intravascular ultrasound.
In both the BMS and DES subgroups, the cumulative incidences of TVR, all-cause death, MI, and MACE were not significantly different between the IVUS guidance group and the angiography guidance group (Table 3). The adjusted risks for TVR, all-cause death, and MACE were also not significantly different between the 2 groups. In the BMS subgroup, the cumulative incidence of definite ST was significantly lower in the IVUS guidance group compared with that in the angiography guidance group. In contrast, the cumulative incidence of definite ST was not significantly different between the 2 groups in the DES subgroup (Table 3). The small number of events in definite ST, however, did not allow adjustment for confounders in both the BMS and DES subgroups.
| Variables | IVUS-guided PCI | Angiography- guided PCI |
IVUS guidance | |||||
|---|---|---|---|---|---|---|---|---|
| No. patients with events (Cumulative incidence) |
No. patients with events (Cumulative incidence) |
Unadjusted | P-value | Adjusted | P-value | |||
| HR | 95% CI | HR | 95% CI | |||||
| Bare-metal stent | n=564 | n=1,859 | ||||||
| TVR | 132 (25) | 477 (28) | 0.86 | 0.71–1.04 | 0.13 | 1.32 | 0.96–1.81 | 0.09 |
| Definite ST | 3 (0.5) | 59 (3.3) | 0.16 | 0.04–0.44 | <0.001 | – | – | – |
| All-cause death | 68 (13) | 271 (16) | 0.86 | 0.67–1.10 | 0.23 | 0.92 | 0.59–1.42 | 0.70 |
| MI | 24 (4.5) | 110 (6.6) | 0.74 | 0.48–1.12 | 0.16 | 0.79 | 0.40–1.57 | 0.50 |
| MACE† | 208 (38) | 723 (41) | 0.93 | 0.80–1.08 | 0.34 | 1.34 | 1.03–1.73 | 0.03 |
| Drug-eluting stent | n=368 | n=237 | ||||||
| TVR | 56 (16) | 47 (22) | 0.73 | 0.51–1.07 | 0.11 | 0.68 | 0.35–1.31 | 0.24 |
| Definite ST | 8 (2.2) | 3 (1.3) | 1.70 | 0.49–7.78 | 0.41 | – | – | – |
| All-cause death | 44 (13) | 40 (17) | 0.77 | 0.52–1.15 | 0.20 | 0.65 | 0.31–1.36 | 0.25 |
| MI | 21 (6.2) | 13 (6.5) | 0.91 | 0.48–1.80 | 0.79 | – | – | – |
| MACE† | 105 (29) | 90 (39) | 0.72 | 0.55–0.95 | 0.02 | 0.7 | 0.43–1.14 | 0.15 |
Cumulative incidence was estimated by the Kaplan-Meier method. Unadjusted HR and 95% CI were estimated by the Cox proportional hazard models. †All-cause death, MI, or TVR. Abbreviations as in Table 1.
The main finding in the current study was that IVUS-guided PCI was not associated with a lower risk for TVR or for ST in STEMI patients who underwent primary PCI.
The benefit of IVUS for TVR has been demonstrated in previous randomized trials in which patients were enrolled in the setting of elective PCI.3–7 IVUS could help in the optimization of stent expansion and detection of incomplete apposition and/or edge dissection, resulting in reduction of restenosis or ST. Indeed, the benefits of IVUS for preventing restenosis were mainly observed in previous studies with BMS and small vessel size, for which large acute lumen gain is crucial in preventing restenosis. In the DES era, in which restenosis was dramatically reduced, the benefits of IVUS are still somewhat controversial due to the absence of definitive randomized trial data,9,10 although the ADAPT-DES study enrolling >8,000 patients reported that IVUS-guided PCI with DES was associated with significantly lower rates of ST, MI, and MACE.12 The role of IVUS might be different in STEMI patients with primary PCI compared with those undergoing elective PCI. In the ADAPT-DES study, 813 STEMI patients were enrolled, and it was found that IVUS use was associated with substantially improved outcomes in STEMI patients.12 In accordance with several previous studies,13,14 however, the current study of 3,028 STEMI patients found no apparent benefit of IVUS for reducing TVR and ST as well as mortality in STEMI patients undergoing primary PCI regardless of stent type (DES or BMS). In STEMI patients, large thrombus burden in the culprit lesion generally makes it difficult to determine the optimal size of stent expansion. In addition, IVUS at the time of AMI was reported not to be able to predict the occurrence of late-acquired incomplete stent apposition (ISA), because late-acquired ISA seemed to result from positive vessel wall remodeling or thrombus dissolution.19 Furthermore, rapid reperfusion would be the most crucial issue, rather than detailed assessment of lesions and optimization of stent implantation during primary PCI. Indeed, door-to-balloon time was significantly longer in IVUS-guided PCI than in angiography-guided PCI in the current study. Therefore, the role of IVUS might be different between primary PCI for STEMI and elective PCI.
Consistent with the ADAPT-DES study,12 in contrast, the cumulative incidence of definite ST in the IVUS-guided PCI group was significantly lower than that in the angiography-guided PCI group in the current study. The adjusted HR for the risk of ST still favored IVUS-guided PCI, although the adjusted risk for ST was no longer statistically significant, possibly due to the lack of adequate sample size. The difference between IVUS- and angiography-guided PCI in the incidence of ST was calculated at <30 days after PCI in the current study, suggesting that IVUS use during primary PCI for STEMI might have the potential to prevent early ST via the detecting edge dissection or residual thrombus. Furthermore, the clinical impact of IVUS might differ according to the timing of IVUS use in primary PCI. Therefore, the present results do not necessarily preclude the use of IVUS during primary PCI.
The current study has several limitations. First and most importantly, the observational study design precluded definitive conclusions because of selection bias and unmeasured confounders. It was notable that the adjusted effect of IVUS-guided PCI relative to angiography-guided PCI for TVR after stratification by center moved further to the opposite direction to the crude effect, suggesting the presence of different procedural strategies, and variable threshold for TVR across centers. Second, we did not collect data on the timing of IVUS (either before or after intervention or both), IVUS parameters, or the rate of complications associated with IVUS use. Third, the low frequency of definite ST did not allow adjustment for confounders in the subgroup analysis. Fourth, it was left to the operator’s discretion whether to use IVUS and how to respond to the information obtained via IVUS, with no specific guidelines for optimal stenting. Therefore, well-conducted prospective randomized studies are needed in order to reach definitive conclusions on the role of IVUS in STEMI patients. Finally, the current practice pattern for STEMI, including the indications for DES for culprit lesions and the use of new P2Y12 inhibitors, has changed from that in the present study.
IVUS-guided PCI was not associated with a lower risk for TVR or ST in STEMI patients who underwent primary PCI.
We appreciate the assistance of the co-investigators at the CREDO-Kyoto AMI registry. The current study was supported by the Health, Labour and Welfare Ministry in Japan and the Pharmaceuticals and Medical Devices Agency (PMDA) in Japan. These organizations had no role in the study design, in the collection, analysis, or interpretation of data, in the writing of the reports, or in the decision to submit the article for publication.
The authors declare no conflicts of interest.
The current study was supported by the Health, Labour and Welfare Ministry in Japan and the Pharmaceuticals and Medical Devices Agency (PMDA) in Japan.
Supplementary File 1
Appendix S1. Participating Centers and Investigators for the CREDO-Kyoto AMI Registry
Appendix S2. Clinical Research Coordinators
Appendix S3. Clinical Event Committee Members
Figure S1. Prevalence of IVUS-guided PCI per center.
Table S1. Multivariate analysis for TVR before stratification by center
Table S2. Multivariate analysis for TVR after stratification by center
Please find supplementary file(s);
http://dx.doi.org/10.1253/circj.CJ-15-0870