論文ID: CJ-16-0125
論文ID: CJ-16-0125
Background: There are little data about cardiovascular shock caused by various diseases. We evaluated the characteristics and predictors of 30-day mortality in patients with cardiovascular shock in Japan.
Methods and Results: The Japanese Circulation Society Cardiovascular Shock registry was a prospective, observational, multicenter, cohort study. Between May 2012 and June 2014, 979 patients with cardiovascular shock were analyzed. The primary endpoint was 30-day all-cause mortality. The mean systolic blood pressure on hospital arrival was 78±18 mmHg. The main causes of shock were acute coronary syndrome (51.0%), non-ischemic arrhythmia (16.4%), and aortic disease (14.9%). The 30-day all-cause mortality was 34.3%. After adjustment for independent predictors of 30-day mortality, the odds ratios for systolic blood pressure (per 10-mmHg decrease), consciousness disturbance, congestive heart failure, out-of-hospital cardiac arrest, estimated glomerular filtration rate (per 10-ml/min/1.73 m2 decrease), and causes of shock (non-ischemic arrhythmia, aortic disease, and myocarditis) were 1.15 (95% confidence interval [CI], 1.08–1.22), 2.62 (95% CI, 1.80–3.82), 2.58 (95% CI, 1.67–3.99), 1.62 (95% CI, 1.05–2.51), 1.20 (95% CI, 1.10–1.30), and 0.48 (95% CI, 0.30–0.77), 3.98 (95% CI, 2.32–6.81), and 3.25 (95% CI, 1.20–8.84), respectively.
Conclusions: The 30-day mortality for cardiovascular shock was still high at 34%. Primary predictors of mortality were cardiorenal function on hospital arrival and shock etiology.
Shock is a manifestation of circulatory failure related to an insufficient supply of oxygenated blood to the tissues. Cardiovascular shock is caused by multiple conditions, and is associated with a high mortality rate despite advances in emergency care systems and treatments for shock.1–5 Although cardiogenic shock is commonly caused by acute myocardial infarction (MI),6 making a diagnosis in the emergency setting is always challenging. Understanding the actual state of cardiovascular shock, such as the distribution of its causes and characteristics, is very helpful in daily clinical practice. Moreover, determining the predictors of short-term mortality in patients with cardiovascular shock is very important for taking measures against cardiovascular shock. However, there are little data about cardiovascular shock caused by various diseases, except for acute coronary syndrome (ACS). Therefore, the aim of the present study was to evaluate the characteristics and predictors of 30-day mortality in patients with cardiovascular shock in Japan.
The Japanese Circulation Society (JCS) Cardiovascular Shock registry was a prospective, observational, multicenter cohort study. Each hospital’s ethics committee approved the registry, which was registered with the University Hospital Medical Information Network Clinical Trials Registry (no.: UMIN000008441; http://www.umin.ac.jp/ctr/index/htm/). Patients diagnosed with cardiovascular shock were enrolled from 82 centers in Japan between May 2012 and June 2014. Cardiovascular shock included ACS, non-ischemic arrhythmia, aortic disease, myocarditis, cardiomyopathy, pulmonary thromboembolism, valvular heart disease (VHD), infective endocarditis, cardiac tamponade and others. Eligible patients had out-of-hospital onset of cardiovascular shock, and they had to meet 1 major criterion and ≥1 minor criteria. Major criteria were (1) systolic blood pressure (SBP) ≤100 mmHg and heart rate <60 beats/min or >100 beats/min, and (2) decrease in SBP >30 mmHg from the usual values. Minor criteria were the presence of cold sweat, pallor of the skin, cyanosis, a capillary refill time ≥2 s, consciousness disturbance, or vital organ hypoperfusion according to the physician. Patients who had out-of-hospital cardiac arrest (OHCA) were eligible if they were still in a state of shock after return of spontaneous circulation (ROSC). Patients younger than 16 years or those without ROSC were prospectively excluded.
Prehospital information about the patients transferred by an ambulance was collected by emergency medical service (EMS) personnel, and patients’ clinical signs, diagnosis, and treatment after hospital arrival were collected by participating physicians. The physician in charge of the patients filled out the data form in cooperation with the EMS personnel. Data were integrated into the registry system on the JCS Shock registry’s database server, and the data were logically checked by the computer system. If the data form was incomplete, the JCS Shock registry’s management group returned it to the respective hospital for data completion.
DefinitionsThe primary endpoint was all-cause mortality at 30 days after hospital arrival. The secondary endpoint was neurological status at hospital discharge, which was assessed using the Cerebral Performance Categories (CPC) scale.7,8 The CPC scale corresponds to the following categories: (1) good cerebral performance (full function of activities of daily living), (2) moderate cerebral disability (disabled but independent), (3) severe cerebral disability (conscious but disabled and dependent), (4) coma/vegetative state (unconscious), and (5) brain death or death. CPC 1–2 was considered a good neurological outcome and CPC 3–5 was considered a poor neurological outcome. All-cause mortality was defined as death from any cause. Local participating clinicians determined the causes of death retrospectively. ACS was defined as ST-segment elevation MI, non-ST-segment elevation MI, and unstable angina. Non-ischemic arrhythmia was defined as arrhythmia, including sick sinus syndrome; first-, second-, and third-degree atrioventricular block; torsades de pointes; ventricular fibrillation; ventricular tachycardia; atrial fibrillation; atrial flutter; atrial tachycardia; and paroxysmal supraventricular tachycardia, except for arrhythmias caused by ACS and/or ischemic heart disease. Aortic disease was defined as a rupture or impending rupture of an aortic aneurysm and acute aortic dissection. Myocarditis was caused by infection (viral and bacterial), drug, autoimmune disease, idiopathic, or sarcoidosis.9 Cardiomyopathy was defined as ischemic cardiomyopathy, dilated cardiomyopathy, hypertrophic (obstructive) cardiomyopathy, takotsubo cardiomyopathy, and cardiac amyloidosis.10 VHD was defined as aortic stenosis, aortic regurgitation, mitral stenosis, and mitral regurgitation. IE was diagnosed according to the Duke criteria.11 Cardiac tamponade was caused by infection (viral, bacterial, mycobacterial, and fungal); neoplastic, uremic, idiopathic, or iatrogenic pericarditis; or connective tissue disease. Consciousness disturbance was defined as Japan Coma Scale12 ≥2 on hospital arrival. Congestive heart failure was diagnosed on hospital arrival according to the Framingham criteria.13 A history of hypertension, dyslipidemia, and diabetes mellitus was defined as follows: hypertension was defined as SBP ≥140 mmHg, diastolic blood pressure ≥90 mmHg, or ongoing therapy for hypertension; dyslipidemia was defined as a serum total cholesterol concentration ≥220 mg/dl, a low-density lipoprotein-cholesterol concentration ≥140 mg/dl, or current treatment with a lipid-lowering therapy; diabetes mellitus was defined as HbA1c ≥6.5%, casual plasma glucose level ≥200 mg/dl, or treatment with oral hypoglycemic agents or an insulin injection.
Statistical AnalysisContinuous variables are summarized as medians and interquartile ranges (IQRs) or mean±standard deviation, and they were compared with the Mann-Whitney U test or Student’s t-test. Binary and categorical variables were calculated as frequencies (percentages), and they were compared with the chi-square test or Fisher’s exact test. Multivariate logistic regression analysis was performed for predictors of the study outcomes. Variables reported by previous studies as clinically important factors such as age, sex, SBP, heart rate, consciousness disturbance, congestive heart failure, OHCA, estimated glomerular filtration rate (eGFR), and causes of shock were entered into a multivariate model.14–18 If the variables were missing in >5% of patients, they were excluded from multivariate analysis. P-values were 2-tailed and considered under 0.05 as statistically significant in all analyses. Data were analyzed using SPSS, version 22.0 (SPSS Inc, Chicago, IL, USA).
Among 1,004 patients enrolled in the JCS Shock registry, 25 were excluded because they had OHCA without ROSC (n=23) or non-shock (n=2). Finally, 979 patients were analyzed. Of them, 336 (34.3%) died within 30 days after hospital arrival (Figure 1).

Flow chart illustrating patients’ movement through the registry. OHCA, out-of-hospital cardiac arrest; ROSC, return of spontaneous circulation.
Patients’ baseline clinical characteristics are shown in Table 1 and Table 2. The median (IQR) age was 72.0 (63.0, 81.0) years and men accounted for 65.9%. More than half of the patients developed symptoms in their home (n=646, 66.0%), and they were in the shock state within 1 h (n=602, 61.4%). Almost all of them were transferred by ambulance (n=837, 85.4%). Chest pain (n=341, 34.9%), syncope (n=240, 24.5%), and dyspnea (n=214, 21.8%) were the most common symptoms. Other symptoms included consciousness disturbance (n=61, 6.2%), general fatigue (n=39, 4.0%), vomiting (n=35, 3.6%), dizziness (n=11, 1.1%), and seizure (n=4, 0.4%). Patients with 30-day mortality had worse clinical characteristics, such as a lower SBP, heart rate, ejection fraction, and eGFR and a higher incidence of consciousness disturbance, congestive heart failure and OHCA, before hospital arrival. Also, they had a higher incidence of chest pain and palpitation and a lower incidence of dyslipidemia and smoking.
| Variable | Overall (n=979) |
30-day survival (n=643) |
30-day mortality (n=336) |
P value |
|---|---|---|---|---|
| Age (years) | ||||
| Median (IQR) | 72.0 (63.0, 81.0) | 71.0 (62.0, 80.0) | 72.0 (64.0, 82.0) | 0.151 |
| Mean±SD | 70.3±14.2 | 69.9±13.8 | 70.9±14.9 | 0.289 |
| Male sex, n (%) | 645 | 425 (65.9) | 220 (34.1) | 0.846 |
| Onset location, n (%) | ||||
| Home | 646 | 413 (63.9) | 233 (36.1) | 0.109 |
| Public space | 144 | 100 (69.4) | 44 (30.6) | 0.303 |
| Street | 76 | 54 (71.1) | 22 (28.9) | 0.304 |
| Other | 113 | 76 (67.3) | 37 (32.7) | 0.707 |
| Transfer method, n (%) | ||||
| Ambulance | 837 | 541 (64.6) | 296 (35.4) | 0.095 |
| Walk-in | 69 | 54 (78.3) | 15 (21.7) | 0.022 |
| Doctor-car | 13 | 9 (69.2) | 4 (30.8) | 0.786 |
| Doctor-heli | 40 | 25 (62.5) | 15 (37.5) | 0.665 |
| Taxi | 4 | 4 (100.0) | 0 (0) | 0.147 |
| Other | 16 | 10 (62.5) | 6 (37.5) | 0.787 |
| Onset to shock time, n (%) | ||||
| <1 h | 602 | 398 (66.1) | 204 (33.9) | 0.718 |
| 1–3 h | 133 | 85 (63.9) | 48 (36.1) | 0.644 |
| 3–6 h | 74 | 50 (67.6) | 24 (32.4) | 0.722 |
| 6–12 h | 46 | 30 (65.2) | 16 (34.8) | 0.946 |
| 12–24 h | 38 | 20 (52.6) | 18 (47.4) | 0.084 |
| 24–48 h | 22 | 14 (63.6) | 8 (36.4) | 0.838 |
| 48–72 h | 12 | 9 (75.0) | 3 (25.0) | 0.494 |
| >72 h | 16 | 10 (62.5) | 6 (37.5) | 0.787 |
| Unknown | 36 | 27 (75.0) | 9 (25.0) | 0.230 |
| Onset to hospital time (min), median (IQR)* |
72.0 (40.0, 284.0) | 81.0 (40.0, 280.0) | 62.0 (38.0, 297.0) | 0.216 |
*Data available for 820 patients. IQR, interquartile range; SD, standard deviation.
| Variable | Overall (n=979) |
30-day survival (n=643) |
30-day mortality (n=336) |
P value |
|---|---|---|---|---|
| Systolic blood pressure (mmHg)* | ||||
| Median (IQR) | 80.0 (70.0, 90.0) | 81.0 (70.0, 90.0) | 76.0 (62.0, 87.0) | <0.001 |
| Mean±SD | 78.2±15.7 | 79.6±14.9 | 74.6±17.2 | <0.001 |
| Heart rate (beats/min)* | ||||
| Median (IQRs) | 75.0 (40.0, 105.0) | 77.0 (43.0, 106.0) | 72.0 (0, 102.0) | <0.001 |
| Mean±SD | 73.0±48.7 | 78.6±47.4 | 62.2±49.6 | <0.001 |
| Respiratory rate (/min), median (IQR)* |
20.0 (12.0, 25.0) | 20.0 (16.0, 25.0) | 16.0 (0, 26.0) | <0.001 |
| SpO2 (%), median (IQR)* | 98.0 (93.0, 100.0) | 98.0 (94.0, 100.0) | 97.0 (89.0, 99.0) | 0.001 |
| Body temperature (℃), median (IQR)* |
35.8 (35.0, 36.4) | 35.9 (35.2, 36.4) | 35.6 (34.6, 36.2) | <0.001 |
| Consciousness disturbance, n (%)* | 526 | 271 (51.5) | 255 (48.5) | <0.001 |
| Congestive heart failure, n (%)* | 637 | 385 (60.4) | 252 (39.6) | <0.001 |
| OHCA before hospital arrival, n (%) | 275 | 123 (44.7) | 152 (55.3) | <0.001 |
| LVEF (%), median (IQR)* | 45.0 (30.0, 60.0) | 49.0 (35.0, 60.0) | 30.0 (20.0, 50.0) | <0.001 |
| eGFR (ml/min/1.73 m2)* | ||||
| Median (IQR) | 43.8 (30.4, 56.8) | 46.4 (33.2, 59.8) | 38.7 (27.1, 51.7) | <0.001 |
| Mean±SD | 45.1±22.3 | 47.6±23.1 | 40.2±19.7 | <0.001 |
| Chief complaint, n (%) | ||||
| Chest pain | 341 | 241 (70.7) | 100 (29.3) | 0.016 |
| Dyspnea | 214 | 139 (65.0) | 75 (35.0) | 0.800 |
| Syncope | 240 | 159 (66.3) | 81 (33.8) | 0.830 |
| Abdominal pain | 39 | 21 (53.8) | 18 (46.2) | 0.112 |
| Back pain | 48 | 33 (68.8) | 15 (31.3) | 0.646 |
| Palpitation | 25 | 24 (96.0) | 1 (4.0) | 0.001 |
| Other | 263 | 162 (61.6) | 101 (38.4) | 0.100 |
| History, n (%) | ||||
| Hypertension | 545 | 363 (66.6) | 182 (33.4) | 0.494 |
| Dyslipidemia | 274 | 200 (73.0) | 74 (27.0) | 0.003 |
| Diabetes mellitus | 252 | 157 (62.3) | 95 (37.7) | 0.190 |
| Smoker | 278 | 197 (70.9) | 81 (29.1) | 0.031 |
*Data available for 959 patients with systolic blood pressure, 956 with heart rate, 777 with respiratory rate, 772 with SpO2, 826 with body temperature, 973 with consciousness disturbance, 975 with congestive heart failure, 554 with LVEF and 952 with eGFR. eGFR, estimated glomerular filtration rate; LVEF, left ventricular ejection fraction; OHCA, out-of-hospital cardiac arrest. Other abbreviations as in Table 1.
The causes of cardiovascular shock and the 30-day mortality are shown in Table 3. ACS (n=499, 51.0%), non-ischemic arrhythmia (n=161, 16.4%) and aortic disease (n=146, 14.9%) were the causative disorders in nearly 80% of cases of cardiovascular shock. Other causes of shock included pulmonary hypertension and myxoma, among others. The 30-day mortality ranged from 20.9% and 57.1%. Patients with non-ischemic arrhythmia had a better outcome, whereas those with aortic disease and myocarditis had worse outcome. Neurological outcome at discharge was available for 975 patients. Overall, 434 patients (44.5%) had a good neurological outcome and 541 (55.5%) had a poor neurological outcome, including CPC3 (n=136, 13.9%), CPC4 (n=31, 3.2%), and CPC5 (n=374, 38.4%).
| Variable | Overall (n=979) |
30-day Survival (n=643) |
30-day mortality (n=336) |
P value |
|---|---|---|---|---|
| ACS, n (%) | 499 | 332 (66.5) | 167 (33.5) | 0.566 |
| Non-ischemic arrhythmia, n (%) | 161 | 120 (74.5) | 41 (25.5) | 0.010 |
| Aortic disease, n (%) | 146 | 78 (53.4) | 68 (46.6) | 0.001 |
| Myocarditis, n (%) | 22 | 10 (45.5) | 12 (54.5) | 0.043 |
| Cardiomyopathy, n (%) | 38 | 28 (73.7) | 10 (26.3) | 0.289 |
| PE, n (%) | 43 | 34 (79.1) | 9 (20.9) | 0.059 |
| VHD, n (%) | 14 | 6 (42.9) | 8 (57.1) | 0.070 |
| IE, n (%) | 5 | 3 (60.0) | 2 (40.0) | 0.789 |
| Cardiac tamponade, n (%) | 13 | 10 (76.9) | 3 (23.1) | 0.390 |
| Other, n (%) | 38 | 22 (57.9) | 16 (42.1) | 0.303 |
ACS, acute coronary syndrome; IE, infective endocarditis; PE, pulmonary thromboembolism; VHD, valvular heart disease.
The multivariate logistic regression analysis of independent predictors for the 30-day mortality is shown in Figure 2. The adjusted odds ratio for 30-day mortality was 1.15 (95% confidence interval [CI], 1.08–1.22; P<0.001) after SBP (per 10-mmHg decrease), 2.62 (95% CI, 1.80–3.82; P<0.001) after consciousness disturbance, 2.58 (95% CI, 1.67–3.99; P<0.001) after congestive heart failure, 1.62 (95% CI, 1.05–2.51; P=0.031) after OHCA before hospital arrival, and 1.20 (95% CI, 1.10–1.30; P<0.001) after eGFR (per 10-ml/min/1.73 m2 decrease). Regarding causes of shock (reference: ACS), the adjusted odds ratio for 30-day mortality was 0.48 [95% CI, 0.30–0.77; P=0.002] in non-ischemic arrhythmia, 3.98 [95% CI, 2.32–6.81; P<0.001] in aortic disease, and 3.25 [95% CI, 1.20–8.84; P=0.021]) in myocarditis. Multivariate logistic regression analysis of independent predictors of neurological outcome at hospital discharge is shown in Figure 3. The results for neurological outcome were similar to the results for 30-day mortality except with regard to age (per 10-year increase).

Multivariate logistic regression analysis of 30-day mortality. Of the study patients, 95.5% (935/979) were entered into the multivariate model. ACS, acute coronary syndrome; CI, confidence interval; eGFR, estimated glomerular filtration rate; IE, infective endocarditis; OHCA, out-of-hospital cardiac arrest; OR, odds ratio; PE, pulmonary thromboembolism; VHD, valvular heart disease.
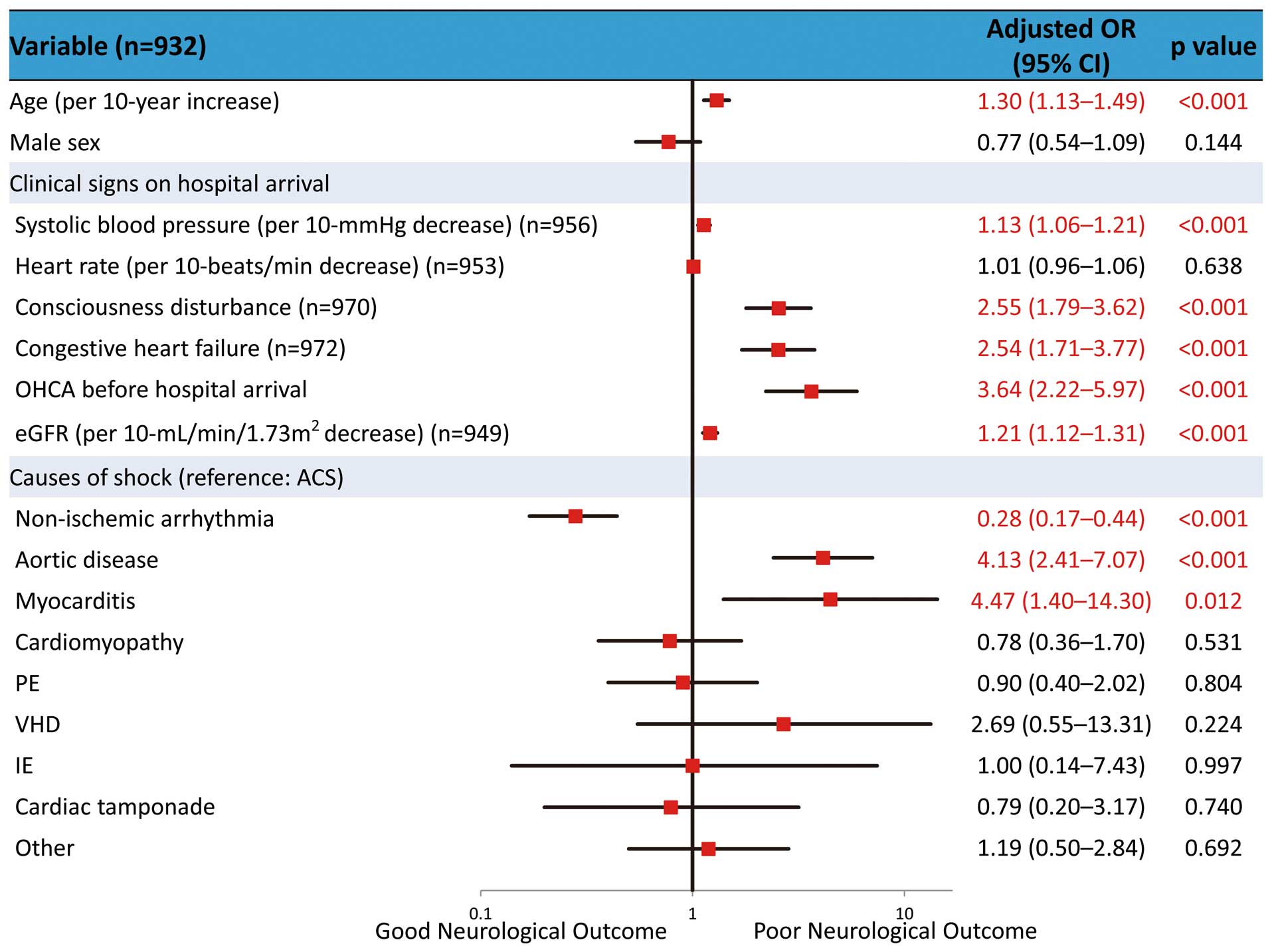
Multivariate logistic regression analysis of neurological outcome at hospital discharge. Of the study patients, 95.2% (932/979) were entered into the multivariate model ACS, acute coronary syndrome; CI, confidence interval; eGFR, estimated glomerular filtration rate; IE, infective endocarditis; OHCA, out-of-hospital cardiac arrest; OR, odds ratio; PE, pulmonary thromboembolism; VHD, valvular heart disease.
This study, the largest nationwide registry of patients with cardiovascular shock caused by ACS and other various cardiovascular diseases, clarified the actual state of cardiovascular shock in Japan. Moreover, this study determined that patients with aortic disease and myocarditis had worse outcomes, and those with non-ischemic arrhythmia had better outcomes than those with ACS, which was the most common cause of cardiovascular shock in this study cohort. As the population of this study reflects the real-world setting, the findings from this study are useful in daily clinical practice, especially when the cause of cardiovascular shock is difficult to determine immediately.
Almost all previous studies of cardiogenic shock have targeted patients with acute MI, or they did not clarify the baseline causes.6 The present study included all consecutive out-of-hospital patients with cardiovascular shock. As a result, we were able to characterize all the causes of shock, including ACS and other cardiovascular diseases. The prevalent causes of shock in our study were ACS, non-ischemic arrhythmia, and aortic disease.
The 30-day mortality of patients with ACS was 33.5% in this study. Between the mid-1970 s and late 1980 s, approximately 70% of patients with cardiogenic shock died during hospitalization, even in the era of the coronary care unit.19 In contrast, in the early 2000 s, mortality decreased to approximately 40% in association with early reperfusion therapy.20 In the early 2010 s, approximately 30–40% of patients died, and these data are consistent with the present study results.21–23 Despite the recent advances in coronary intervention techniques and devices, the mortality rate of patients with ACS with cardiogenic shock may plateau over time. Future research and new treatment techniques are needed to further improve the mortality rate.
Although cardiogenic shock was previously reported to be associated with a low incidence (3–6%) of supraventricular and ventricular arrhythmia,24 there are little data about the proportion and mortality rate of arrhythmia as a cause of cardiogenic shock. In the present study, the proportion of non-ischemic arrhythmia (supraventricular arrhythmia; n=28, ventricular arrhythmia; n=90) was next to that of ACS, and patients with non-ischemic arrhythmia had better outcomes than those with ACS, as shown in the multivariate logistic regression analysis. The favorable outcome of these patients may have been associated, at least partly, with immediate use of antiarrhythmic drugs, defibrillation, and temporary pacing in the emergency room.
The present study showed that shock caused by aortic disease had a significantly higher mortality rate than ACS. Previous studies have reported high mortality rates in patients in the shock state with aortic disease; acute type A aortic dissection has a mortality rate of 40–50%,17,25,26 type B aortic dissection is 43%,27 and ruptured aortic aneurysm is 53%.28 Furthermore, preoperative shock is reportedly an independent prognostic factor.29,30 Goda et al reported that the mortality rate was reduced by shortening the circulatory collapse time.31 In the present study, the median onset to hospital time (111 min) was relatively longer in patients with aortic disease than in the overall patient group. One of the measures to improve the prognosis of aortic disease may be to shorten the onset to treatment time.
Although myocarditis and VHD were relatively minor in our cohort, these diseases are important because they were associated with a worse prognosis. Fulminant myocarditis, which is characterized by extensive inflammatory infiltration and numerous necrotic myocytes, is a rapidly progressive life-threatening disease. The mortality rate in our cohort was slightly higher (54.5%) than in previous reports (≈40%).18,32 Moreover, approximately half of the patients with myocarditis required extracorporeal membrane oxygenation (n=10). VHD included various valve diseases such as mitral regurgitation (n=4), mitral stenosis (n=1), aortic stenosis (n=7), and aortic regurgitation (n=1). The 30-day mortality rate of aortic stenosis was 57.1% and that of mitral regurgitation was 75%. Most of the patients did not undergo cardiac surgery within 24 h, which may have led to the high mortality rate.
Sleeper et al reported that age, SBP, renal dysfunction, and end-organ hypoperfusion were independent predictors of in-hospital mortality in the SHOCK trial and registry data,14 as reported by other previous studies that included patients with cardiogenic shock caused by ACS.21,33–35 However, age was not an independent predictor of the 30-day mortality in the present study, which was not consistent with previous studies.36,37 This discordance may be related to some bias such as the small number of elderly patients, and elderly patients are less likely to receive treatment. Furthermore, this may also be associated with the heterogeneity of etiology among the enrolled patients in the present study, which included various cardiovascular diseases. Of note, consciousness disturbance on hospital arrival was a strong predictor of 30-day mortality. A Glasgow coma scale of 3 was reported as a predictor of in-hospital mortality in patients with OHCA caused by ACS;38 however, there are little data about the effect of consciousness disturbance on hospital arrival on the outcome of patients with cardiovascular shock. Although congestive heart failure was reported as a variable associated with poor mortality in patients with ACS,15,39 our results underscore heart failure as an important determinant of prognosis in patients with shock with a wide range of cardiovascular diseases in general. As previously documented, OHCA is associated with poor outcome in patients with ACS,16,40 and this is consistent with the result found in the present study. However, Ostenfeld et al reported that OHCA was not an independent predictor of death in patients with cardiogenic shock caused by ACS.41 The effect of OHCA on patients’ outcome has to be investigated in a further analysis. In the present study, eGFR was a strong predictor of 30-day mortality. A history of renal disease, renal function on admission, and acute renal dysfunction have also been reported as independent predictors of 30-day mortality in previous studies.21,35,42,43 Renal dysfunction on hospital arrival may reflect baseline renal function and systemic hypoperfusion caused by the shock state.
The predictors of poor neurological outcome were similar to those of 30-day mortality in the present study. However, age was an independent predictor of poor neurological outcome (but not mortality) at discharge, possibly because elderly patients were likely to decrease their activities of daily living following potentially fatal conditions such as cardiovascular shock, as previously noted.44
Study LimitationsFirst, in all clinical studies, data integrity, validity, and ascertainment bias are potential limitations. However, uniform data collection, as in the JCS Shock registry, minimizes these potential sources of bias. Second, some variables, including the onset to hospital time, vital signs, and left ventricular ejection fraction, could be determinant variables of short-term mortality in patients with cardiovascular shock; however, such variables were not necessarily available for all patients, because it is often difficult to obtain these data for critically ill patients in the real-world setting. Moreover, we could not incorporate these variables in multiple logistic regression analysis because of the lack of data; thus, whether these clinically important variables are associated with 30-day mortality remains to be seen. Third, details of the treatment were left to the physician’s discretion at each hospital, although treatment such as the administration of fluids, adrenergic agents, mechanical support device, and coronary intervention was based on the guidelines of the Japanese Circulation Society. Fourth, the mortality rate of patients with cardiovascular shock may be different in each country, region, and hospital, because the causes of shock, which may differ in each country, region, and hospital, were significantly associated with 30-day mortality. Finally, only overall data were analyzed in the present study. Further subanalyses regarding the characteristics and predictors of each cause and treatment are needed.
The 30-day mortality rate for cardiovascular shock was still high at 34%. The primary predictors of mortality were cardiorenal function on hospital arrival and the etiology of shock.
We thank Makoto Kobayashi for the administration work done for the subcommittee of the Japanese Circulation Society Cardiovascular Shock registry and the staff of the following hospitals for data collection (in alphabetical order): Asahikawa Medical University Hospital, Chikamori Hospital, Dokkyo Medical University, Ebara Hospital, Ehime University Hospital, Fuchu Hospital, Fukuoka University Hospital, Fukushima Medical University Aizu Medical Center, Harasanshin Hospital, Hirosaki University School of Medicine and Hospital, Hiroshima Prefectural Hospital, Hokkaido Cardiovascular Hospital, Hyogo Prefectural Amagasaki General Medical Center, International Goodwill Hospital, Itami City Hospital, IUHW Atami Hospital, JA Hiroshima General Hospital, Japanese Red Cross Kyoto Daini Hospital, Japanese Red Cross Nagoya Daiichi Hospital, Japanese Red Cross Okayama Hospital, JCHO Kyushu Hospital, JCHO Yokohama Chuo Hospital, Jichi Medical University Hospital, Joetsu General Hospital, Juntendo University Shizuoka Hospital, Kashiwa Municipal Hospital, Kawaguchi Municipal Medical Center, Kawasaki Hospital, Kita-Harima Medical Center, Kitano Hospital, Kouseikai Takai Hospital, Kumamoto University Hospital, Kyorin University Hospital, Kyushu University Hospital, Matsue City Hospital, Matsue Red Cross Hospital, Matsumoto Kyoritsu Hospital, Mito Medical Center, Musashino Red Cross Hospital, Nagasaki University Hospital, Nagoya University Graduate School of Medicine, National Hospital Organization Kanazawa Medical Center, National Hospital Organization Kyoto Medical Center, Nihon University Hospital, Nippon Medical School Chiba Hokusoh Hospital, Nishitokyo Central General Hospital, NTT Medical Center Tokyo, Osaka Police Hospital, Osaka Saiseikai Senri Hospital, Osaka University Hospital, Otemae Hospital, Saiseikai Futsukaichi Hospital, Saiseikai Hita Hospital, Saiseikai Kawaguchi General Hospital, Saiseikai Kumamoto Hospital, Saiseikai Niigata Daini Hospital, Saiseikai Yokohamashi Nanbu Hospital, Saitama Medical University International Medical Center, Sakaide City Hospital, Sakakibara Heart Institute, Sasebo City General Hospital, Shiga University of Medical Science Hospital, Shinshu University Hospital, Steel Memorial Muroran Hospital, Sumitomo Hospital, Tohoku Rosai Hospital, Tokai University Hachioji Hospital, Tokushima University Hospital, Tokyo Dental College Ichikawa General Hospital, Tokyo Medical and Dental University Hospital of Medicine, Tokyo Medical University Hospital, Tokyo Metropolitan Tama Medical Center, Tokyo Metropolitan Hiroo Hospital, Toyonaka Municipal Hospital, Tsukazaki Hospital, Yamaguchi Grand Medical Center, Yamaguchi Rosai Hospital, Yokohama City University Hospital, Yokohama Municipal Citizen’s Hospital, Yokohama Rosai Hospital, and Yokohama City University Medical Center.
None.