論文ID: CJ-16-0035
論文ID: CJ-16-0035
Background: Obstructive sleep apnea (OSA) is associated with activation of the sympathetic nervous system, and patients with this condition often experience elevated blood pressure (BP), increased BP variability, and nocturnal BP surges.
Methods and Results: The SYMPLICITY HTN-3 trial was a large prospective, randomized, blinded, sham-controlled trial of renal denervation for treatment of uncontrolled, apparently treatment-resistant hypertension. In a post hoc analysis, we examined the effect of renal denervation vs. sham control on office and ambulatory (including nocturnal) systolic BP in patients with and without OSA. 26% (94/364) of renal denervation subjects and 32% (54/171) of sham control subjects had OSA. Baseline office and nighttime systolic BP values were similar in both arms, including in subjects with and without OSA. Compared with sham control, renal denervation reduced the 6-month office systolic BP in subjects with (−17.0±22.4 vs. −6.3±26.1 mmHg, P=0.01) but not in subjects without OSA (−14.7±24.5 vs. −13.4±26.4 mmHg, P=0.64), P=0.07 for the interaction between treatment arm and OSA status. In those with sleep apnea, renal denervation was also associated with a reduction in maximum (−4.8±21.8 vs. 4.5±24.6 mmHg, P=0.03) and average peak (−5.6±20.4 vs. 3.2±22.4 mmHg, P=0.02) nighttime systolic BP.
Conclusions: OSA subjects appeared to be responsive to renal denervation therapy. However, this hypothesis requires prospective testing.
Obstructive sleep apnea (OSA) affects 3–7% of the adult population1 and is associated with increased risk for cardiovascular events, including ischemic heart disease, heart failure, stroke, and death.2–4 OSA is an independent risk factor for resistant hypertension,5 with a prevalence of 65–90% in this patient population.6–8 Several biological mechanisms have been proposed to explain the association of OSA with increased cardiovascular risk, including intermittent hypoxia leading to increased oxidative stress and endothelial dysfunction, as well as increased nighttime sympathetic tone with persistent blood pressure (BP) elevation.9,10
Editorial p ????
A nondipping pattern of 24-h ambulatory systolic BP (SBP) is common in OSA patients, because of high sympathetic tone, and it is also a risk factor for stroke and advanced target organ damage.11,12 Likewise, a common characteristic of ambulatory BP in OSA patients is uncontrolled nocturnal BP.12 Variability in nocturnal SBP, because of the high BP peaks, is in itself a risk factor for stroke and cardiovascular events. Pringle et al reported that for every within-subject standard deviation increase of 5 mmHg in nighttime SBP in an elderly hypertensive population, the risk of stroke increased by 80%.13
In some settings, percutaneous renal sympathetic denervation (RDN) therapy using radiofrequency energy has been associated with a reduction in SBP as well as attenuated central sympathetic outflow.14–18 This is evidenced by reductions in both renal noradrenaline spillover19 and muscle sympathetic nerve activity.20 We hypothesized that nocturnal SBP might be a sensitive indicator of response to RDN, particularly in patients with OSA, and analyzed the outcomes of the Renal Denervation in Patients With Uncontrolled Hypertension (SYMPLICITY HTN-3) trial. Although the SYMPLICITY HTN-3 trial, which randomized subjects to RDN or a sham procedure, did not demonstrate superiority of RDN over sham control based on the 6-month change in office BP,21 further evaluation of the trial identified several potentially confounding factors that may have affected the overall results.22 We examined the effect of RDN on 6-month 24-h SBP in resistant hypertensive subjects with OSA in the SYMPLICITY HTN-3 trial.
The SYMPLICITY HTN-3 trial enrolled 535 subjects from 88 sites in the USA between October 2011 and May 2013. The study design and overall outcomes16,21,23 have been reported. Briefly, the trial enrolled 18–80-year-old subjects with uncontrolled, apparently treatment-resistant hypertension (office SBP ≥160 mmHg despite 3 or more antihypertensive medications, including a diuretic, at maximum tolerable dose). Baseline 24-h ambulatory BP monitoring was used to exclude subjects with white coat hypertension (24-h SBP <135 mmHg despite office SBP ≥160 mmHg). Subjects were randomized 2:1 to RDN or a sham procedure (renal angiography without RDN) with both the subject and BP assessor blinded to treatment arm. The trial was approved by the institutional review board of each site, and all subjects provided written informed consent.
At the time of enrollment, subjects self-reported whether they currently had sleep apnea, or OSA, and whether they currently used continuous or bilevel positive airway pressure (CPAP/BiPAP) for nocturnal respiratory support. Individuals requiring chronic oxygen support or mechanical ventilation, including CPAP/BiPAP, for reasons other than nocturnal respiratory support because of sleep apnea were excluded from the study.
Ambulatory BP MonitoringAll ambulatory BP measurements were performed with the Spacelabs 24 Ambulatory Blood Pressure Monitoring System (Spacelabs Healthcare, Snoqualmie, WA, USA). The 24-h ambulatory BP monitoring was performed every 30 min throughout the day and night at both baseline and 6 months after randomization.
DefinitionsIn this post hoc analysis, we defined the nighttime period as 01:00 to 05:59 hours, as defined previously, to capture sleeping hours.24 Daytime was defined as 09:00 to 20:59 hours. Average peak nighttime BP was the average of the 3 highest BP measurements over the nighttime period. Maximum (and minimum) nocturnal BP values were the maximum (and minimum) readings over the nighttime period. A dipping BP pattern was defined as an average decrease in SBP at nighttime of at least 10% of average daytime SBP; all other patterns were defined as nondipping.
Statistical AnalysisContinuous variables are presented as mean±standard deviation. Between-group differences were compared using t-tests, and within-group differences from baseline to follow-up were assessed using the paired t-test. Categorical variables, presented as counts and percentages, were compared using Fisher’s exact test and the chi-square test for multilevel variables. The trial primary endpoint analysis25 applied a modified intention-to-treat principle: patients with qualifying medication changes used the BP measurements immediately prior to the medication change for the 6-month BP measurement used in the original analysis of SYMPLICITY HTN-3. The present analyses did not apply this carry-forward principle, and the current 6-month analysis only includes patients with a BP measurement obtained at 6 months. The P value for the interaction between treatment arm (RDN vs. control) and OSA status (OSA vs. non-OSA) was calculated for various BP measures. P<0.05 was considered statistically significant. All analyses were performed using SAS version 9.1 or higher (SAS Institute, Inc, Cary, NC, USA).
Patient disposition in SYMPLICITY HTN-3 is shown in Figure 1. Among the 535 resistant hypertensive subjects enrolled, OSA was present in 26% (94/364) of subjects in the RDN arm and in 32% (54/171) of subjects in the sham control arm (P=0.18). Table 1 provides baseline demographics for subjects with and without OSA in the RDN and sham arms. In the RDN arm, subjects with OSA had higher body mass index and were more likely to have type II diabetes mellitus and left ventricular hypertrophy as compared with non-OSA subjects. Among subjects with OSA, baseline characteristics were similar between those in the RDN and control arms, although OSA subjects in the RDN arm were older. Two-thirds (62/94) of subjects with OSA in the RDN arm and in the control arm (37/54) were using CPAP/BiPAP for nocturnal respiratory support because of sleep apnea. Baseline demographics were similar regardless of current usage of CPAP/BiPAP in both the RDN and control arms.

Patient disposition in SYMPLICITY HTN-3. ABPM, ambulatory blood pressure monitoring; M, month; OSA, obstructive sleep apnea; RDN, renal denervation.
| OSA | Non-OSA | |||||
|---|---|---|---|---|---|---|
| RDN (n=94) | Sham (n=54) | P value | RDN (n=270) | Sham (n=117) | P value | |
| Age, years | 56.1±10.4 | 52.2±10.5 | 0.03 | 58.6±10.4 | 58.1±11.1 | 0.69 |
| Male | 63 (67.0) | 41 (75.9) | 0.27 | 152 (56.3) | 69 (59.0) | 0.66 |
| Race | 0.64 | 0.19 | ||||
| White | 74 (78.7) | 39 (72.2) | 191 (71.0) | 80 (68.4) | ||
| Black or African American | 18 (19.1) | 13 (24.1) | 72 (26.8) | 37 (31.6) | ||
| Other | 2 (2.1) | 2 (3.7) | 6 (2.2) | 0 (0.0) | ||
| Body mass index, kg/m2 | 37.0±6.0 | 35.1±6.4 | 0.07 | 33.3±6.4 | 33.3±6.4 | 0.94 |
| Chronic kidney disease (eGFR <60 ml/min/1.73 m2) |
12 (12.8) | 10 (18.5) | 0.35 | 23 (8.5) | 7 (6.0) | 0.54 |
| Stroke | 9 (9.6) | 5 (9.3) | >0.99 | 21 (7.8) | 14 (12.0) | 0.25 |
| Current smoker | 8 (8.5) | 10 (18.5) | 0.12 | 28 (10.4) | 11 (9.4) | 0.86 |
| Left ventricular hypertrophy | 25 (26.6) | 15 (27.8) | >0.99 | 40 (14.8) | 95 (19.7) | 0.24 |
| History of coronary artery disease | 31 (33.0) | 14 (25.9) | 0.46 | 70 (25.9) | 30 (25.6) | >0.99 |
| Type 2 diabetes mellitus | 54 (57.4) | 26 (48.1) | 0.31 | 117 (43.3) | 44 (37.6) | 0.31 |
| Taking antihypertensive medication for >10 years | 64 (68.1) | 38 (70.4) | 0.85 | 184 (68.1) | 79 (67.5) | 0.91 |
| History of hospitalization for hypertensive crisis | 27 (28.7) | 14 (25.9) | 0.85 | 57 (21.1) | 24 (20.5) | >0.99 |
Results presented as mean±standard deviation or no. (%). eGFR, estimated glomerular filtration rate; OSA, obstructive sleep apnea; RDN, renal denervation.
Table S1 shows the antihypertensive medication prescriptions at baseline and 6 months among subjects with and without OSA in SYMPLICITY HTN-3. At baseline, the number of antihypertensive medications prescribed in the RDN and sham control arms among OSA subjects was 5.1±1.2 vs. 5.4±1.5 (P=0.14) and remained similar at 6 months (5.0±1.4 vs. 5.4±1.7, P=0.09). Compared with OSA subjects in the sham control arm, OSA subjects in the RDN arm were more likely to be prescribed an angiotensin-converting enzyme inhibitor but less likely to be prescribed an aldosterone antagonist at baseline.
BPNo differences in baseline SBP (office and 24-h ambulatory) between subjects in the OSA and non-OSA groups were observed at 6 months (Table 2). At 6 months, the change in office SBP among OSA subjects was greater in the RDN group as compared with the sham group (−17.0±22.4 vs. −6.3±26.1 mmHg, P=0.01); however, the change in 24-h ambulatory SBP was not significantly different between the 2 groups (−5.0±14.7 vs. −0.8±17.9 mmHg, P=0.14). There was no difference among non-OSA subjects between the RDN and control arms in both office and 24-h ambulatory SBP (Figure 2). The greater response to treatment in OSA as compared with non-OSA subjects was driven in part by the smaller response in OSA control subjects as compared with non-OSA control subjects (office SBP −6.3 vs. −13.4 mmHg, P=0.11, P=0.07 for the interaction between treatment arm and OSA status; 24-h ambulatory SBP −0.8 vs. −6.6 mmHg, P<0.05, P=0.30 for the interaction term).
| OSA | Non-OSA | |||||
|---|---|---|---|---|---|---|
| RDN (n=94) | Sham (n=54) | P value | RDN (n=270) | Sham (n=117) | P value | |
| Baseline BP, mmHg | ||||||
| Office | n=94 | n=54 | n=270 | n=117 | ||
| Systolic | 181.9±17.6 | 182.3±17.2 | 0.90 | 179.0±15.5 | 179.2±16.7 | 0.91 |
| Diastolic | 96.5±15.4 | 104.9±15.9 | <0.01 | 96.5±17.0 | 96.1±15.0 | 0.82 |
| 24-h ambulatory | n=93 | n=54 | n=267 | n=113 | ||
| Systolic | 160.3±12.3 | 163.0±17.0 | 0.31 | 158.7±13.5 | 157.8±14.2 | 0.59 |
| Diastolic | 88.2±12.4 | 96.1±13.5 | <0.01 | 88.0±14.5 | 88.5±14.2 | 0.78 |
| Average daytime systolic | 164.4±13.0 | 167.9±15.5 | 0.15 | 163.0±13.8 | 163.2±15.0 | 0.92 |
| Nighttime ambulatory systolic | ||||||
| Average | 152.3±15.7 | 152.6±23.7 | 0.92 | 150.5±18.2 | 147.1±17.2 | 0.09 |
| Maximum | 170.0±18.4 | 168.6±25.0 | 0.72 | 168.5±20.7 | 165.4±19.9 | 0.18 |
| Minimum | 134.8±16.6 | 136.6±23.9 | 0.62 | 134.6±18.4 | 131.3±17.7 | 0.10 |
| Average peak | 164.6±17.3 | 163.3±23.8 | 0.74 | 162.5±19.4 | 159.4±18.3 | 0.15 |
| 6-mo change in BP, mmHg | ||||||
| Office | n=91 | n=52 | n=259 | n=117 | ||
| Systolic | −17.0±22.4 | −6.3±26.1 | 0.01 | −14.7±24.5 | −13.4±26.4 | 0.64 |
| Diastolic | −6.7±11.7 | −4.7±15.5 | 0.42 | −6.5±12.0 | −4.6±12.7 | 0.17 |
| 24-hour ambulatory | n=83 | n=50 | n=242 | n=109 | ||
| Systolic | −5.0±14.7 | −0.8±17.9 | 0.14 | −7.3±15.2 | −6.6±16.7 | 0.69 |
| Diastolic | −3.7±9.1 | −1.8±9.7 | 0.27 | −4.3±9.3 | −3.7±10.3 | 0.62 |
| Daytime ambulatory systolic | ||||||
| Average | −5.2±14.6 | −2.1±17.7 | 0.28 | −7.9±16.8 | −8.4±18.7 | 0.77 |
| Nighttime ambulatory systolic | ||||||
| Average | −5.5±19.6 | 1.6±21.5 | 0.06 | −6.3±17.8 | −3.1±18.7 | 0.13 |
| Maximum | −4.8±21.8 | 4.5±24.6 | 0.03 | −7.1±21.9 | −3.8±21.4 | 0.19 |
| Minimum | −4.1±22.1 | 1.4±21.4 | 0.17 | −6.7±18.8 | −3.7±19.3 | 0.17 |
| Average peak | −5.6±20.4 | 3.2±22.4 | 0.02 | −6.8±19.8 | −2.8±19.9 | 0.08 |
BP, blood pressure. Other abbreviations as in Table 1.
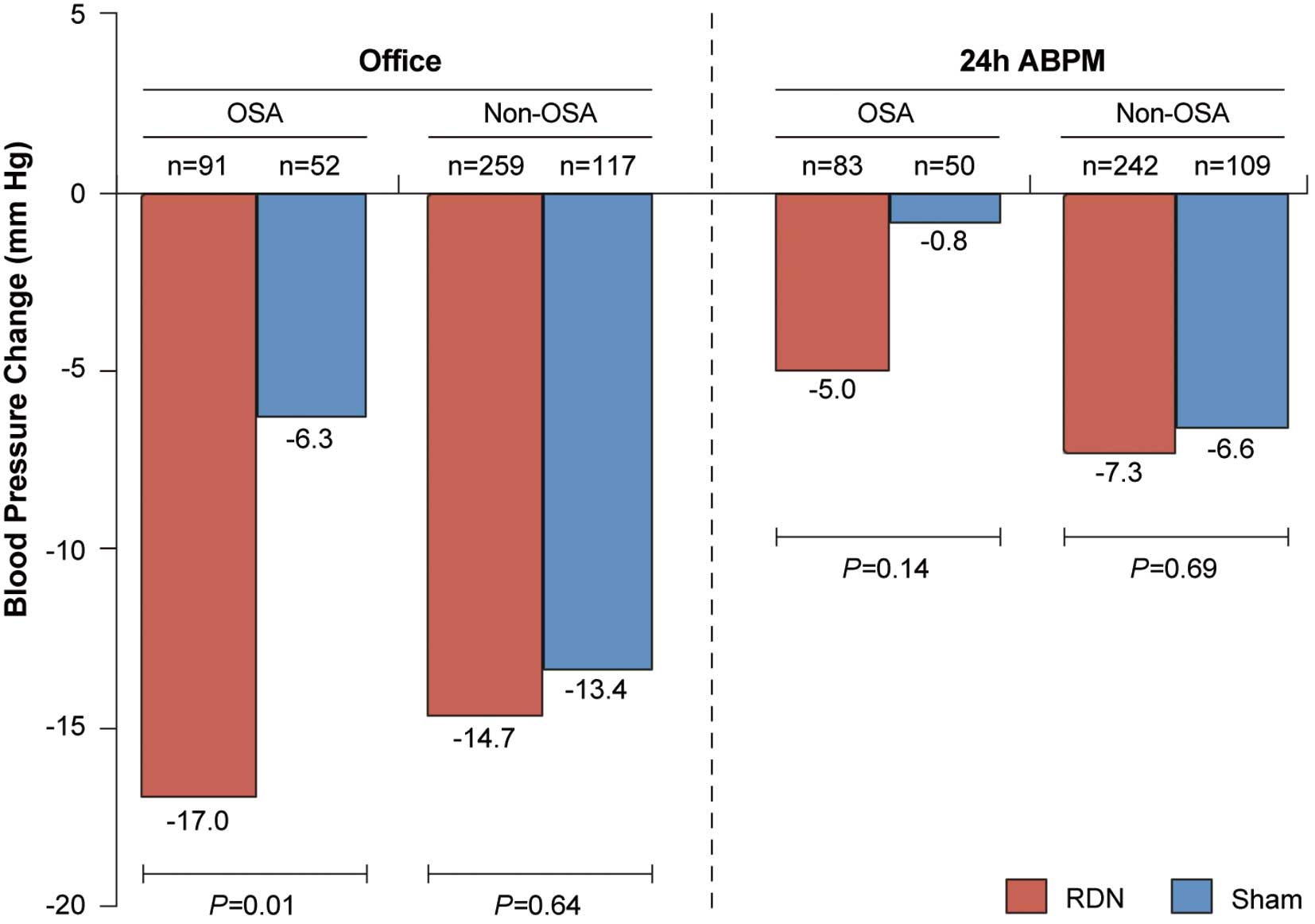
The 6-month change in office and 24-h ambulatory systolic blood pressure values in subjects treated with renal denervation (RDN) or a sham procedure among those with and without obstructive sleep apnea (OSA). ABPM, ambulatory blood pressure monitoring.
Greater differences between RDN and sham group subjects with OSA were observed in peak nighttime (−5.6±20.4 vs. 3.2±22.4, P=0.02) and maximum nighttime (−4.8±21.8 vs. 4.5±24.6, P=0.03) SBP, as was a trend favoring RDN in average nighttime SBP (−5.5±19.6 vs. 1.6±21.5, P=0.06) (Figure 3A). The P values for the interaction between treatment arm and OSA status for average, maximum, and peak nighttime SBP values were all nonsignificant. These differences between RDN and sham group subjects were not observed in the non-OSA subgroup (Figure 3B).

The 6-month change in systolic blood pressure in the renal denervation (RDN) and sham control groups, stratified by daytime and nighttime, in subjects with (A) and without (B) obstructive sleep apnea.
The improvement in nocturnal BP in RDN compared with sham control OSA subjects was more pronounced in the subgroup of OSA patients using CPAP/BiPAP (Figure 4), although no difference in the 6-month change in office SBP between OSA subjects using or not using CPAP/BiPAP was observed.

The 6-month change in nighttime systolic blood pressure among subjects with obstructive sleep apnea who were receiving or not receiving continuous/bilevel positive airway pressure (CPAP/BiPAP), stratified by renal denervation (RDN) and sham control.
A nocturnal nondipping pattern was observed at baseline in 72% of OSA subjects (74% of RDN subjects and 65% of sham control subjects). A significant increase in the proportion of subjects with a nondipping pattern was observed between baseline and 6 months in the OSA control subjects (56% vs. 70%, P=0.01) but not the RDN subjects (Figure 5). Among all subjects with a nondipping pattern at baseline, the treatment difference on the 6-month change in peak nighttime SBP in RDN and control subjects was more pronounced (−10.7 vs. −4.5 mmHg, P=0.02) (Figure 6A). A similar relationship was observed among OSA subjects, though it was not statistically significant (Figure 6B).

Dipper status at baseline and 6 months among patients with obstructive sleep apnea, stratified by renal denervation (RDN) and sham control. There was a significant increase in the number of nondippers between baseline and 6 months among obstructive sleep apnea subjects in the sham control group but not in the RDN group. M, months.

The 6-month change in systolic blood pressure among patients who were nondippers at baseline, stratified by (A) all patients and (B) patients with obstructive sleep apnea. RDN, renal denervation.
The main finding of our post hoc analysis was that the subset of OSA resistant hypertensive patients treated with RDN in SYMPLICITY HTN-3 showed a greater reduction in office SBP compared with the sham control group at 6 months post-treatment. Furthermore, the change in maximum nighttime SBP was greater among OSA subjects treated with RDN than among control subjects, as was the average peak nighttime SBP (−5.6±20.4 vs. 3.2±22.4 mmHg, P=0.02). This difference in nocturnal BP between RDN and sham control subjects was also observed in OSA subjects using CPAP/BiPAP.
During sleep, OSA patients experience repetitive apneic events that result in hypoxia and hypercapnia, which act via chemoreflexes to elicit high levels of sympathetic nerve traffic.26 Reducing sympathetic activity in OSA patients, as has been demonstrated with CPAP (reducing both muscle sympathetic nerve activity27 and norepinephrine clearance28), leads to a reduction in BP29 and the severity of OSA.30 By reducing efferent renal sympathetic drive, RDN might reduce chronic fluid overload and, possibly, peripharyngeal fluid accumulation that may predispose to upper airway obstruction.31 Previously, Witkowski et al evaluated the 6-month effect of sleep apnea severity in 10 patients with resistant hypertension and OSA treated with RDN and reported decreases in office SBP, apnea-hypopnea index, oxygen desaturation index, Epworth Sleepiness Scale score, and plasma glucose concentration.32 This decrease in the apnea-hypopnea index was also observed in a meta-analysis that included 49 OSA patients treated with RDN.33 Furthermore, Linz et al showed in a preclinical model that the increased sympathetic response associated with tracheal obstruction was attenuated with RDN but not with β-blockade.34
OSA is also associated with a nocturnal surge in BP variability because of high BP peaks, a nondipping BP pattern, and therefore increased cardiovascular risk.12 Even OSA patients using CPAP/BiPAP may still experience a nocturnal BP surge,35 which may explain the observed treatment difference in nighttime SBP, including peak nighttime SBP, even in OSA subjects using CPAP/BiPAP. Additionally, among nondippers, the 6-month change in peak nighttime SBP in the SYMPLICITY HTN-3 trial among all subjects was −10.7±19.5 mmHg in subjects treated with RDN, which was greater than the change in the control arm (−4.5±22.1 mmHg) P=0.02), with a similar (but nonsignificant) order of magnitude in OSA subjects. Because the nondipping pattern may be mediated by the autonomic nervous system, and high sympathetic tone in particular, the present results suggested that OSA subjects may be particularly responsive to RDN therapy. Furthermore, although there was a significant increase in the percent of nondippers vs. dippers in the sham control OSA cohort between baseline and 6 months, perhaps because of a natural progression of disease, no change in dipper status was observed in the RDN OSA cohort, which again suggested that RDN reduced the sympathetic tone in these patients. However, this hypothesis requires prospective testing.
The treatment difference among OSA subjects was primarily associated with differences in the sham control response between OSA and non-OSA subjects (office SBP −6.3 vs. −13.4 mmHg, P=0.11) rather than in RDN subjects with and without OSA (office SBP −17.0 vs. −14.7 mmHg). It has been hypothesized that increased medication adherence at 6 months in sham control subjects decreased the ability to discern the effect of RDN on BP in SYMPLICITY HTN-3.22 However, OSA subjects tend to be more adherent to prescribed pharmacological treatments.36 Thus, OSA subjects may have allowed for better discrimination of the effect of RDN on BP by providing a more “controlled” comparator group than with non-OSA subjects. The importance of medication adherence is also supported by the fact that other studies of RDN that appeared to have improved medication adherence showed a greater treatment effect.37,38
The reduction in 24-h ambulatory SBP after RDN was less pronounced than the reduction in office SBP, in both OSA and non-OSA subjects, a phenomenon observed not only in the SYMPLICITY HTN-3 trial but in other studies of RDN.38,39 This difference in office and ambulatory BP reductions appears to be greater with increasing baseline BP.40 Compared with 24-h ambulatory BP, office BP is more susceptible to white coat hypertension, Hawthorne effect, measurement bias, and measurement variability.41 The 24-h ambulatory BP also includes nighttime measurements when BP tends to be lower than during the daytime, when office BP is measured.
Study LimitationsThe results of this analysis should be interpreted in the context of several limitations. OSA was self-reported by subjects, and polysomnography was not performed at baseline. However, 80% of OSA subjects required CPAP/BiPAP machines, suggesting that these subjects indeed had OSA. Interestingly, the subset of patients treated with CPAP had a more pronounced response to RDN. It is therefore possible that CPAP prescription was a more specific identifier of the OSA subpopulation. The definitions of daytime and nighttime were not actigraphy-guided individual determinations. This post hoc analysis was undertaken in a clinical trial that did not meet its primary efficacy endpoint. Furthermore, no adjustment was made for multiple subgroup and endpoint comparisons. Lastly, all patients in the SYMPLICITY HTN-3 trial had treatment-resistant hypertension, and outcomes after RDN in patients with OSA who do not have resistant hypertension are unknown.
In conclusion, OSA subjects treated with RDN in the SYMPLICITY HTN-3 trial had a significant 6-month reduction in office SBP compared with sham control subjects. Nighttime ambulatory BP analysis showed a significant and marked 6-month reduction in maximum and average peak nocturnal BP values with RDN compared with sham control among OSA subjects. The present results suggested that patients with OSA may be particularly responsive to RDN therapy. RDN also reduced nighttime SBP in nondippers, which also suggested that RDN affected the sympathetic drive in these patients. Whether these specific subgroup results may be partly related to differences in baseline sympathetic tone, drug adherence, randomization bias, or to pure chance is unknown. However, this analysis suggested that when medications only cannot suppress nocturnal hypertension and BP peaks in OSA hypertensive patients, a strategy combined with RDN may be an effective approach to test. Our analysis of BP outcomes in OSA subjects after RDN requires confirmation in future prospective clinical research of subjects randomized to RDN therapy or to a sham procedure. The SPYRAL HTN Global Clinical Trial Program, now under way in its first phase, will examine outcomes in the absence (SPYRAL HTN OFF-MED, ClinicalTrials.gov Identifier: NCT02439749) and presence (SPYRAL HTN ON-MED, NCT02439775) of antihypertensive therapy and will likely include OSA subjects.42 Both trials will use the SymplicityTM Spyral multielectrode renal denervation catheter to provide automated 4-quadrant ablations, and both trials will allow RDN treatments in renal artery branches and accessories in addition to the main renal artery. These trials will test whether this change in denervation device and procedure, in addition to studying a more typical hypertensive trial population and assessing medication adherence, will address the limitations identified from the SYMPLICITY HTN-3 trial, leading to a greater treatment effect. Additionally, the Renal Denervation in Patients With Resistant Hypertension and Obstructive Sleep Apnea trial (clinicaltrials.gov Identifier: NCT01366625) has completed enrollment of 60 subjects with OSA and resistant hypertension and will assess BP reduction, sleep apnea response, metabolic markers, and cardiac changes after RDN.
We thank Minglei Liu, PhD, and Martin Fahy, MS, for data analysis support; Nicole Brilakis, MS, MBA, and Sidney A. Cohen, MD, for editorial support; and Vanessa DeBruin, MS, and Denise Jones, RN, BSN, for research support (all of Medtronic).
K.K. has received honoraria from Daiichi Sankyo Co, Ltd, Mochida Pharmaceutical Co, Ltd, Dainippon Sumitomo Pharma, Astellas Pharma, Bristol-Myers Squibb, Teijin Pharma Ltd, Novartis, Omron Healthcare, and Medtronic. D.L.B. discloses the following relationships – Advisory Board: Cardax, Elsevier Practice Update Cardiology, Medscape Cardiology, Regado Biosciences; Board of Directors: Boston VA Research Institute, Society of Cardiovascular Patient Care; Chair: American Heart Association Get With the Guidelines Steering Committee; Data Monitoring Committees: Duke Clinical Research Institute, Harvard Clinical Research Institute, Mayo Clinic, Population Health Research Institute; Honoraria: American College of Cardiology (Senior Associate Editor, Clinical Trials and News, ACC.org), Belvoir Publications (Editor in Chief, Harvard Heart Letter), Duke Clinical Research Institute (clinical trial steering committees), Harvard Clinical Research Institute (clinical trial steering committee), HMP Communications (Editor in Chief, Journal of Invasive Cardiology), Journal of the American College of Cardiology (Guest Editor; Associate Editor), Population Health Research Institute (clinical trial steering committee), Slack Publications (Chief Medical Editor, Cardiology Today’s Intervention), WebMD (CME steering committees); Other: Clinical Cardiology (Deputy Editor); Research Funding: Amarin, AstraZeneca, Bristol-Myers Squibb, Eisai, Ethicon, Forest Laboratories, Ischemix, Medtronic (co-principal investigator of SYMPLICITY HTN-3), Pfizer, Roche, Sanofi Aventis, The Medicines Company; Site Co-Investigator: Biotronik, Boston Scientific, St. Jude Medical; Trustee: American College of Cardiology; Unfunded Research: FlowCo, PLx Pharma, Takeda. D.E.K. receives research and grant support and consulting honoraria from Medtronic CardioVascular and Boston Scientific. S.B. and C.G. are employees of Medtronic. J.M.F. is a consultant for BackBeat Medical, Medtronic, Bayer, Forest, Regeneron, and Sanofi; he receives research support from Medtronic. S.O. is an advisor to and receives research support from Medtronic. M.R. has nothing to disclose. R.R.T. receives consultant fees from Medtronic, Janssen, and Relypsa, and royalties from UpToDate. G.B. is a consultant for Bayer AG, Boehringer Ingelheim GmbH, Bristol-Myers Squibb Co, Daiichi Sankyo Co, Ltd, Janssen Pharmaceuticals, Inc, Medtronic, and Relypsa, Inc, and has received university-directed research support from Takeda Pharmaceutical Co, Ltd.
This work was supported by Medtronic. Clinical trial registration – www.clinicaltrials.gov; Unique identifier: NCT01418261.
Supplementary File 1
Table S1. Antihypertensive medication prescription among patients with obstructive sleep apnea at baseline and 6 months
Please find supplementary file(s);
http://dx.doi.org/10.1253/circj.CJ-16-0035