Abstract
Background:
The pharmacological advantage of combining physiotherapy with anticoagulants for the prevention of venous thromboembolism (VTE) after total knee arthroplasty (TKA) is not fully known. Herein we investigated the potential benefit of this combination therapy in patients undergoing TKA.
Methods and Results:
The 38 patients were randomly assigned to a physiotherapy group (n=19) or a physiotherapy plus 30 mg/day edoxaban group (n=19). The occurrence of VTE was evaluated, as were serial changes in parameters measured by the Total Thrombus-formation Analysis System, a novel system for quantitatively analyzing thrombus formation using microchips with thrombogenic surfaces (collagen plus tissue factor, atheroma [AR]-chip). Combination therapy significantly reduced the incidence of VTE after TKA compared with monotherapy (P=0.038). The area under the curve (AUC) of thrombus formation for the AR-chip (AR10-AUC30) was significantly lower in the combination group (P=0.001) on Day 7 after TKA than before TKA, but no significant change was observed with monotherapy (P=0.809). In 13 VTE-positive patients, AR10-AUC30
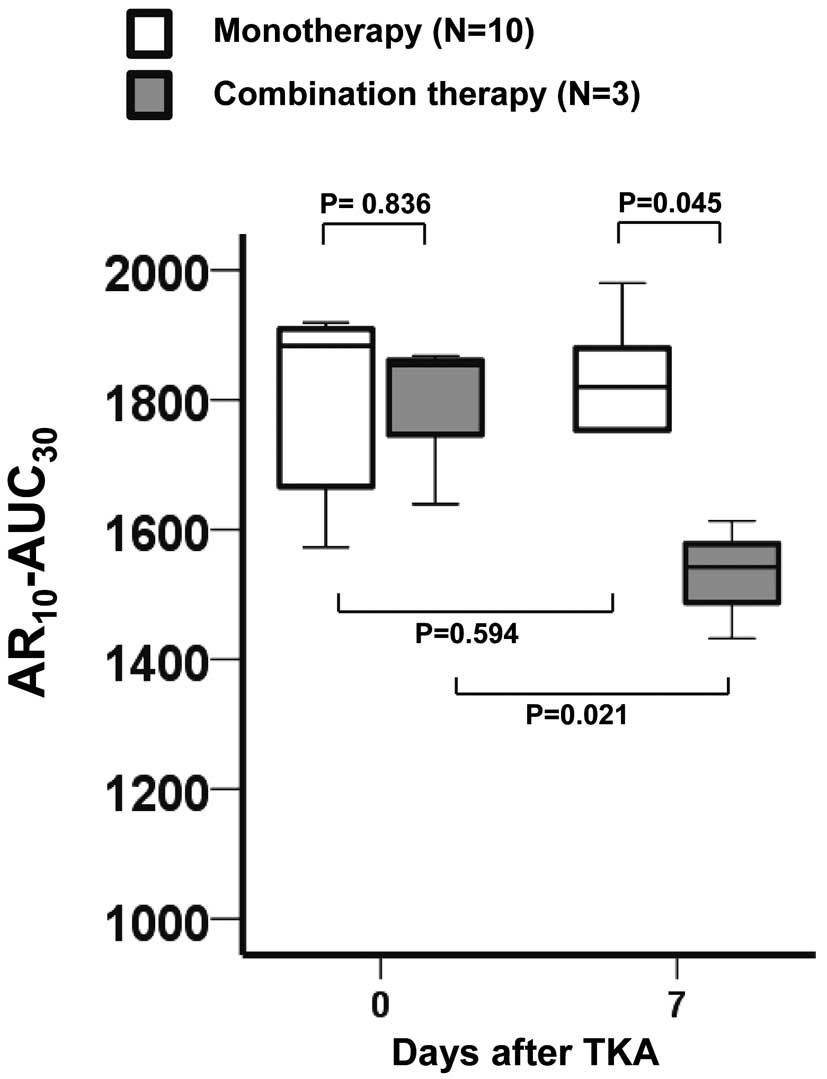
was significantly lower in the combination group (n=3) than in the monotherapy group (n=10) on Day 7 (P=0.045).
Conclusions:
The combination of physiotherapy and edoxaban significantly reduced the incidence of VTE after TKA compared with physiotherapy alone. However, it is possible that VTE occurrence after TKA is not only associated with thrombogenicity, but also rheological factors.
The disease state characterized by the sequential development of deep vein thrombosis (DVT) and pulmonary thromboembolism (PTE) is referred to as venous thromboembolism (VTE). In orthopedic surgery, VTE often occurs during the perioperative period after total hip arthroplasty (THA), total knee arthroplasty (TKA), and hip fracture surgery (HFS), and many cases are fatal once PTE occurs. Therefore, VTE is one of the biggest concerns in the field of orthopedic surgery during the perioperative period.
The Total Thrombus-formation Analysis System (T-TAS; Fujimori Kogyo), a microchip-based flow chamber system capable of evaluating whole-blood thrombogenicity, was developed as an easy-to-use system for the quantitative analysis of thrombus formation. We have established the validity of the T-TAS in patients receiving a vitamin K antagonist (VKA), direct oral anti-coagulants (DOACs),1,2
and antiplatelet agents.3,4
Recently, we reported that visual analysis of the AR-chip in the T-TAS could identify differential inhibitory patterns of VKA and DOACs of thrombus formation under flow conditions.5
Furthermore, we have previously demonstrated the usefulness of the T-TAS in distinguishing the pharmacological effects of edoxaban (DU-176b; a DOAC sold under the trade names Savaysa and Lixiana [Daiichi Sankyo, Tokyo, Japan]) in patients undergoing TKA.6
However, we did not directly compare these effects with results from a control group (i.e., a group that was not treated with edoxaban), so the possibility that the surgery affected the measured outcomes could not be ruled out.6
Importantly, previous studies demonstrated the effectiveness of antithrombotic therapy using edoxaban to prevent the occurrence of VTE in patients after TKA,7,8
but these findings were based on comparisons with anticoagulants. The effectiveness of the combination of physiotherapy and edoxaban treatment compared with physiotherapy alone has not yet been investigated. Thus, the aim of the present study was to examine the efficacy of combining edoxaban with physiotherapy in reducing the incidence of VTE in the lower extremities following surgery, as evaluated using ultrasonography and contrast-enhanced computed tomography (CT). Moreover, we sought to evaluate the association between thrombosis formation in the lower extremities following surgery and T-TAS parameters; that is, we investigated whether T-TAS could be a predictor of VTE after TKA. To test the hypothesis that combination therapy (i.e., physiotherapy plus edoxaban) would reduce the incidence of VTE in the lower extremities after surgery, we designed the Efficacy Study of the COmbination of Edoxaban and Physiotherapy on the PRevention of Venous-Thromboembolism in patients after Total Knee Arthroplasty (ESCORT-TKA) trial to evaluate the antithrombotic effects and bleeding complications associated with the addition of edoxaban to physiotherapy. The results for patients under going combination therapy were compared with those undergoing physiotherapy monotherapy, and the incidence of VTE in patients undergoing TKA was determined.
Methods
The ESCORT-TKA was a prospective single-center open-label randomized controlled clinical trial that explored the efficacy of edoxaban in reducing the incidence of VTE after TKA. The protocol of the ESCORT-TKA trial has been described in detail previously.9
Ethics Statement
All procedures were conducted in accordance with the Declaration of Helsinki and its amendments. The study protocol was approved by the Institutional Review Board of Kumamoto University, and written informed consent was obtained from each patient or the family of the patient.
Study Population
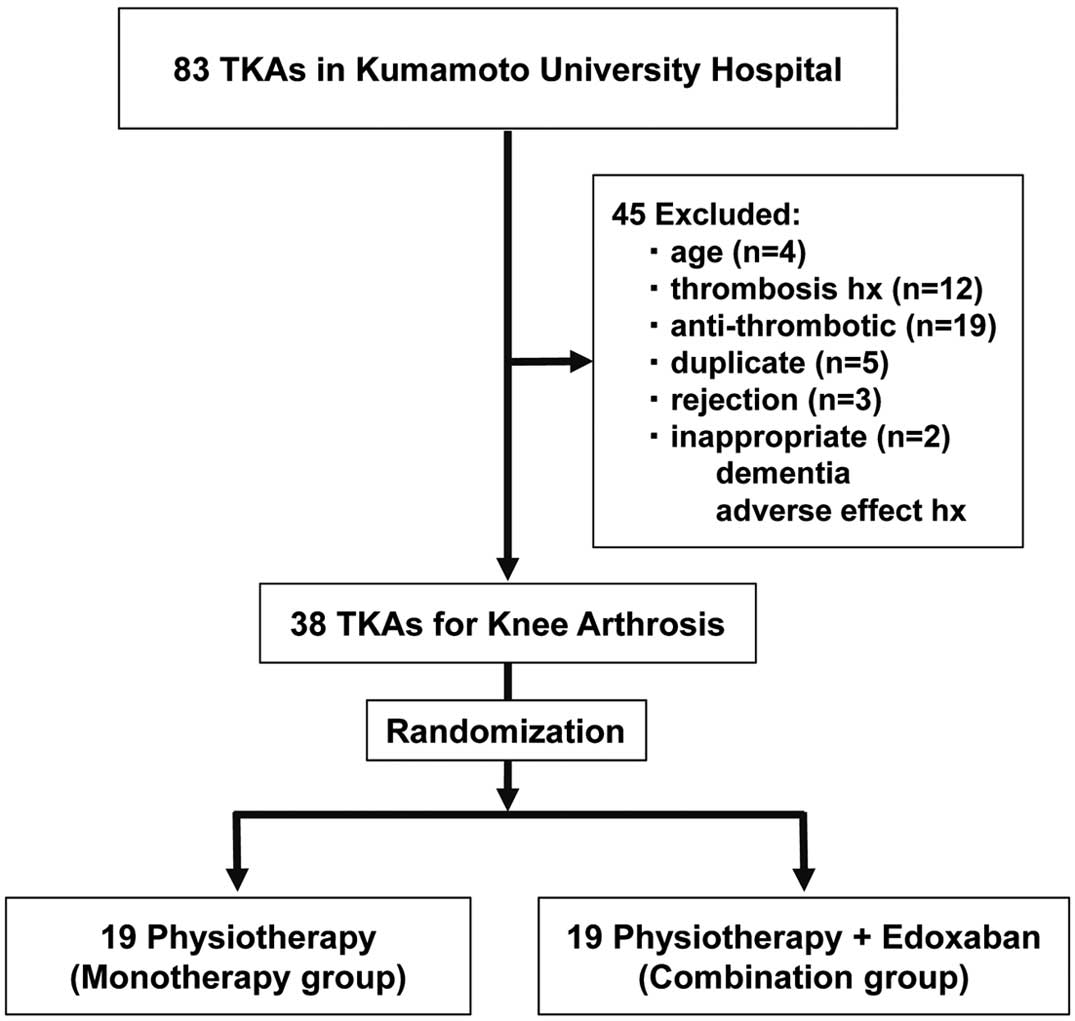
In all, 83 consecutive patients undergoing TKA at Kumamoto University Hospital between April 2015 and December 2016 were enrolled in the study. Of these, 45 patients were excluded because of old age (n=4), a history of thrombosis (n=12), taking antithrombotic agents (n=19), declining consent to the study (n=3), duplicate cases (n=5), and other reasons (n=2; e.g., dementia, a history of adverse effects with DOAC). The remaining 38 patients were randomly divided into 2 groups (Figure 1): (1) patients who received physiotherapy alone (monotherapy group; n=19); and (2) patients treated with physiotherapy and edoxaban (combination group; n=19). Although we had planned to study 80 patients based on a sample size calculation from a previous study,9
we discontinued this study after 38 patients have been enrolled because the differences in the primary endpoint had reached statistical significance.
TKA for Patients With Knee Arthrosis
All TKAs were performed by the same surgeon who used a measured resection technique, as described previously.10
Briefly, the patella was not resurfaced. Using a mobile-bearing TKA (NexGen LPS Flex Mobile; Zimmer, Warsaw, IN, USA), a device was used intraoperatively to place 4 0.8-mm tantalum beads at predefined sites in a polyethylene insert. The Hospital for Special Surgery (HSS) score was used for preoperative and postoperative clinical evaluations. Radiographic evaluations were performed using standing anteroposterior radiographs for the tibiofemoral angle and the Knee Society rating system for component alignment.11
The surgeon was blinded to the allocation results.
Physiotherapy
Physiotherapy consisted of 3 components: (1) walking and active exercise;12–14
(2) elastic stockings (ES);15–17
and (3) intermittent pneumatic compression (IPC).18–24
The preventive effects of these therapies for high-risk patients have been comprehensively reviewed elsewhere.25
Walking training was initiated on Day 3 after TKA; ES were applied at the time of hospitalization (i.e., before TKA surgery), and IPC was performed as appropriate (e.g., when the start of walking training was delayed).
Periprocedural Anticoagulation Regimen and Blood Sample Collection
Oral administration of 30 mg edoxaban, once daily, was initiated on Day 2 after TKA to prevent VTE, according to the STARS E-3 Trial.8
Blood samples were obtained the day before TKA and on Day 7 after TKA (trough point). Therefore, edoxaban was prescribed for 5 days unless the presence of VTE was recognized during the evaluation on Day 7 after TKA. This anticoagulation regimen is shown in
Figure 2.
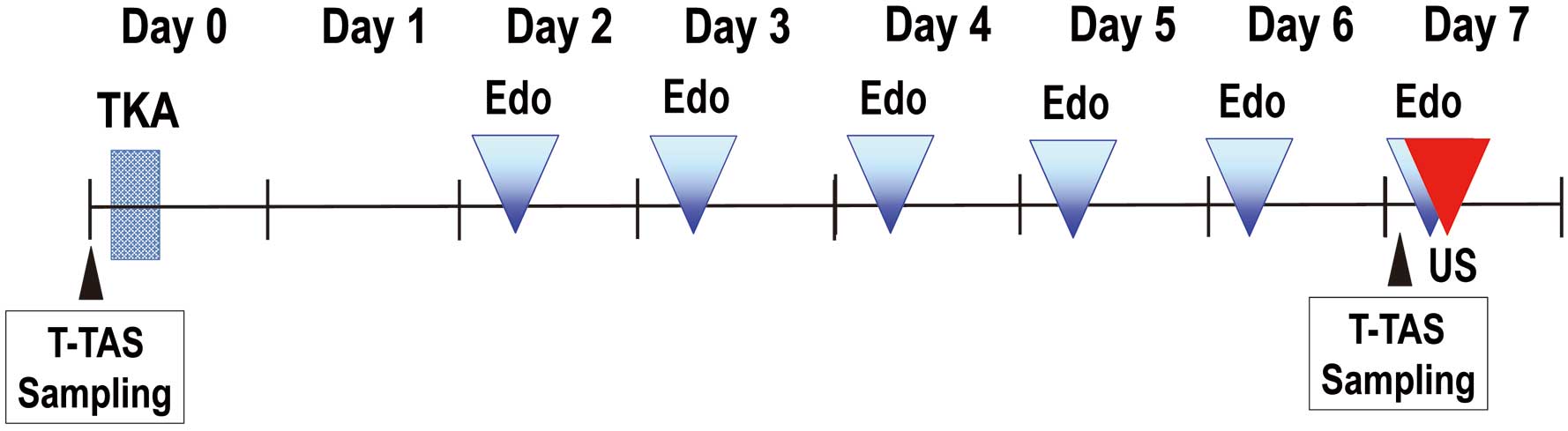
Blood samples were collected as described previously.26
Briefly, blood samples were collected from the antecubital vein using a 21-gauge butterfly needle into a Hirudin Blood Tube (MP0600; Verum Diagnostia, Munich, Germany; final hirudin concentration 25 μg/mL), blood collection tubes (VP-CA050K70; Venoject II, Terumo, Japan), and a syringe containing 0.11 mL of 3.8% sodium citrate solution. Each sample was immediately centrifuged at 1,800 g for 10 min at room temperature, and plasma was collected for biochemical analysis. The prothrombin time-international normalized ratio (PT-INR) and activated partial thromboplastin time (APTT) were measured using commercially available thromboplastin reagents (Coagupia PT-N and Coagupia APTT-N, respectively; Sekisui Medical, Tokyo, Japan) according to the manufacturer’s instructions. Both PT-INR and APTT are well-known indicators of anticoagulation effects, and these parameters are known to vary with DOAC administration. Therefore, these parameters were examined in order to confirm the effect of edoxaban.
Measurement of Thrombogenicity by T-TAS
The T-TAS is an automated microchip-based flow chamber system developed for the easy and quick assessment of platelet thrombus formation under flow conditions.26–28
Details of the T-TAS, including its appearance and components, have been reported previously and the validity of the method under DOAC use has been established.2,6
Briefly, this system analyzes different thrombus formation processes with a simple procedure using 2 microchips with different thrombogenic surfaces. One chip, the platelet (PL)-chip, is coated with Type I collagen. Inside the microchip, platelets adhere and aggregate onto the surface of the collagen and the microchip capillaries are occluded. The other chip, the atheroma (AR)-chip, is coated with Type I collagen plus tissue thromboplastin. Inside the microchip, platelets and the coagulation system are activated simultaneously by collagen and tissue thromboplastin, respectively. The process of thrombus formation inside the 2 chips was analyzed by monitoring changes in flow pressure. The area under the flow-pressure curve (AUC) was computed to assess platelet thrombogenicity inside the microchips, with the AUC for the first 10 min for the PL-chip tested at flow rate of 24 μL/min (PL24-AUC10) and the AUC for the first 30 min for the AR-chip tested at flow rate of 10 μL/min (AR10-AUC30) calculated.
Efficacy and Safety Endpoints
Primary Outcome Measures
The primary endpoint for the determination of treatment efficacy in the present study was the prevalence of VTE, as shown by follow-up ultrasonography on Day 7 after TKA. If a symptomatic DVT or PTE was suspected based on signs or symptoms that occurred between the initiation of drug treatment and ultrasonography at the completion (discontinuation) of drug treatment, appropriate imaging was performed to confirm the event. A suspected VTE was evaluated by ultrasonography and contrast-enhanced CT. A suspected PTE was re-evaluated by contrast-enhanced CT. A definite diagnosis of symptomatic DVT or PTE was based on the findings of this clinical imaging. The other primary endpoint in the determination of treatment efficacy was changes in T-TAS parameters.
Secondary Outcome Measures
The secondary endpoints in the determination of treatment efficacy were changes in PT-INR and APTT levels from the day before surgery to Day 7 after TKA, and the safety assessment described above.
Evaluation of Thrombosis
Ultrasonography of the whole lower limbs was performed on Day 7 by different skilled examiners blinded to the treatment group. Suspected VTE was evaluated by ultrasonography and contrast-enhanced CT, whereas suspected PTE was evaluated by contrast-enhanced CT. A definite diagnosis of a symptomatic VTE or PTE was based on the findings of clinical imaging.
Statistical Analysis
Continuous variables are described as the mean±SD or as median values and the interquartile range for asymmetrically distributed data. Categorical variables are presented using frequency distributions. Univariate analyses were conducted using Chi-squared tests and t-tests to compare independent samples. All serial changes were analyzed using non-parametric tests because the data were not normally distributed, as identified by the Shapiro-Wilk test. P<0.05 was considered significant. All statistical analyses were performed using SPSS version 23 (IBM Corp., Armonk, NY, USA).
Results
Baseline Characteristics, T-TAS Parameters, PT, and APTT
The baseline characteristics of patients treated with monotherapy (n=19) and combination therapy (n=19) are listed in
Table 1. The baseline characteristics did not differ between the 2 groups, except for body mass index, which was low in the group undergoing combination therapy.
Table 1.
Comparison of Baseline Demographics and Clinical Parameters Between the Physiotherapy Plus Edoxaban (Combination) and Physiotherapy Monotherapy Groups
| |
Monotherapy |
Combination |
P value |
| No. subjects |
19 |
19 |
|
| Age (years) |
71.3±8.7 |
74.1±7.1 |
0.284 |
| Males |
3 (15.8) |
2 (10.5) |
0.642 |
| BMI (kg/m2) |
24.1±3.6 |
21.4±3.2 |
<0.001 |
| ABI |
1.1±0.1 |
1.1±0.1 |
0.507 |
| DM |
5 (26.3) |
3 (15.8) |
0.440 |
| Hyperlipidemia |
4 (21.1) |
3 (15.8) |
0.686 |
| Ccr (mL/min) |
86.3±37.6 |
72.4±20.9 |
0.166 |
| PT (INR) |
0.97 [0.93–1.00] |
1.00 [0.96–1.02] |
0.172 |
| APTT (s) |
31.0 [28.4–33.1] |
31.3 [27.9–34.7] |
0.931 |
| D-dimer (μg/mL) |
2.1±3.0 |
1.1±0.6 |
0.143 |
| AR10-AUC30 |
1,809 [1,692–1,909] |
1,782 [1,645–1,866] |
0.544 |
| PL24-AUC10 |
377.7 [242.0–421.2] |
342.2 [278.8–398.5] |
0.297 |
Data are given as the mean±SD, n (%) or as the median [interquartile range]. ABI, ankle-brachial index; APTT, activated partial thrombin time; AR10-AUC30, area under the curve (AUC) of thrombus formation for the AR-chip; BMI, body mass index; Ccr, creatinine clearance; DM, diabetes mellitus; INR, international normalized ratio; PL24-AUC10, AUC for the first 10 min for the PL-chip tested at flow rate of 24 μL/min; PT, prothrombin time.
The incidence of VTE on Day 7 was significantly lower in the combination than monotherapy group (15.8% vs. 52.6%, respectively; P=0.0382). Ultrasonography revealed that all VTEs were in the lower legs, and additional contrast-enhanced CT confirmed these findings. Detailed information concerning the location of venous thrombosis is summarized in
Table 2. Fatal pulmonary embolisms were not observed in either group. Characteristics of patients with (n=13) and without (n=25) VTE are given in
Table 3. A lower rate of edoxaban administration and higher D-dimer levels were observed on Day 7 after TKA in the VTE-positive compared with VTE-negative group.
Table 2.
Location of Venous Thrombosis in Patients in the Physiotherapy Plus Edoxaban (Combination) and Physiotherapy Monotherapy Groups
Treatment
group |
Age
(years) |
Sex |
Side |
Location |
| Monotherapy |
70 |
F |
Affected |
SV |
| Monotherapy |
72 |
F |
Affected |
SV+PeV |
| Monotherapy |
83 |
M |
Affected |
SV |
| Monotherapy |
69 |
F |
Affected |
SV+PTV |
| Monotherapy |
79 |
F |
Both |
SV |
| Monotherapy |
56 |
F |
Affected |
SV |
| Monotherapy |
72 |
F |
Affected |
SV |
| Monotherapy |
63 |
F |
Affected |
SV+PeV |
| Monotherapy |
78 |
F |
Affected |
SV |
| Monotherapy |
78 |
F |
Affected |
SV |
| Combination |
80 |
F |
Affected |
SV |
| Combination |
72 |
F |
Affected |
SV |
| Combination |
66 |
F |
Affected |
SV |
PeV, peroneal vein; PTV, posterior tibial vein; SV, soleal vein.
Table 3.
Patient Characteristics According to the Presence of VTE Before and After TKA
| |
VTE absent |
VTE present |
P value |
| No. subjects |
25 |
13 |
|
| Edoxaban |
16 (64.0) |
3 (23.1) |
0.016 |
| Age (years) |
72.9±8.2 |
72.1±7.6 |
0.782 |
| Male sex |
4 (16.0) |
1 (7.7) |
0.486 |
| BMI (kg/m2) |
26.3±4.0 |
24.7±2.9 |
0.227 |
| ABI |
1.1±0.07 |
1.1±0.12 |
0.642 |
| DM |
5 (20.0) |
3 (23.1) |
0.831 |
| Hyperlipidemia |
3 (12.0) |
4 (30.8) |
0.165 |
| Ccr (mL/min) |
80.4±33.2 |
77.3±26.9 |
0.782 |
| Before TKA |
| PT (INR) |
1.00 [0.96–1.02] |
0.97 [0.93–1.03] |
0.429 |
| APTT (s) |
30.1 [27.6–34.8] |
31.3 [28.5–33.1] |
0.738 |
| D-dimer (μg/mL) |
1.3±1.1 |
2.3±3.4 |
0.188 |
| AR10-AUC30 |
1,761 [1,702–1,856] |
1,857 [1,653–1,909] |
0.377 |
| PL24-AUC10 |
367.4 [261.2–399.0] |
370.8 [262.7–421.2] |
0.761 |
| Day 7 after TKA |
| PT (INR) |
1.12 [1.05–1.21] |
1.06 [0.98–1.15] |
0.125 |
| APTT (s) |
35.0 [31.5–40.3] |
33.9 [30.0–34.9] |
0.150 |
| D-dimer (μg/mL) |
9.2±2.3 |
11.5±2.8 |
0.013 |
| AR10-AUC30 |
1,681 [1,512–1,771] |
1,778 [1,525–1,868] |
0.159 |
| PL24-AUC10 |
367.4 [291.9–408.4] |
374.8 [316.2–428.5] |
0.429 |
Data are given as the mean±SD, n (%) or as the median [interquartile range]. TKA, total knee arthroplasty; VTE, venous thromboembolism. Other abbreviations as in Table 1.
Of the 38 subjects enrolled in the present, 1 experienced serious bleeding from surgical wounds that required a blood transfusion due to anemia caused by the bleeding.
Effects of Edoxaban on T-TAS Parameters, PT, and APTT
Figure 3
shows serial changes in T-TAS parameters (AR10-AUC30
and PL24-AUC10), PT-INR, and APTT in the 2 treatment groups. Although AR10-AUC30
had decreased significantly in the combination group by Day 7 compared with before TKA (P=0.001), AR10-AUC30
did not change significantly in the monotherapy group (P=0.809). Moreover, there was no significant change in PL24-AUC10
over time. Both PT-INR and APTT were significantly prolonged in the 2 treatment groups on Day 7 compared with before TKA, but PT-INR on Day 7 were significantly higher in the combination than monotherapy group.
Serial Changes in AR10-AUC30
in the Presence of VTE
Figure 4
shows serial changes in AR10-AUC30
in the presence and absence of VTE. Specifically, there were no significant changes in AR10-AUC30
from before to 7 days after TKA in either the VTE-positive or VTE-negative group.
Discussion
The main features and findings of the present study are that: (1) edoxaban combined with physiotherapy significantly enhances the efficacy of physiotherapy by reducing the incidence of VTE after TKA; and (2) although edoxaban significantly decreased AR10-AUC30
measured by T-TAS, some VTE-positive patients had a low AR10-AUC30
on Day 7 in the combination therapy group.
After artificial joint surgery, VTE occurs due to the complex interplay of Virchow’s triad (endothelial injury, stasis, and hypercoagulability). Thrombus formation after TKA has been associated with immobility and resting after surgery, blood stagnation due to swelling, bone resection, treatment of the meniscus, vascular endothelial cell disorders due to intraoperative vein towing (such as tibia anterior dislocation), and the enhancement of blood coagulation caused by surgical stress. Regarding the efficacy of edoxaban, Fuji et al7
reported that the incidence of VTE was 48.3% in the placebo group compared with 29.5%, 26.1%, 12.5%, and 9.1% in groups treated with 5, 15, 30, and 60 mg edoxaban daily, respectively.7
In the present study, the incidence of VTE in patients treated with both physiotherapy and 30 mg edoxaban daily after TKA was 15.8 %, compared with an incidence of 52.6% in patients treated with physiotherapy alone.
According to Western29
and Japanese30
guidelines for the diagnosis, treatment, and prevention of PTE and DVT, physiotherapy or anticoagulation therapy are the recommended treatments to prevent VTE in high-risk patients, including after TKA. The guidelines recommend unfractionated heparin (UFH), a VKA, low-molecular weight heparin (LMWH), fondaparinux, and enoxaparin as anticoagulants. UFH is an injected drug associated with several disadvantages, such as pain and necessary dose adjustments. Although the VKA is an oral drug, its use is also associated with disadvantages, such as a delay before the drug reaches therapeutic concentrations, individual differences in drug efficacy and a narrow safety margin, and the need for periodic monitoring. Moreover, the efficacy of VKA is affected by other drugs and the diet. Although the use of fondaparinux or enoxaparin in the clinical setting does not require blood coagulation monitoring, these drugs require daily subcutaneous administration. Therefore, these drugs place a burden on both patients and healthcare workers, so agents that address the drawbacks of these currently available therapies are desired.
In screening for DVT in whole lower limbs and the pelvis in the present study, only localized-type DVTs of the lower limb were found. Isolated soleal vein thrombosis occurred in the central and inner veins at a rate of 90%, but most were relapsed. It is believed that fluctuations in soleal vein thrombosis are repeated recurrences due to sustained risk factors and can present proximal development or an embolic source.31–34
DOACs are innovative drugs that circumvent complex patient management issues, such as frequent blood sampling, diet restriction, or drug interactions, which were problems in the warfarin-only era.6
Four DOACs are clinically available for the prevention of VTE after orthopedic surgery. Most importantly, accumulating clinical evidence has shown that DOACs such as dabigatran,35,36
apixaban,37,38
rivaroxaban,39,40
and edoxaban41
are beneficial because they significantly reduce the incidence of VTE after orthopedic surgery, similar to the VKA. Moreover, DOACs are associated with fewer complications, such as major bleeding, than the VKA. These data have been systematically reviewed elsewhere.42
Clinical development tests in Japan have reported VTE incidence after TKA of 65.3%, 21.3%, and 16.2% in groups receiving placebo, 1.5 mg fondaparinux daily, or 2.5 mg fondaparinux daily, respectively.43
In another study, the incidence of VTE after TKA in the placebo and 40 mg enoxaparin daily groups was 60.8% and 29.8%, respectively.44
This study also suggested the usefulness of edoxaban for the prevention of VTE after TKA.
In the present study, we demonstrated the efficacy and safety of edoxaban after TKA, although we were forced to discontinue the study. Therefore, the administration of edoxaban after TKA should be mandatory for the prevention of VTE. Based on the findings of the present study, we strongly recommended combination therapy with physiotherapy and edoxaban for the prevention of VTE after TKA. Moreover, although edoxaban administration decreased both the incidence of VTE and the AR10-AUC30
(Figure 3), VTE-positive patients who had a low AR10-AUC30
on Day 7 were found in the combination group (Figure 4). Based on these results, it is possible that rheological factors, such as vascular endothelial dysfunction and blood flow disturbances, or other thrombogenic mechanisms (except Factor Xa suppression) may be involved in the development of VTE in patients treated with combination therapy. Large-scale clinical studies using the T-TAS parameters may be needed to detect differences in thrombogenicity or bleeding risk between VTE-positive and VTE-negative patients in monotherapy and combination therapy groups.
We reported previously that the AR10-AUC30
as determined by T-TAS is a useful marker for the prediction of periprocedural bleeding events in patients with atrial fibrillation.1
However, in the present study only one of the 38 patients experienced a serious bleeding event, as described above. Therefore, we cannot definitively state the usefulness of the AR10-AUC30
as a hemorrhagic marker after TKA surgery. Furthermore, the risk of VTE persists even with physiotherapy and anticoagulation therapy. Because residual risks are considered intricately intertwined with many factors, as stated above, they require further investigation.
Study Limitations
The present study has several limitations. First, it was a single-center study. Moreover, compared with previous studies, the number of patients was relatively small. Specifically, we had planned to enroll 80 patients, but discontinued the study because the differences in the primary endpoint reached statistical significance. Second, we were unable to measure blood edoxaban concentrations. Consequently, we could not correlate the significant decreases in AR10-AUC30
and the incidence of VTE observed in the present study with edoxaban concentrations. Third, we did not examine the effects of warfarin or DOACs (e.g., dabigatran,45
apixaban,46,47
and rivaroxaban48–50) other than edoxaban. Fourth, as mentioned above, thrombosis in the lower limbs was evaluated by different skilled sonographers, but the accuracy of a distal DVT diagnosis among the different sonographers was not confirmed. Therefore, further pathophysiological and molecular physiological studies, including animal experiments, are warranted.
Conclusions
In the present study we have demonstrated the efficacy and safety of edoxaban for the prevention of VTE after TKA. To the best of our knowledge, this study is the first to show that edoxaban enhances the efficacy of physiotherapy for thromboprophylaxis after TKA.
Acknowledgments
The authors thank Kazuya Hosokawa and Tomoko Ohnishi from the Research Institute of Fujimori Kogyo Co. (Yokohama, Japan) for their excellent technical support in the measurement of T-TAS parameters. The authors also thank all paramedical staff and clinical secretaries for their kind support during this work.
Authorship Contributions
D.S., K.K., H.O. and E.N. conceived of and designed the research; N.O., S.Y. and S.I. performed the experiments; D.S., M. Ishii, Y.A. and Y.O. prepared the figures; H.M. and K.T. approved the final version of the manuscript; M. Ito, T.M., and S.H. analyzed the data; K.K. edited and revised the manuscript; D.S. and K.K. drafted the manuscript.
Source of Funding
This study was supported, in part, by a Grant-in-Aid for Scientific Research (#15K09089) from the Ministry of Education, Culture, Sports, Science and Technology of Japan.
Conflict of Interest
All authors declare no conflicts of interest.
References
- 1.
Ito M, Kaikita K, Sueta D, Ishii M, Oimatsu Y, Arima Y, et al. Total thrombus-formation analysis system (T-TAS) can predict periprocedural bleeding events in patients undergoing catheter ablation for atrial fibrillation. J Am Heart Assoc 2016; 5: e002744.
- 2.
Sueta D, Kaikita K, Ogawa H. Letter by Sueta et al regarding article, “Urgent need to measure effects of direct oral anticoagulants”. Circulation 2016; 134: e496–e497.
- 3.
Arima Y, Kaikita K, Ishii M, Ito M, Sueta D, Oimatsu Y, et al. Assessment of platelet-derived thrombogenicity by the total thrombus-formation analysis system in coronary artery disease patients on antiplatelet therapy. J Thromb Haemost 2016; 14: 850–859.
- 4.
Oimatsu Y, Kaikita K, Ishii M, Mitsuse T, Ito M, Arima Y, et al. Total thrombus-formation analysis system predicts periprocedural bleeding events in patients with coronary artery disease undergoing percutaneous coronary intervention. J Am Heart Assoc 2017; 6: e005263.
- 5.
Ishii M, Kaikita K, Ito M, Sueta D, Arima Y, Takashio S, et al. Direct oral anticoagulants form a thrombus different from warfarin in a microchip flow chamber system. Sci Rep 2017; 7: 7399.
- 6.
Sueta D, Kaikita K, Okamoto N, Arima Y, Ishii M, Ito M, et al. A novel quantitative assessment of whole blood thrombogenicity in patients treated with a non-vitamin K oral anticoagulant. Int J Cardiol 2015; 197: 98–100.
- 7.
Fuji T, Fujita S, Tachibana S, Kawai Y. A dose-ranging study evaluating the oral Factor Xa inhibitor edoxaban for the prevention of venous thromboembolism in patients undergoing total knee arthroplasty. J Thromb Haemost 2010; 8: 2458–2468.
- 8.
Fuji T, Wang CJ, Fujita S, Kawai Y, Nakamura M, Kimura T, et al. Safety and efficacy of edoxaban, an oral Factor Xa inhibitor, versus enoxaparin for thromboprophylaxis after total knee arthroplasty: The STARS E-3 trial. Thromb Res 2014; 134: 1198–1204.
- 9.
Sueta D, Kaikita K, Okamoto N, Yamabe S, Ishii M, Arima Y, et al. Efficacy Study of the COmbination of Edoxaban and Physiotherapy on the PRevention of Venous-Thromboembolism in patients after Total Knee Arthroplasty (ESCORT-TKA Trial): Study protocol for a randomized controlled trial. Clin Trials Regul Sci Cardiol 2016; 19: 1–4.
- 10.
Okamoto N, Nakamura E, Nishioka H, Karasugi T, Okada T, Mizuta H. In vivo kinematic comparison between mobile-bearing and fixed-bearing total knee arthroplasty during step-up activity. J Arthroplasty 2014; 29: 2393–2396.
- 11.
Ewald FC. The Knee Society total knee arthroplasty roentgenographic evaluation and scoring system. Clin Orthop Relat Res 1989; 248: 9–12.
- 12.
Hartman JT, Altner PC, Freeark RJ. The effect of limb elevation in preventing venous thrombosis. J Bone Joint Surg Am 1970; 52: 1618–1622.
- 13.
Ishii M, Kawaji H, Hamasaki M, Ida H, Takagi M, Kobayashi S. Examination of maximum velocity of femoral vein with various methods for prevention of deep vein thrombosis. Hip Joint 2001; 27: 557–559.
- 14.
McNally MA, Cooke EA, Mollan RA. The effect of active movement of the foot on venous blood flow after total hip replacement. J Bone Joint Surg Am 1997; 79: 1198–1201.
- 15.
Hirai M, Iwata H, Hayakawa N. Effect of elastic compression stockings in patients with varicose veins and healthy controls measured by strain gauge plethysmography. Skin Res Technol 2002; 8: 236–239.
- 16.
Hui A, Heras-Palou C, Dunn I, Triffitt P, Crozier A, Imeson J, et al. Graded compression stockings for prevention of deep-vein thrombosis after hip and knee replacement. J Bone Joint Surg Br 1996; 78: 550–554.
- 17.
Levine MN, Gent M, Hirsh J, Weitz J, Turpie AG, Powers P, et al. Ardeparin (low-molecular-weight heparin) vs graduated compression stockings for the prevention of venous thromboembolism: A randomized trial in patients undergoing knee surgery. Arch Intern Med 1996; 156: 851–856.
- 18.
Nicolaides A, Miles C, Hoare M, Jury P, Helmis E, Venniker R. Intermittent sequential pneumatic compression of the legs and thromboembolism-deterrent stockings in the prevention of postoperative deep venous thrombosis. Surgery 1983; 94: 21–25.
- 19.
Warwick D, Harrison J, Glew D, Mitchelmore A, Peters TJ, Donovan J. Comparison of the use of a foot pump with the use of low-molecular-weight heparin for the prevention of deep-vein thrombosis after total hip replacement. A prospective, randomized trial. J Bone Joint Surg Am 1998; 80: 1158–1166.
- 20.
Siddiqui AU, Buchman TG, Hotchkiss RS. Pulmonary embolism as a consequence of applying sequential compression device on legs in a patient asymptomatic of deep vein thrombosis. Anesthesiology 2000; 92: 880–880.
- 21.
Kaempffe FA, Lifeso RM, Meinking C. Intermittent pneumatic compression versus coumadin: Prevention of deep vein thrombosis in lower-extremity total joint arthroplasty. Clin Orthop Relat Res 1991; 269: 89–97.
- 22.
Hull R, Delmore T, Hirsh J, Gent M, Armstrong P, Lofthouse R, et al. Effectiveness of intermittent pulsatile elastic stockings for the prevention of calf and thigh vein thrombosis in patienis undergoing elective knee surgery. Thromb Res 1979; 16: 37–45.
- 23.
McKenna R, Galante J, Bachmann F, Wallace D, Kaushal P, Meredith P. Prevention of venous thromboembolism after total knee replacement by high-dose aspirin or intermittent calf and thigh compression. Br Med J 1980; 280: 514–517.
- 24.
Haas SB, Insall JN, Scuderi GR, Windsor R, Ghelman B. Pneumatic sequential-compression boots compared with aspirin prophylaxis of deep-vein thrombosis after total knee arthroplasty. J Bone Joint Surg Am 1990; 72: 27–31.
- 25.
Geerts WH, Heit JA, Clagett GP, Pineo GF, Colwell CW, Anderson FA, et al. Prevention of venous thromboembolism. Chest 2001; 119(Suppl): 132S–175S.
- 26.
Yamaguchi Y, Moriki T, Igari A, Matsubara Y, Ohnishi T, Hosokawa K, et al. Studies of a microchip flow-chamber system to characterize whole blood thrombogenicity in healthy individuals. Thromb Res 2013; 132: 263–270.
- 27.
Hosokawa K, Ohnishi T, Kondo T, Fukasawa M, Koide T, Maruyama I, et al. A novel automated microchip flow-chamber system to quantitatively evaluate thrombus formation and antithrombotic agents under blood flow conditions. J Thromb Haemost 2011; 9: 2029–2037.
- 28.
Hosokawa K, Ohnishi T, Fukasawa M, Kondo T, Sameshima H, Koide T, et al. A microchip flow-chamber system for quantitative assessment of the platelet thrombus formation process. Microvasc Res 2012; 83: 154–161.
- 29.
Guyatt GH, Akl EA, Crowther M, Gutterman DD, Schuünemann HJ. Executive summary: Antithrombotic therapy and prevention of thrombosis: American College of Chest Physicians evidencebased clinical practice guidelines. Chest 2012; 141(Suppl): 7S–47S.
- 30.
JCS Joint Working Group. Guidelines for the diagnosis, treatment and prevention of pulmonary thromboembolism and deep vein thrombosis (JCS 2009): Digest version. Circ J 2011; 75: 1258–1281.
- 31.
Lohr JM, Kerr TM, Lutter KS, Cranley RD, Spirtoff K, Cranley JJ. Lower extremity calf thrombosis: To treat or not to treat? J Vasc Surg 1991; 14: 618–623.
- 32.
Ohgi S, Tachibana M, Ikebuchi M, Kanaoka Y, Maeda T, Mori T. Pulmonary embolism in patients with isolated soleal vein thrombosis. Angiology 1998; 49: 759–764.
- 33.
Lautz TB, Abbas F, Walsh SJN, Chow C, Amaranto DJ, Wang E, et al. Isolated gastrocnemius and soleal vein thrombosis: Should these patients receive therapeutic anticoagulation? Ann Surg 2010; 251: 735–742.
- 34.
Ohgi S, Ohgi N. Relation between isolated venous thrombi in soleal muscle and positive anti-nuclear antibody. Ann Vasc Dis 2012; 5: 321–327.
- 35.
Schulman S, Kearon C, Kakkar AK, Mismetti P, Schellong S, Eriksson H, et al. Dabigatran versus warfarin in the treatment of acute venous thromboembolism. N Engl J Med 2009; 361: 2342–2352.
- 36.
Schulman S, Kakkar AK, Goldhaber SZ, Schellong S, Eriksson H, Mismetti P, et al. Treatment of acute venous thromboembolism with dabigatran or warfarin and pooled analysis. Circulation 2014; 129: 764–772.
- 37.
Agnelli G, Buller HR, Cohen A, Curto M, Gallus AS, Johnson M, et al. Oral apixaban for the treatment of acute venous thromboembolism. N Engl J Med 2013; 369: 799–808.
- 38.
Nakamura M, Nishikawa M, Komuro I, Kitajima I, Uetsuka Y, Yamagami T, et al. Apixaban for the treatment of Japanese subjects with acute venous thromboembolism (AMPLIFY-J Study). Circ J 2015; 79: 1230–1236.
- 39.
Agnelli G, Berkowitz S, Bounameaux H, Büller H, Cohen A, Gallus A, et al. Oral rivaroxaban for symptomatic venous thromboembolism. N Engl J Med 2010; 363: 2499–2510.
- 40.
Büller HR, Prins MH, Lensing AW, Decousus H, Jacobson BF, Minar E, et al. Oral rivaroxaban for the treatment of symptomatic pulmonary embolism. N Engl J Med 2012; 366: 1287–1297.
- 41.
Büller HR, Décousus H, Grosso MA, Mercuri M, Middeldorp S, Prins MH, et al. Edoxaban versus warfarin for the treatment of symptomatic venous thromboembolism. N Engl J Med 2013; 369: 1406–1415.
- 42.
van Es N, Coppens M, Schulman S, Middeldorp S, Buller HR. Direct oral anticoagulants compared with vitamin K antagonists for acute venous thromboembolism: Evidence from phase 3 trials. Blood 2014; 124: 1968–1975.
- 43.
Fuji T, Fujita S, Ochi T. Fondaparinux prevents venous thromboembolism after joint replacement surgery in Japanese patients. Int Orthop 2008; 32: 443–451.
- 44.
Fuji T, Ochi T, Niwa S, Fujita S. Prevention of postoperative venous thromboembolism in Japanese patients undergoing total hip or knee arthroplasty: Two randomized, double-blind, placebo-controlled studies with three dosage regimens of enoxaparin. J Orthop Sci 2008; 13: 442–451.
- 45.
Eriksson BI, Dahl OE, Rosencher N, Kurth AA, van Dijk CN, Frostick SP, et al. Dabigatran etexilate versus enoxaparin for prevention of venous thromboembolism after total hip replacement: A randomised, double-blind, non-inferiority trial. Lancet 2007; 370: 949–956.
- 46.
Lassen MR, Raskob GE, Gallus A, Pineo G, Chen D, Portman RJ. Apixaban or enoxaparin for thromboprophylaxis after knee replacement. N Engl J Med 2009; 361: 594–604.
- 47.
Lassen MR, Raskob GE, Gallus A, Pineo G, Chen D, Hornick P. Apixaban versus enoxaparin for thromboprophylaxis after knee replacement (ADVANCE-2): A randomised double-blind trial. Lancet 2010; 375: 807–815.
- 48.
Lassen MR, Ageno W, Borris LC, Lieberman JR, Rosencher N, Bandel TJ, et al. Rivaroxaban versus enoxaparin for thromboprophylaxis after total knee arthroplasty. N Engl J Med 2008; 358: 2776–2786.
- 49.
Turpie AG, Lassen MR, Davidson BL, Bauer KA, Gent M, Kwong LM, et al. Rivaroxaban versus enoxaparin for thromboprophylaxis after total knee arthroplasty (RECORD4): A randomised trial. Lancet 2009; 373: 1673–1680.
- 50.
Turpie AG, Haas S, Kreutz R, Mantovani LG, Pattanayak CW, Holberg G, et al. A non-interventional comparison of rivaroxaban with standard of care for thromboprophylaxis after major orthopaedic surgery in 17,701 patients with propensity score adjustment. Thromb Haemost 2014; 111: 94–102.