Abstract
Background:
The therapeutic efficacy of bone marrow mononuclear cells (BM-MNC) autotransplantation in critical limb ischemia (CLI) has been reported. Variable proportions of circulating monocytes express low levels of CD34 (CD14+CD34low
cells) and behave in vitro as endothelial progenitor cells (EPCs). The aim of the present randomized clinical trial was to compare the safety and therapeutic effects of enriched circulating EPCs (ECEPCs) with BM-MNC administration.
Methods and Results:
ECEPCs (obtained from non-mobilized peripheral blood by immunomagnetic selection of CD14+
and CD34+
cells) or BM-MNC were injected into the gastrocnemius of the affected limb in 23 and 17 patients, respectively. After a mean of 25.2±18.6-month follow-up, both groups showed significant and progressive improvement in muscle perfusion (primary endpoint), rest pain, consumption of analgesics, pain-free walking distance, wound healing, quality of life, ankle-brachial index, toe-brachial index, and transcutaneous PO2. In ECEPC-treated patients, there was a positive correlation between injected CD14+CD34low
cell counts and the increase in muscle perfusion. The safety profile was comparable between the ECEPC and BM-MNC treatment arms. In both groups, the number of deaths and major amputations was lower compared with eligible untreated patients and historical reference patients.
Conclusions:
This study supports previous trials showing the efficacy of BM-MNC autotransplantation in CLI patients and demonstrates comparable therapeutic efficacy between BM-MNC and EPEPCs.
Peripheral arterial disease (PAD) is due primarily to an atherosclerotic obstruction of the arteries of the lower limbs. Disease prevalence ranges between 3% and 10%, and increases up to 20% in patients over 70 years of age.1,2
When the obstruction becomes critical, a typical cluster of signs and symptoms occurs, such as ischemic leg pain at rest and an incident ulcerative or gangrenous lesion of the affected extremity. Only 1–2% of the all PAD patients over the 50 years of age progresses to critical limb ischemia (CLI).1,2
The fast and spontaneous deterioration occurring in most CLI patients unavoidably leads to amputation, unless a surgical or procedural intervention can restore arterial flow. Therefore, a timely attempt to achieve effective arterial revascularization should always be made. Nonetheless, quite often many patients are not eligible for any surgical or percutaneous intervention.3
In patients progressing to CLI, the development of an effective net of collateral vessels is often lacking, especially in diabetic patients, affecting the fate of the ischemic leg. This compensatory mechanism consists of microcirculatory neovascularization and may be accomplished by circulating endothelial progenitor cells (EPCs) and vascular progenitor cells.3,4
Over the past 2 decades, stimulation of angiogenesis has been attempted using gene transfer of growth or hypoxia-inducible factors, with some indication of efficacy but also with important limitations due primarily to poor yield of gene transfer.4
Another approach has been the use of bone marrow (BM) stem cells (SC) that may differentiate towards endothelium5–7
or also stimulate resident endothelial cells through the release of growth factors.7
After the pioneering study of Tateishi-Yuyama et al,8
several reports confirmed the clinical efficacy of the intramuscular transplantation of autologous BM mononuclear cells (BM-MNC) or of circulating CD34+
SC mobilized by granulocyte colony stimulating factor (G-CSF) or granulocyte-macrophage colony stimulating factor (GM-CSF).9–16
Editorial p ????
In a previous study, we demonstrated the existence of a subpopulation of circulating CD14+
cells in healthy subjects that exhibited low expression of CD34 (CD14+CD34low), being the major source of circulating EPCs.17
We assumed that these cells may represent an accessible and convenient source of EPC obtained without mobilization. To verify this possibility, we evaluated the number of these cells in the peripheral blood (PB) of healthy subjects at different ages, and in patients with chronic vascular diseases. We then developed a cell-enrichment system based on the immunomagnetic isolation of both CD14+
(including CD14+CD34low) and CD34+
circulating cells, defined “enriched circulating (EC) EPCs” (patent no. FI2004A0000238). We then designed a single-center randomized unblinded clinical trial, the Stem Cell Emergency Life Threatening Arteriopathy (SCELTA) study, with the aim of comparing the safety and efficacy of intramuscular transplant of autologous ECEPCs vs. BM-MNCs in the limbs of CLI patients. The final analysis performed on 40 patients (17 treated with BM-MNCs and 23 treated with ECEPCs) demonstrated similar safety and efficacy of the 2 treatments, as revealed by clinical, functional, and imaging evaluation.
Methods
Study Design
The SCELTA study was a single-center randomized unblinded clinical trial with the aim of assessing the safety and therapeutic efficacy of an intramuscular injection of autologous ECEPCs in 30 CLI patients compared with 30 CLI patients injected with BM-MNCs. Eligible subjects were selected among men and women aged >40 years with persistent rest pain requiring systemic analgesic treatment during the last 15 days and/or the presence of trophic lesions amenable to occluding arteriopathy, and only if an ankle-brachial index (ABI) <0.40 (ankle systolic pressure <50–70 mmHg), a toe-brachial index (TBI) <0.40 (toe systolic pressure <30–50 mmHg), and transcutaneous (TC) PO2
<30 mmHg could be demonstrated. Patients were enrolled in the study only if, according to vascular Duplex and computed tomography angiography (CTA) or arteriography findings, intravascular or surgical revascularization was not feasible or when the patient refused to undergo surgical treatment, and after written informed consent had been obtained. ECEPCs or BM-MNCs were inoculated into the gastrocnemius muscle of the affected limb through 40 simultaneous injections of 1 mL cellular suspension (if both limbs were affected, only the worst was injected). The randomization list (1:1, without stratification) was generated using a computerized random number generator by one of the investigators (E. Mannucci), who was not involved in the enrolment or clinical management of patients.
Data were collected on a dedicated electronic medical record, with controlled access, at the Immunology and Cell Therapy Unit, Azienda Ospedaliero-Universitaria Careggi. Detailed methods for all procedures are provided in
Methods S1, available as Supplementary Material to this paper.
This study was approved by the Italian Ministry of Health in 2007 (Approval D.G. PREV. VII/19820/9/I.8.F.u/9).
Results
Circulating CD14+CD34low
Cell Counts in Healthy Subjects and Patients With Chronic Vascular Disorders
Before starting the SCELTA trial, the relative recovery of CD14+CD34low
cells using immunomagnetic isolation from healthy subjects at different ages and patients with chronic vascular disorders was calculated. The number of CD14+CD34low
cells was variable, independent of age or the presence of PAD (Figure S1). The absolute number of CD14+CD34low
and CD34+
cells recoverable from leukapheresis after immunomagnetic cell isolation was also calculated in donors from 3 different blood banks. The number of CD14+CD34low
cells in patients ranged from 30×106
to 840×106, which is much higher than the number of classic CD34+
SC, which ranged from 0.56×106
to 11×106
(Table S1).
Enrolled Patients and Dropouts
Patient enrolment started in December 2009 and the evaluation ended on October 2015; the trial was stopped before 60 patients were enrolled in the study (30 in each of the BM-MNC and ECEPC groups) because of time limits and for the achievement of a primary endpoint. The SCELTA trial design is shown in
Figure 1. By October 2015, 44 of the 126 patients screened were found to be eligible for the study and had therefore been enrolled and randomized. An additional 18 patients were considered eligible for inclusion, but they were not randomized for different reasons, including late refusal to undergo treatment, the presence of monoclonal gammopathy of undetermined significance (MGUS; 8 patients), rapid mind deterioration (1 patient), and the absence of accessible venous sources (1 patient). Forty of 44 enrolled patients were treated according to the inclusion and exclusion criteria (4 patients dropped out). Follow-up visits were performed at 30, 150, and 360 days. According to treatment randomization, 23 patients received intramuscular injections of ECEPCs and 17 received intramuscular injections of BM-MNCs. Baseline clinical, functional, and biochemical data are summarized in
Table 1.

Table 1.
Baseline Characteristics of Patients Treated With Enriched Circulating ECEPC or BM-MNC
| |
ECEPCs |
BM-MNC |
P value |
| No. PAD patients |
23 |
17 |
NS |
| Age (years) |
71.2±9.4 |
68.7±10.8 |
NS |
| Sex (M/F) |
20/3 |
13/4 |
NS |
| Trophic lesions |
16 (69) |
14 (82) |
NS |
| Previous endovascular therapy |
16 (70) |
11 (64) |
NS |
| Previous bypass surgery |
17 (74) |
8 (47) |
NS |
| Rutherford class |
| C4 |
7 (30) |
5 (30) |
NS |
| C5 |
8 (35) |
6 (35) |
NS |
| C6 |
8 (35) |
6 (35) |
NS |
| Hypertension |
17 (74) |
8 (47) |
NS |
| Diabetes mellitus |
12 (52) |
5 (29) |
NS |
| Dyslipidemia |
16 (69) |
9 (52) |
NS |
| Past smoker |
18 (78) |
13 (76) |
NS |
| Therapy with statins |
18 (78) |
9 (52) |
NS |
| Ejection fraction (%) |
56±6.5 |
58.1±6.8 |
NS |
| Creatinine (mg/dL) |
0.95±0.3 |
0.86±0.2 |
NS |
| Hemoglobin (g/dL) |
13.1±1.5 |
13.5±1.6 |
NS |
| Previous CAD (<6 months) |
15 (65) |
6 (35) |
NS |
| Previous TIA or stroke |
4 (17) |
0 |
NS |
| Infected lesions |
10 (43) |
8 (47) |
NS |
Unless indicated otherwise, data are given as the mean±SD or as n (%). BM-MNC, bone marrow mononuclear cells; CAD, coronary artery disease; ECEPC, endothelial progenitor cells; TIA, transient ischemic attack.
Details of the patients who dropped out of the study are given in
Results S1.
Historical Reference Group
A placebo control group was not envisaged in the study design. Instead, in order to obtain a comparable sample of untreated CLI patients, outcome data (at 1 year) were collected for 40 patients in a historical clinical reference group (HCRG) with similar disease severity (Rutherford classification), matched for age, sex, and comorbidities, whose medical records clinical and instrumental data were retrieved from medical records (see
Methods S1). The HCRG cohort also included CLI patients who were not eligible for surgical or interventional revascularization procedures (Table S2).
Adverse Events (AEs)
AEs Occurring Within 5 Days After Procedures
The AEs that occurred within 5 days after the procedures included dyspnea with transient O2
desaturation (1 patient) and a hyperkinetic state inducing non-ST-elevation myocardial infarction (NSTEMI) without variations in cardiac contractility during apheresis (1 patient), pain at the site of intramuscular injection (which resolved within 24 h; 2 patients), angina (chest pain) 3 h after injection (1 patient), hematoma at the injection site (1 patient), fever within the 5 days after injection (1 patient), and transient kidney failure after injection of contrast medium (1 patient). The post-procedural AEs and their possible relationship to the entire procedure are reported in
Table S3.
AEs Occurring >5 Days After Procedures
AEs that occurred more than 5 days after procedure included the death of 1 patient randomized to ECEPC 5 months after treatment due to myocardial infarction and major amputations in 7 patients (5 in the ECEPC-treated group and 2 in the BM-MNC-treated group; P=NS). Major amputations were required exclusively in patients showing progressive extension of local infection, untreatable pain, and/or presepticemic conditions (just before or soon after treatment; 18 patients). No amputations were required in patients in whom there was no infection (0/22 patients; P<0.001;
Table S3). Minor amputations were required in 7 patients (2 in the BM-MNC-treated group, 5 in the ECEPC-treated group) and had been all planned at the beginning of the study due to persisting trophic or gangrenous lesions. No amputations were required in the remaining 18 (of 23; 78.3%) patients in the ECEPC group and the remaining 15 (of 17; 88.2%) patients in the BM-MNC group (P=NS). During the follow-up period, 3 of 17 patients (17.6%) in the BM-MNC-treated group developed new trophic lesions, compared with 2 of 23 patients (8.7%) in the ECEPC-treated group (P=NS). Of these patients, 3 (1 in the BM-MNC group, 2 in the EPEPC group) subsequently underwent major amputation.
Comparison of Clinical Results Between the ECEPC and BM-MNC Groups
Clinical comparisons were made between the ECEPC and BM-MNC groups for 5 parameters: rest pain, analgesic drug consumption, pain-free walking distance (PFWD), trophic limb lesions (TLL), and quality of life (QoL).
Rest Pain
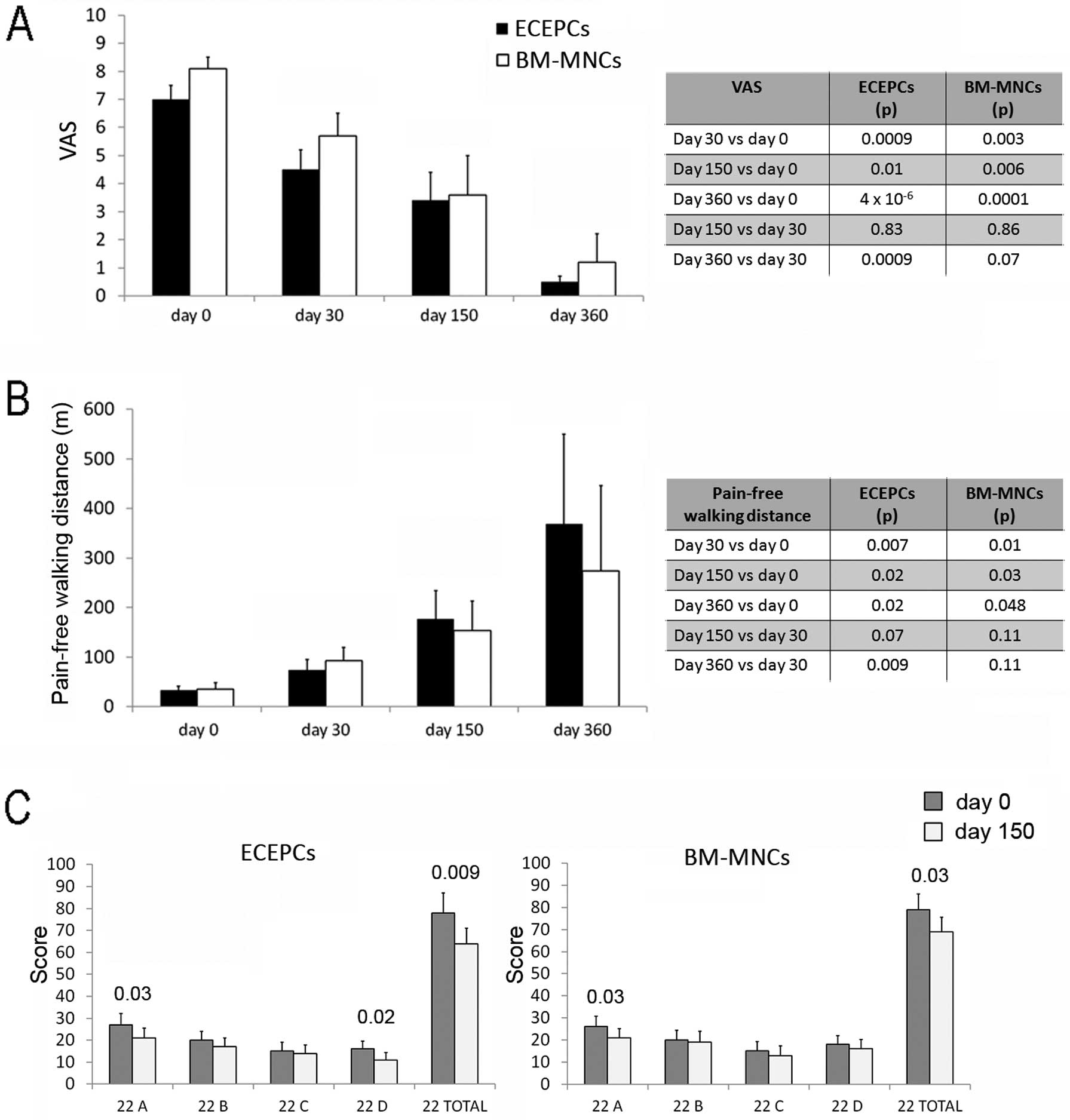
The mean pain intensity score showed no differences between patients randomized to treatment with ECEPCs or BM-MNCs at any follow-up visit. Pain scores, as evaluated using a visual analog scale (VAS), were significantly lower than at Time 0 at all follow-up times in both groups; in addition, pain scores were significantly reduced between the 1st and the 3rd follow-up visits in the ECEPC arm (Figure 2A).
Consumption of Analgesic Drugs
No difference was found in mean analgesic drug consumption between patients randomized to treatment with ECEPCs or BM-MNCs at any time point (data not shown).
Pain-Free Walking Distance
There was no difference between the ECEPC and BM-MNC groups with regard to PFWD at Time 0 and at subsequent follow-up visits, but mean PFWD increased gradually and significantly in both groups after treatment (Figure 2B).
Wound Evaluation
The number of patients with TLL before treatment was not significantly different in the ECEPC compared to the BM-MNC group: 16/23 (69.6%) vs 14/17 (82.4%) respectively (P=NS). In both groups, the number of patients with TLL decreased significantly after treatment (6/17 [35%] and 6/15 [40%] in the ECEPC and BM-MNC groups, respectively; P<0.05 and P<0.01, respectively). In order to evaluate the evolution of single trophic lesions, as assessed by Wagner grades, those lesions with a grade ≤2 were checked before and after cell treatment, and there was a tendency for an improvement in the grades of the lesions in both groups. There was difference between the 2 groups (Table S4).
QoL
The mean QoL scores did not differ between the 2 groups before treatment, and there was a marked increase in QoL scores (to the same extent) in the 2 groups at the second follow-up examination (Figure 2C).
Comparison of Instrumental Vascular Tests Between the ECEPC and BM-MNC Groups
ABI and TBI
Mean ABI and TBI values were comparable in all patients before treatment, and they improved significantly (to a similar extent) in both treatment groups at each follow-up visit compared with baseline. No differences were found after treatment between the BM-MNCs and ECEPCs groups (Figure 3A,B).
TC PO2
Mean TC PO2
values were similarly low in both groups before treatment, and increased significantly at each follow-up examination, without any difference between the BM-MNC and ECEPC groups (Figure 3C).
Muscle Perfusion
Muscle perfusion, as measured by contrast-enhanced ultrasound sonography (CEUS) at the upper calf and forefoot of the ischemic limb, was similar in the 2 groups. There was a gradual, significant increase in muscle perfusion at both the upper calf and forefoot seen in all follow-up examinations in both groups (Figure 4). Since we believe muscle represents the most direct functional test to define recovery of limb vascularization,29
we chose this parameter as a primary endpoint in the present study. As expected, there was a significant correlation between muscle perfusion and other vascular parameters, particularly TC PO2
(Figure S2).

Anatomical Vessel Assessment
CTA was used to characterize the anatomy of arteries within the limbs at the time of screening or enrolment examination, at the 1st and 3rd follow-up visits. No differences in collateral circulation were found between the BM-MNC and ECEPC groups before and after treatment, whereas a significant increase in microcirculation was noted at the 3rd compared with the 1st follow-up visit (P<0.05 in the BM-MNC group; P<0.01 in ECEPC group;
Figure 5A).
Figure 5B
shows 1 representative case from the ECEPC-treated group.
Correlations Between the Number of CD14+CD34low
Cells Inoculated and Improvement in Muscle Perfusion
Because the injected ECEPC suspension also included high numbers of CD14+CD34−
cells and low numbers of CD34+
SC in addition to the CD14+CD34low
cells, the total number of circulating or BM-derived cells was counted, as was the number of each of these three 3 cell subpopulations, in order to investigate their possible relationship with improvements in muscle perfusion. As shown in
Figure 6, the number of CD14+CD34low
cells from PB was positively correlated with calf time to peak (TTP), whereas there was no correlation between TTP and total leukocytes or CD34+
SC. No appreciable correlation was found between muscle perfusion and BM-MNC. Similar data were obtained for forefoot TTP (data not shown).
Clinical Outcomes in Treated Patients and the HCRG
The outcomes observed in the treated patients from both groups (present study) and the HCRG are reported in
Table 2. The 1-year death rate (all-cause mortality) was significantly higher in the HCRG than in the treated patient group, with 18/40 (45%) and 1/40 (2.5%) of HCRG and treated patients dying, respectively (P<0.0001). Interestingly, sepsis was the cause of death in 9 of 18 deaths (50%) in the HCRG, whereas acute myocardial infarction was the cause of death for the only treated patient who died. The number of major amputations was higher in the HCRG than treated patient group (12/40 [30%] vs. 7/40 [17.5%], respectively) although the difference did not reach statistical significance. However, the proportion of patients who died dead after a major amputation was higher in HCRG than treated patient group (7/12 [58.3%] vs. 0/7 [0%], respectively; P<0.001).
Table 2.
Comparison Between All Treated Patients: Both ECEPC and BM-MNC (Present Study) and the HCRG
| |
No.
patients |
Follow-up
duration
(months) |
No. deaths |
Major amputations |
Improvements in |
| Sepsis |
Other causes |
Died |
Survived |
PFWD |
Drug
consumption |
Trophic
lesions |
| +Amp |
−Amp |
+Amp |
−Amp |
| Patients |
| Treated |
40 |
12 |
0 |
0 |
0 |
1 |
0 |
7 |
17/32 (53) |
13/39 (33.3) |
10/28 (36) |
Untreated
HCRG |
40 |
12 |
4 |
5 |
2 |
7 |
7 |
5 |
0/17 |
2/22 (9) |
3/30 (10) |
| P value |
NS |
NS |
<0.001 |
<0.001 |
<0.05 |
<0.001 |
<0.001 |
NS |
<0.001 |
<0.001 |
<0.001 |
Unless indicated otherwise, data are given as n (%) or as the number of patients in each group. +Amp, with amputation; −Amp, without amputation; HCRG, historical clinical reference group; PFWD, pain-free walking distance.
With regard to clinical parameters, the proportion of survivors in the non-amputated group with healed trophic lesions was higher for treated patients than the HCRG (10/28 [37.7%] vs. 3/30 [10%], respectively). The PFWD for 53% of treated patients was >200 m, whereas none of the HCRG patients had a PFWD during the 1-year follow-up period. During the follow-up period, 2 patients worsened compared to enrollment (6%), 14 were stable (43.7%), and 17 improved (51.5%) in the treated group, compared with 2 (11.7%), 15 (88.2%), and 0 (0%; P<0.001) surviving, non-amputated patients, respectively, in the HCRG. Analgesic drug consumption, evaluated in survivors, decreased significantly in the treated patients compared with the HCRG (13/39 [33.3%] vs. 2/22 [9%], respectively; P<0.001;
Table 2). These results are in keeping with other studies in the field.7–15,45–47
Discussion
Several studies have shown that autologous or even allogeneic implantation of BM-MNCs promotes therapeutic angiogenesis in patients with CLI, although some review of the existing literature dose not fully support this correlation.8,9,12,13,16,27–32
Alternative techniques have also been attempted, such as implantation of autologous circulating CD34+
SC, following G-CSF mobilization, with evidence of some positive results.10,11,14,15
However, SC mobilization may be failing in approximately one-third of subjects,32
and it virtually does not happen in diabetic patients.33,34
Some years ago, we demonstrated the existence of a subpopulation of CD14+
cells exhibiting low CD34 expression (CD14+CD34low) that appeared to be the major source of circulating EPCs.17
The proportion of these cells in the PB varies individually, ranging from 10% to 70% of total CD14+
cells. The relative amount of circulating CD14+CD34low
cells was not dependent on age and was unaffected in chronic vascular diseases.
To demonstrate the therapeutic efficacy of ECEPCs in CLI patients, we designed a single-center randomized clinical trial (SCELTA) in which 2 groups of patients received intramuscular leg injections of autologous ECEPCs or BM-MNCs.
At present, the mechanisms regulating SC homing, tissue incorporation, survival, and differentiation are partially known,35,36
and studies have not reported any significant differences between intra-arterial or intramuscular injections.37–39
Thus, we chose to use the multiple-site (n=40) gastrocnemius injection method because it results in a cellular depot in the ischemic tissue.38
In the present study, 23 patients were treated with ECEPCs and 17 were treated with BM-MNCs, and the absolute number of viable injected EPCPCs and BM-MNCs was comparable to that reported in other studies using the same source of cells and route of administration.8,32,41,42
However, it should be noted that the number of recovered CD34+
SC was in the order of 106, compared with 109
CD14+CD34low
cells, in both the BM-MNC and ECEPC suspensions. As expected, CD34+
SC were present at 40-fold lower levels in the ECEPC than in the BM-MNC suspensions, but the number of CD14+CD34low
cells was approximately 4-fold higher in the ECEPC than in the BM-MNC suspensions. Although the number of CD14+CD34low
cells present in the injected ECEPC suspension was positively correlated with the increase in muscle perfusion after treatment, there was no correlation between the number of CD14+CD34low
cells or the number of CD34+
SC present in the injected BM-MNC suspension with muscle perfusion. Thus, it is probable that other cell types in the BM-MNC suspension account for the positive effects of the treatment, possibly mesenchymal SC.16,18,42
Although the safety of BM-MNC treatment has been largely demonstrated by previous studies,8,9,12,13
we could not draw definitive conclusions about ECEPCs due to the insufficient number of patients treated; however, treatment with autologous PB MNCs after G-CSF mobilization has been demonstrated to be safe by previous studies.10,11,14,15
A placebo control group was not envisaged in the present study because many studies have already demonstrated the efficacy of BM-MNC implantation;14,27–33,43
these data were recently confirmed by Pignon et al,47
who demonstrated the efficacy of BM-derived MNCs in reducing the frequency of major amputations in CLI patients in a randomized double-blind placebo-controlled trial. Thus, in the present study we considered the BM-MNC arm as the control condition, in accordance with the decision of the Ethics Committee. We also planned an internal control represented by the longitudinal evaluation of each patient before the injection procedure: (1) screening time (3–4 months before the procedure); (2) enrollment time (28 days before the procedures); and (3) procedure time (24 h before the injection). The data clearly show that in this preprocedural period, all parameters evaluated were stable if not worsening. In some cases the worsening was statistically significant due to the typical clinical instability of CLI patients. Moreover, we took advantage of an HCRG comprising patients (matched for number, disease severity, therapy regimen, and general characteristics) who had previously been evaluated for CLI in our institutions (Careggi University Hospital and San Giovanni di Dio Hospital). This close similarity in patient characteristics and the standard clinical management can overcome, at least in part, the well-known limitations in interpreting the results after comparison with a retrospective reference group rather than with a standard prospective control group. Indeed, the patients in the HCRG represent the classic history of CLI patients in our institutions in the absence of cell therapy. In the HCRG, a major incidence of deaths, major amputations, and a worsening of ulcer scores was observed over a 12-month follow-up compared with patients in both treatment arms in the present study, indicating that both cell regimens are useful in CLI. Similarly, there was a significantly higher incidence of deaths and major amputations in the cohort of screened eligible patients that did not undergo treatment compared with treated subjects (data not shown). It is also of note that major amputations in treated patients were required exclusively for patients with extensive local infections and/or presepticemic conditions before treatment.
The trial was stopped after the enrolment of 40 patients because the statistical improvement of the single primary endpoint (i.e., muscle perfusion) was largely obtained before 60 patients had been enrolled in the study. The trial was not powered for treatment efficacy; nevertheless, all the clinical parameters evaluated (i.e., rest pain, analgesic drug consumption, PFWD, wound healing and QoL), improved significantly in both treatment arms at the earliest follow-up visit (30 days after treatment), with additional progressive improvements at the 2nd (150 days) and 3rd (360 days) follow-up examinations. The favorable clinical effects were consistent with the objective instrumental results of different vascular and metabolic tests (ABI, TBI, TC PO2, TTP). CTA revealed data favoring an improvement in macrovessel and collateral vessel representation at the 3rd follow-up visit, being an expression of neovasculogenesis, which is a late event. These data suggest that injection of EPCs induces a widening of the microcirculatory net and a run-off microvascular bed that can be considered prerequisites for the later development of larger proximal vessels.
All the clinical improvements in the treated patients persisted even beyond 360 days (3rd follow-up visit) and some patients in both groups are still in good conditions up to 6 years after treatment. Moreover, none of the patients surviving at the 1-year follow-up examination died or underwent major amputations in the following period, with a mean (±SD) follow-up for the entire group of 25.2±18.6 months. The question of whether vascular regeneration induced by EPCs is due to their differentiation into endothelial cells remains to be answered. SC-tracing studies in animal models have shown that injected cells are retained for only a short period in the limb,43
and that incorporation into the vascular bed does not contribute to the observed proangiogenic effects.43,44
SCs could act through a paracrine effect, either directly on the local endothelium43
or indirectly through the recruitment of angiogenic monocytes.44
Conclusions
The results of the present trial do not only confirm the results of several previous studies on the therapeutic efficacy of autologous transplantation of BM-MNCs in CLI,8–16,47
but they also provide evidence of the efficacy of an additional procedure, based on the injection of a circulating cell population consisting of monocytes (containing the CD14+CD34low
population) and of CD34+
cells, in absence of G-CSF mobilization. These cells, particularly the CD14+CD34low
population, have been shown to be prone towards differentiation, at least in vitro, into endothelial cells.17
Thus, the results of the present study provide strong support for the “non inferiority” of non-mobilized ECEPCs vs. BM-MNCs treatment, and encourage the use of an ECEPC method as a possible source of EPCs for therapeutic purposes as an alternative to BM.
Acknowledgments
This study was fully supported by a special grant to S.R. from the Tuscany Ministry of Health (DGR. n. 525 of 17 July 2006). The authors thank Susanna Bormioli (Azienda Ospedaliero-Universitaria Careggi and Center of Excellence Denothe, University of Florence) for the English language revision of the manuscript.
Supplementary Files
Supplementary File 1
Supplementary Methods
Supplementary Results
Figure S1.
Recoverable CD14+CD34low
cells from the blood of critical limb ischemia (CLI) patients and healthy donors.
Figure S2.
Correlation between muscle perfusion, shown as time to peak (TTP) at the level of the calf and transcutaneous (TC) PO2
on Days 30, 150, and 360 of follow-up.
Table S1.
Number of cells injected
Table S2.
Baseline characteristics of the treated with both ECEPC and BM-MNC patients and HCRG
Table S3.
Adverse events (AEs)
Table S4.
Evaluation of the evolution of TLL
Please find supplementary file(s);
http://dx.doi.org/10.1253/circj.CJ-17-0720
References
- 1.
Wolfe JH, Wyatt MG. Critical and subcritical ischaemia. Eur J Vasc Endovasc Surg 1997; 13: 578–582.
- 2.
Norgren L, Hiatt WR, Dormandy JA, Nehler MR, Harris KA, Fowkes FG; TASC II Working Group. Inter-Society Consensus for the Management of peripheral arterial disease (TASC II). J Vasc Surg 2007; 45(Suppl S): S5–S67.
- 3.
Attanasio S, Snell J. Therapeutic angiogenesis in the management of critical limb ischemia: Current concepts and review. Cardiol Rev 2009; 17: 115–120.
- 4.
Isner JM, Pieczek A, Schainfeld R, Blair R, Haley L, Asahara T, et al. Clinical evidence of angiogenesis after arterial gene transfer of phVEGF165 in patient with ischaemic limb. Lancet 1996; 348: 370–374.
- 5.
Aranguren XL, McCue JD, Hendrickx B, Zhu XH, Du F, Chen E, et al. Multipotent adult progenitor cells sustain function of ischemic limbs in mice. J Clin Invest 2008; 118: 505–514.
- 6.
Kamihata H, Matsubara H, Nishiue T, Fujiyama S, Tsutsumi Y, Ozono R, et al. Implantation of bone marrow mononuclear cells into ischemic myocardium enhances collateral perfusion and regional function via side supply of angioblasts, angiogenic ligands, and cytokines. Circulation 2001; 104: 1046–1052.
- 7.
Sprengers RW, Lips DJ, Moll FL, Verhaar MC. Progenitor cell therapy in patients with critical limb ischemia without surgical options. Ann Surg 2008; 247: 411–420.
- 8.
Tateishi-Yuyama E, Matsubara H, Murohara T, Ikeda U, Shintani S, Masaki H, et al. Therapeutic angiogenesis for patients with limb ischaemia by autologous transplantation of bone-marrow cells: A pilot study and a randomised controlled trial. Lancet 2002; 360: 427–435.
- 9.
Kajiguchi M, Kondo T, Izawa H, Kobayashi M, Yamamoto K, Shintani S, et al. Safety and efficacy of autologous progenitor cell transplantation for therapeutic angiogenesis in patients with critical limb ischemia. Circ J 2007; 71: 196–201.
- 10.
Kawamoto A, Losordo DW. Endothelial progenitor cells for cardiovascular regeneration. Trends Cardiovasc Med 2008; 18: 33–37.
- 11.
Lara-Hernandez R, Lozano-Vilardell P, Blanes P, Torreguitart-Mirada N, Galmés A, Besalduch J. Safety and efficacy of therapeutic angiogenesis as a novel treatment in patients with critical limb ischemia. Ann Vasc Surg 2010; 24: 287–294.
- 12.
Powell RJ, Comerota AJ, Berceli SA, Guzman R, Henry TD, Tzeng E, et al. Interim analysis results from the RESTORE-CLI, a randomized, double-blind multicenter Phase II trial comparing expanded autologous bone marrow-derived tissue repair cells and placebo in patients with critical limb ischemia. J Vasc Surg 2011; 54: 1032–1041.
- 13.
Walter DH, Krankenberg H, Balzer JO, Kalka C, Baumgartner I, Schlüter M, et al. Intraarterial administration of bone marrow mononuclear cells in patients with critical limb ischemia: A randomized-start, placebo-controlled pilot trial (PROVASA). Circ Cardiovasc Interv 2011; 4: 24–37.
- 14.
Losordo DW, Kibbe MR, Mendelsohn F, Marston W, Driver VR, Sharafuddin M, et al. A randomized, controlled pilot study of autologous CD34+ cell therapy for critical limb ischemia. Circ Cardiovasc Interv 2012; 5: 821–830.
- 15.
Fujita Y, Kinoshita M, Furukawa Y, Nagano T, Hashimoto H, Hirami Y, et al. Phase II clinical trial of CD34+ cell therapy to explore endpoint selection and timing in patients with critical limb ischemia. Circ J 2014; 78: 490–501.
- 16.
Gupta PK, Chullikana A, Parakh R, Desai S, Das A, Gottipamula S, et al. A double blind randomized placebo controlled Phase I/II study assessing the safety and efficacy of allogeneic bone marrow derived mesenchymal stem cell in critical limb ischemia. J Transl Med 2013; 11: 143–153.
- 17.
Romagnani P, Annunziato F, Liotta F, Lazzeri E, Mazzinghi B, Frosali F, et al. CD14+CD34low cells with stem cell phenotypic and functional features are the major source of circulating endothelial progenitors. Circ Res 2005; 97: 314–322.
- 18.
Dal Pozzo S, Urbani S, Mazzanti B, Luciani P, Deledda C, Lombardini L, et al. High recovery of mesenchymal progenitor cells with non-density gradient separation of human bone marrow. Cytotherapy 2010; 12: 579–586.
- 19.
Scheffold A, Assenmacher M, Reiners-Schramm L, Lauster R, Radbruch A. High-sensitivity immunofluorescence for detection of the pro- and anti-inflammatory cytokines gamma interferon and interleukin-10 on the surface of cytokine-secreting cells. Nat Med 2000; 6: 107–110.
- 20.
Lenk K, Adams V, Lurz P, Erbs S, Linke A, Gielen S, et al. Therapeutical potential of blood-derived progenitor cells in patients with peripheral arterial occlusive disease and critical limb ischemia. Eur Heart J 2005; 26: 1903–1909.
- 21.
Huang P, Li S, Han M, Xiao Z, Yang R, Han ZC. Autologous transplantation of granulocyte colony-stimulating factor-mobilized peripheral blood mononuclear cells improves critical limb ischemia in diabetes. Diabetes Care 2005; 28: 2155–2160.
- 22.
Wagner FWJ. The dysvascular foot: A system for diagnosis and treatment. Foot Ankle 1981; 2: 64–122.
- 23.
Silvestro A, Bacchieri A, Bucur R, Di Donato AM, Costantino C, Corrado S, et al. A new questionnaire for measuring quality of life in patients with intermittent claudication. Minerva Cardioangiol 2000; 48: 455–465 (in Italian).
- 24.
Norgren L, Hiatt WR, Dormandy JA, Nehler MR, Harris KA, Fowkes FG. Inter-society consensus for the management of peripheral arterial disease. Int Angiol 2007; 312: 237–246.
- 25.
Dormandy JA, Rutherford RB. Management of peripheral arterial disease (PAD). TransAtlantic Inter-Society Consensus (TASC). J Vasc Surg 2000; 31: S1–S296.
- 26.
Duerschmied D, Zhou Q, Rink E, Harder D, Freund G, Olschewski M, et al. Simplified contrast ultrasound accurately reveals muscle perfusion deficits and reflects collateralization in PAD. Atherosclerosis 2009; 202: 505–512.
- 27.
Sun X, Ying J, Wang Y, Li W, Wu Y, Yao B, et al. Meta-analysis on autologous stem cell transplantation in the treatment of limb ischemic. Int J Clin Exp Med 2015; 8: 8740–8748.
- 28.
Compagna R, Amato B, Massa S, Amato M, Grande R, Butrico L, et al. Cell therapy in patients with critical limb ischemia. Stem Cells Int 2015; 2015: 931420.
- 29.
Arici V, Perotti C, Fabrizio C, Del Fante C, Ragni F, Alessandrino F, et al. Autologous immuno magnetically selected CD133+ stem cells in the treatment of no-option critical limb ischemia: Clinical and contrast enhanced ultrasound assessed results in eight patients. J Transl Med 2015; 13: 342.
- 30.
Huang PP, Yang XF, Li SZ, Wen JC, Zhang Y, Han ZC. Randomised comparison of G-CSF-mobilized peripheral blood mononuclear cells versus bone marrow-mononuclear cells for the treatment of patients with lower limb arteriosclerosis obliterans. Thromb Haemost 2007; 98: 1335–1342.
- 31.
Amato B, Compagna R, Della Corte GA, Martino G, Bianco T, Coretti G, et al. Peripheral blood mono-nuclear cells implantation in patients with peripheral arterial disease: A pilot study for clinical and biochemical outcome of neoangiogensis. BMC Surg 2012; 12(Suppl 1): S1.
- 32.
Hirsch AT, Haskal ZJ, Hertzer NR, Bakal CW, Creager MA, Halperin JL, et al. American Associations for Vascular Surgery/Society for Vascular Surgery, Society for Cardiovascular Angiography and Interventions, Society for Vascular Medicine and Biology, Society of Interventional Radiology, ACC/AHA Task Force on Practice Guidelines – summary of recommendations. J Vasc Interv Radiol 2006; 17: 1383–1397.
- 33.
Moazzami K, Moazzami B, Roohi A, Nedjar S, Dalmatova E. Local intramuscular injection of autologous mononuclear cells for critical lower limb ischemia. Cochrane Database Syst Rev 2014; 12: CD008347.
- 34.
Fadini GP, Avogaro A. Diabetes impairs mobilization of stem cells for the treatment of cardiovascular disease: A meta-regression analysis. Int J Cardiol 2013; 168: 892–897.
- 35.
Rookmaaker MB, Verhaar MC, Loomans CJ, Verloop R, Peters E, Westerweel PE, et al. CD34+ cells home, proliferate, and participate in capillary formation, and in combination with CD34− cells enhance tube formation in a 3-dimensional matrix. Arterioscl Thromb Vasc Biol 2005; 25: 1843–1850.
- 36.
Cooke JP, Losordo DW. Modulating the vascular response to limb ischemia angiogenic and cell therapies. Circ Res 2015; 116: 1561–1578.
- 37.
Franz RW, Shah KJ, Johnson JD, Pin RH, Parks AM, Hankins T, et al. Short- and mid-term results using autologous bone-marrow mononuclear cell implantation therapy as a limb salvage procedure in patients with severe peripheral arterial disease. Vasc Endovascular Surg 2011; 45: 398–406.
- 38.
Malyar NM, Radtke S, Malyar K, Arjumand J, Horn PA, Kröger K, et al. Autologous bone marrow mononuclear cell therapy improves symptoms in patients with end-stage peripheral arterial disease and reduces inflammation-associated parameters. Cytotherapy 2014; 16: 1270–1279.
- 39.
Van Tongeren RB, Hamming JF, Fibbe WE, Van Weel V, Frerichs SJ, Stiggelbout AM, et al. Intramuscular or combined intramuscular/intra-arterial administration of bone marrow mononuclear cells: A clinical trial in patients with advanced limb ischemia. J Cardiovasc Surg (Torino) 2008; 49: 51–58.
- 40.
Matoba S, Tatsumi T, Murohara T, Imaizumi T, Katsuda Y, Ito M, et al. Long-term clinical outcome after intramuscular implantation of bone marrow mononuclear cells in patients with chronic limb ischemia. Am Heart J 2008; 156: 1010–1018.
- 41.
Kamata Y, Takahashi Y, Iwamoto M, Matsui K, Murakami Y, Muroi K, et al. Local implantation of autologous mononuclear cells from bone marrow and peripheral blood for treatment of ischaemic digits in patients with connective tissue diseases. Rheumatology (Oxford) 2007; 46: 882–884.
- 42.
Liew A, O’Brien T. Therapeutic potential for mesenchymal stem cell transplantation in critical limb ischemia. Stem Cell Res Ther 2012; 3: 28.
- 43.
Schwarz TM, Leicht SF, Radic T, Rodriguez-Arabaolaza I, Hermann PC, Berger F, et al. Vascular incorporation of endothelial colony-forming cells is essential for functional recovery of murine ischemic tissue following cell therapy. Arterioscler Thromb Vasc Biol 2012; 32: 13–21.
- 44.
Kinnaird T, Stabile E, Burnett MS, Epstein SE. Bone-marrow-derived cells for enhancing collateral development: Mechanisms, animal data, and initial clinical experiences. Circ Res 2004; 95: 354–363.
- 45.
Ruiz-Salmeron R, de la Cuesta-Diaz A, Constantino-Bermejo M, Pérez-Camacho I, Marcos-Sánchez F, Hmadcha A, et al. Angiographic demonstration of neoangiogenesis after intra-arterial infusion of autologous bone marrow mononuclear cells in diabetic patients with critical limb ischemia. Cell Transplant 2011; 20: 1629–1639.
- 46.
Schiavetta A, Maione C, Botti C, Marino G, Lillo S, Garrone A, et al. A Phase II trial of autologous transplantation of bone marrow stem cells for critical limb ischemia: Results of the Naples and Pietra Ligure Evaluation of Stem Cells study. Stem Cells Transl Med 2012; 1: 572–578.
- 47.
Pignon B, Sevestre MA, Kanagaratnam L, Pernod G, Stephan D, Emmerich J, et al. Autologous bone marrow mononuclear cell implantation and its impact on the outcome of patients with critical limb ischemia: Results of a randomized, double-blind, placebo-controlled trial. Circ J 2017; 81: 1713–1720.