論文ID: CJ-17-0940
論文ID: CJ-17-0940
Background: Patients with intermediate-risk acute pulmonary embolism (APE) are a heterogeneous group with an early mortality rate of 2–15%. The tricuspid annulus plane systolic excursion (TAPSE) and tricuspid regurgitation peak gradient (TRPG) can be used for risk stratification, so we analyzed the prognostic value of a new echo parameter (TRPG/TAPSE) for prediction of APE-related 30-day death or need for rescue thrombolysis in initially normotensive APE patients.
Methods and Results: The study group consists of 400 non-high-risk APE patients (191 men, age: 63.1±18.9 years) who had undergone echocardiography within the first 24 h of admission. The TRPG/TAPSE parameter was calculated. The clinical endpoint (CE) was a combination of 30-day APE-related death and/or rescue thrombolysis. The CE occurred in 8 (2%) patients. All patients with TAPSE ≥20 mm (n=193, 48.2%) had a good prognosis. Among 206 patients with TAPSE <20 mm, 8 cases of the CE occurred (3.9%). NPV and PPV for TRPG/TAPSE >4.5 were 0.2 and 0.98, respectively. The CE was significantly more frequent in 19 (9.2%) patients with TRPG/TAPSE >4.5 than in 188 (90.8%) with TRPG/TAPSE ≤4.5 (4 (21.1%) vs. 4 (2.1%), P=0.0005). Among normotensive APE patients with TAPSE <20 mm, TRPG/TAPSE >4.5 was associated with 21.1% risk of APE-related death or rescue thrombolysis.
Conclusions: TRPG/TAPSE, a novel echocardiographic parameter, may be useful for stepwise echocardiographic risk stratification in normotensive patients with APE, and it identifies patients with a poor prognosis.
Acute pulmonary embolism (APE) is the most serious clinical presentation of venous thromboembolism (VTE). According to registries and hospital discharge databases of unselected patients with APE and VTE, 30-day all-cause mortality rates are between 9% and 10%.1–3 According to the recent European Society of Cardiology (ESC) guidelines on the diagnosis and treatment of APE patients, clinical classification of the severity of an episode of APE is based on the estimated 30-day APE-related mortality risk.4 Patients with cardiogenic shock caused by APE comprise a high-risk group for early death, which is estimated at more than 15%.4,5 Fortunately most APE patients are hemodynamically stable at admission but the early mortality risk is different in this population. Risk stratification of non-high-risk APE patients is based on clinical presentation, cardiac laboratory biomarkers, and signs of right ventricular (RV) dysfunction on echocardiography or computed tomography.4,6 Low-risk patients require a short hospital stay and can be early discharged home or even treated as outpatients.7 Intermediate-risk subjects comprise a very heterogeneous group in which the early mortality ranges between 2% and 15%.4 More of these patients stabilize hemodynamically during anticoagulation, but in some of them clinical deterioration occurs and therefore they may require rescue thrombolysis or surgical or percutaneous embolectomy. Echocardiography is a useful diagnostic tool to detected RV dysfunction. We have previously reported that tricuspid annulus plane systolic excursion (TAPSE) can be used for risk stratification of normotensive APE patients.8 The tricuspid regurgitation peak gradient (TRPG) is an echocardiographic sign of RV overload and it can also be used for risk stratification in APE.4,9,10 The aim of this study was to analyses the prognostic value of a new echocardiographic parameter, TRPG/TAPSE, for prediction of APE-related 30-day death or the need for rescue thrombolysis in initially normotensive APE patients.
The retrospective study group consisted of 400 consecutive patients diagnosed with APE in a single referral center during the period January 2013–December 2016. All patients were hemodynamically stable at admission with systolic blood pressure ≥90 mmHg and without signs of peripheral hypoperfusion. Pulmonary embolism was confirmed by contrast-enhanced multidetector computed tomography (MDCT) when thromboemboli were visualized in at least 1 segmental pulmonary artery. APE was diagnosed when symptoms of pulmonary embolism had been present for no longer than 14 days before diagnosis.
According to the ESC guidelines, low-risk APE was diagnosed when the simplified pulmonary embolism severity index (sPESI) was 0 and all RV dysfunction and myocardial injury markers were negative.4 Normotensive patients with sPESI ≥1 have intermediate-risk APE. Within this category further risk assessment was done. Patients with evidence of both RV dysfunction (by echocardiography or MDCT) and elevated cardiac biomarkers levels (particularly, a positive cardiac troponin test) were classified into the intermediate-high-risk category. Patients in whom RV was normal and/or had normal cardiac biomarkers levels formed the intermediate-low-risk group.4
Patients diagnosed with chronic thromboembolic hypertension and participants in therapeutic clinical trials were not included in this study.
EchocardiographyEchocardiographic examination was performed with a Philips iE 33 machine (Philips Medical System, USA) with 2.5–3.5 MHz transducers, within the first 24 h of admission, and results were digitally recorded. Patients were examined in the left lateral position. The dimensions of the right and left ventricles were measured in the parasternal long-axis view and in the RV focused view at the level of the mitral and tricuspid valve tips in late diastole, as defined by the R wave of continuous ECG tracing. After recording the tricuspid valve peak systolic velocity with continuous-wave Doppler echocardiography, TRPG was calculated using the simplified Bernoulli equation. Pulmonary ejection acceleration time (AcT) was measured using pulsed wave Doppler with the sample volume placed in the RV outflow tract just below the pulmonary valve. Measurements were averaged over 3 consecutive heart cycles. In M-mode presentation, RV function was assessed by TAPSE measurement. We measured the distance (mm) of the systolic excursion of the RV annular segment along its longitudinal plane in a standard apical 4-chamber view. Predefined RV dysfunction criteria have been published previously.8,11 The TRPG/TAPSE parameter was also calculated.
Left ventricular ejection fraction (LVEF) was calculated according to Simpson’s formula using a 2D image of the LV chamber during systole and diastole in the 4- and 2-chamber apical views.12
The agreement analysis showed high interclass and intraclass correlations for Doppler 2D or M-mode data parameters, which have been published previously.10
Clinical Endpoint (CE) of the StudyThe CE of the study was defined as a combination of 30-day APE-related death and/or rescue thrombolysis in patients with hemodynamic deterioration, which was defined by the occurrence of at least one of the following: (1) need for cardiopulmonary resuscitation; (2) systolic BP <90 mmHg for at least 15 min, with signs of end-organ hypoperfusion; or (3) need for intravenous catecholamines in vasopressor doses.
This observational study was approved by the local ethics committee.
Statistical AnalysisThis was a single-center retrospective study. Normal distribution of the analyzed parameters was verified by the Shapiro-Wilk test. Data characterized by a normal distribution are presented as mean and standard deviation, and comparisons between groups were performed using Student’s t-test. Parameters with non-normal distribution are expressed as median and minimum-maximum ranges. For comparison of parameters between groups with such a distribution, the Mann-Whitney U-test was used. The Chi-square test was used for comparisons of discrete values (with Yates’s correction when needed). Positive (PPV) and negative prognostic values (NPV) were calculated for the chosen cut-off values. Receiver-operating curves (ROC) were used for assessment of optimal cut-off values for prediction of the CE. Comparison of the areas under the curve (AUC) was made pairwise according to DeLong et al.13
A Cox proportional hazard risk model was used for univariable and multivariable prediction of outcome in the analyzed group of patients. We decided to include TRPG and RV/LV in the multivariable model without selecting a P-value criterion in the univariable analysis because of the potential role in prediction of APE patients outcome presented in past publications.4,6,14,15 TRPG/TAPSE, TAPSE, TRPG and RV/LV were included as dichotomous parameters in the univariable and multivariable Cox proportional hazard risk models. The cut-off values for TRPG and RV/LV were chosen as 30 mmHg and 0.9, respectively, according to the European Society of Cardiology guidelines.4 Net reclassification index with a cut-off value of risk category specified as 20% was computed for comparison between univariate proportional hazard risk models. Kaplan-Meier curves were used to present 30-day APE-related death and/or rescue thrombolysis.
All tests were 2-sided. Differences between groups were considered as statistically significant with P<0.05. All calculations were performed using STATISTICA data analysis software system (2011, version 10, StatSoft, Tulsa, OK, USA).
The study group consisted of 400 consecutive patients with APE (191 men, 209 women, mean age: 63.1±18.9 years, median 66 (20–101) years). Intermediate-risk APE was diagnosed in 298 patients (58 intermediate-low risk, 240 intermediate-high risk), and low-risk APE in 102 patients. Initially, all patients received body mass-adjusted low-molecular-weight heparin, activated partial thromboplastin time-adjusted unfractionated heparin intravenous infusion or rivaroxaban 15 mg twice daily.
Rescue thrombolysis was performed in 3 (0.75%) intermediate-risk APE patients, and 2 of them survived. The 30-day APE-related mortality rate was 1.5% (6 patients) and the all-cause mortality rate was 3.75% (15 patients). The 9 non-APE related deaths were from neoplasm (3 patients), pneumonia (3 patients), aortic stenosis (1 patient), lower gastrointestinal tract hemorrhage (1 patient), and respiratory failure (1 patients).
The CE, which included APE-related death (n=6, 1.5%) and/or thrombolysis (n=3, 0.75 %), occurred in 8 (2%) patients.
The clinical characteristics of the APE subjects are provided in Table 1. Patients who experienced the CE were older, and had a higher serum NT-proBNP level than subjects with a favorable clinical course.
| All patients (n=400) |
APE-related death and thrombolysis (n=8) |
Remaining patients (n=392) |
P value | |
|---|---|---|---|---|
| Sex, F/M | 209/191 | 6/2 | 203/189 | 0.35 |
| Age, years | 66 (20–101) | 79 (37–87) | 66 (20–101) | 0.03 |
| HR, 1 beat/s | 85 (37–180) | 100 (74–160) | 85 (37–180) | 0.09 |
| Systemic BP, mmHg | 130 (90–186) | 117 (90–160) | 130 (90–186) | 0.23 |
| Comorbidities (COPD, CHF, neoplasm), % | 31.75% | 0% | 32.4% | 0.12 |
| Troponin, ng/mL | 0.035 (0–116) | 0.045 (0.02–0.98) | 0.035 (0–116) | 0.39 |
| Troponin positive, %* | 64% | 100% | 63% | 0.08 |
| NT-proBNP, pg/mL | 748.5 (16–29071) | 9511 (2043–25000) | 715.8 (16–29071) | <0.001 |
| sPESI | 0 (0–4) | 1 (0–3) | 0 (0–4) | 0.27 |
*Troponins considered positive at levels >0.01 ng/mL. APE, acute pulmonary embolism; BP, blood pressure; CHF, congestive heart failure; COPD, chronic obstructive pulmonary disease; HR, heart rate; NT-proBNP, N-terminal pro-B-type natriuretic peptide; sPESI, simplified pulmonary embolism severity index.
Echocardiographic parameters of the study population are summarized in Table 2. The mean value of TAPSE was lower in APE patients with the CE than in the others.
| All patients (n=400) |
APE-related death and thrombolysis (n=8) |
Remaining patients (n=392) |
P value | |
|---|---|---|---|---|
| RV4C, mm | 38.5 (21–65) | 37 (23–55) | 39 (21–65) | 0.87 |
| LV4C mm | 42 (25–72) | 41.5 (32–54) | 42 (25–72) | 0.97 |
| RV/LV 4C | 0.89 (0.49–2.5) | 0.9 (0.53–1.67) | 0.9 (0.49–2.5) | 0.80 |
| AcT, ms | 80 (34–166) | 73.5 (45–111) | 80 (34–166) | 0.32 |
| TRPG, mmHg | 33 (7–90) | 44.5 (23–56) | 33 (7–90) | 0.09 |
| TAPSE, mm | 20.4±5.3 | 11.5 (6–20) | 20 (5–34) | <0.0001 |
AcT, acceleration time; LV4C, left ventricle 4-chamber diameter; RV4C, right ventricle 4-chamber diameter; TAPSE, tricuspid annulus plane systolic excursion; TRPG, tricuspid regurgitation peak gradient. Other abbreviation as in Table 1.
ROC analysis showed that the AUC for TAPSE in the prediction of the CE was (0.94, 95% confidence interval (CI) 0.8–1.0).
We defined the cut-off value of TAPSE as >20 mm with 100% NPV for the CE. Therefore, all patients with TAPSE >20 mm (193 patients, 48.25%) comprised a low-risk group with a good prognosis. In the group of 207 patients with TAPSE ≤20 mm, 8 cases of the CE were observed (3.9%).
As a second echocardiographic step for risk stratification we chose the TRPG/TAPSE parameter. ROC analysis revealed significantly higher AUC for TRPG/TAPSE than for RV/LV and TRPG alone (0.74 vs. 0.41, P=0.009, 0.74 vs. 0.57, P<0.0001) in 207 APE patients with TAPSE ≤20 mm. There was no significant difference between the ROC AUC for TAPSE and TRPG/TAPSE in patients with TAPSE ≤20 mm: respectively, 0.811, (95% CI: 0.6009–1) and 0.7372, (95% CI: 0.5146–0.9597); P=0.06. The ROC curves for TRPG/TAPSE, RV/LV and TRPG are shown in Figure 1.

Receiver-operating characteristic curves for TRPG/TAPSE, RV/LV and TRPG for the clinical endpoint in 207 APE patients with TAPSE ≤20 mm. LV4C, left ventricle 4-chamber diameter; NPV, negative predictive value; PPV, positive predictive value; RV4C, right ventricle 4-chamber diameter; TAPSE, tricuspid annulus plane systolic excursion; TRPG, tricuspid regurgitation peak gradient.
We identified that the PPV and NPV for TRPG/TAPSE >4.5 mmHg/mm were 0.2 and 0.98, respectively, in this group of patients. The CE was significantly more frequent in the 19 (9.2%) patients with TRPG/TAPSE >4.5 than in the 188 (90.8%) with TRPG/TAPSE ≤4.5 mmHg/mm (4 (21.1%) vs. 4 (2.1%), P=0.0005).
The algorithm of risk stratification based on echocardiography is presented in Figure 2.
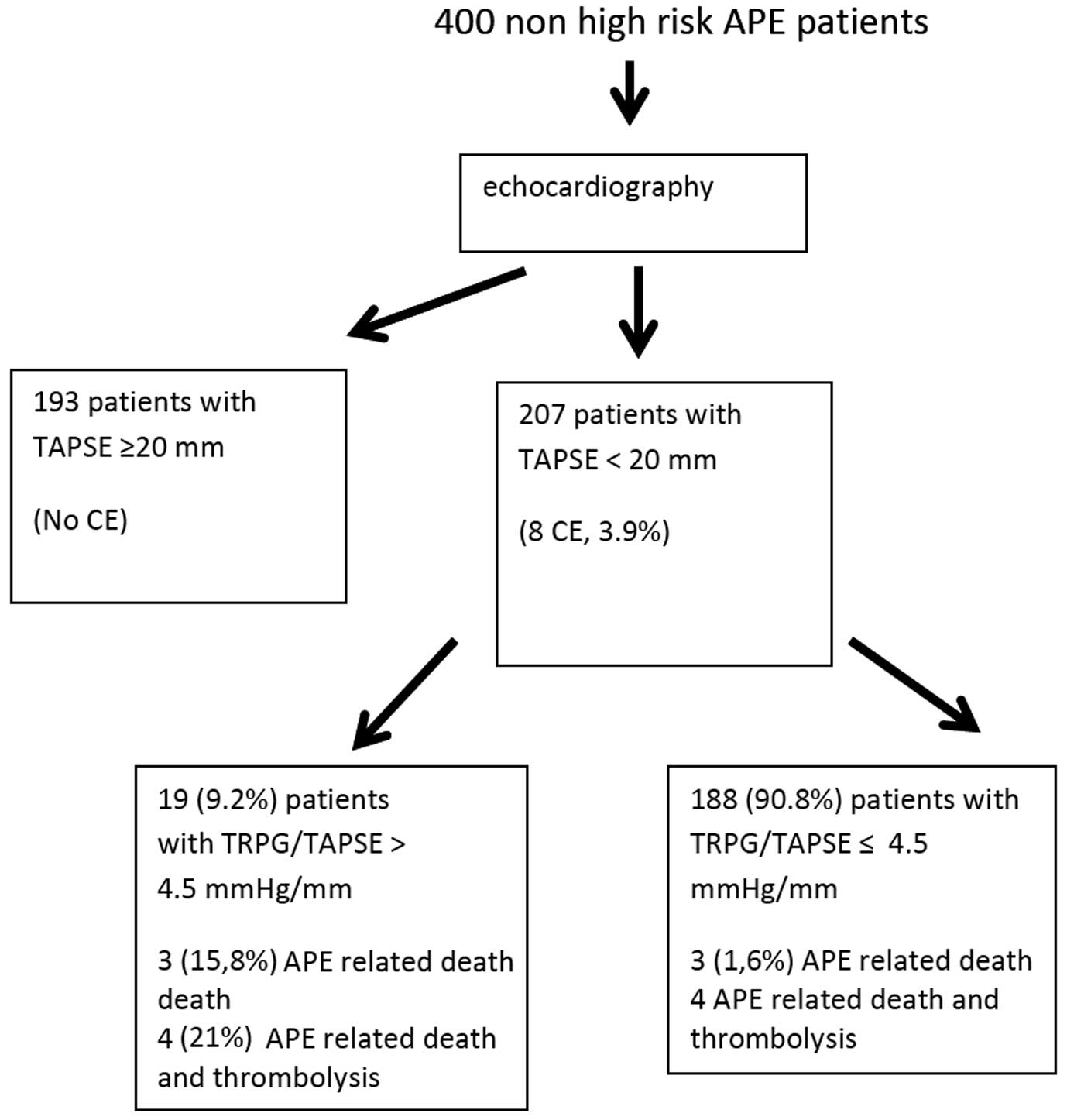
Algorithm of risk stratification based on echocardiography in normotensive patients with acute pulmonary embolism (APE). CE, clinical endpoint. Other abbreviations as in Figure 1.
We found that NT-proBNP, TRPG and TRPG/TAPSE was significantly higher, and that TAPSE, and AcT were significantly lower in patients with TRPG/TAPSE >4.5 mmHg/mm than in patients with TRPG/TAPSE ≤4.5 mmHg/mm. The comparison between these groups is shown in Table 3.
| Patients with TRPG/TAPSE ≤4.5 (n=188) |
Patients with TRPG/TAPSE >4.5 (n=19) |
P value | |
|---|---|---|---|
| Sex, F/M | 108/80 | 11/8 | 0.95 |
| Age, years | 70 (20–100) | 73 (22–86) | 0.85 |
| HR, 1/s | 88 (60–180) | 92 (66–180) | 0.52 |
| Systemic BP, mmHg | 127.5±19.6 | 132.2±15.7 | 0.55 |
| Troponin, ng/mL | 0.063 (0–112) | 0.05 (0–109) | 0.45 |
| Troponin positive, %* | 71% | 74% | 0.84 |
| NT-proBNP, pg/mL | 1957 (20–29071) | 6209 (1694–26685) | <0.001 |
| RV4C, mm | 40±8.1 | 42.4±8.5 | 0.1 |
| LV4C mm | 41 (25–72) | 39 (27–62) | 0.4 |
| RV/LV4c | 0.97 (0.5–2.5) | 1.1 (0.61–1.9) | 0.18 |
| AcT, ms | 74 (36–160) | 58 (37–85) | <0.001 |
| TRPG, mmHg | 36 (7–86) | 58 (37–90) | <0.001 |
| TAPSE, mm | 17 (6–19) | 11 (5–18) | <0.001 |
| TRPG/TAPSE, mmHg/mm | 2.3 (0.4–4.5) | 5.2 (4.6–8.7) | <0.001 |
*Troponins considered positive at levels >0.01 ng/mL. HR, heart rate. Other abbreviations as in Tables 1,2.
We performed univariable Cox proportional hazards regression analysis for TRPG, TAPSE, RV/LV and TRPG/TAPSE as dichotomous parameters using cut-off values of 30 mmHg, 14 mm, 0.9 and 4.5, respectively. Using the ROC, TAPSE=14 mm was chosen as the point with highest sensitivity and specificity (0.75 and 0.835). In the univariable Cox analysis, only TRPG/TAPSE and TAPSE predicted the clinical outcome with respective hazard ratios of 11.4 (95% CI: 2.8–45.6, P<0.0006) and 0.07 (95% CI: 0.01–0.35, P=0.0012) (Table 4). Univariable Cox proportional hazard risk models including TRPG/TAPSE (as new model) and TAPSE (as standard model) were compared using the net reclassification index (NRI) with cut-off value of risk category specified as 20%. NRI was 0.425 with NRI(event)=0.5 and NRI(nonevent)=−0.075. Thus the univariable Cox risk model including TRPG/TAPSE was considered as better than the univariable Cox model with TAPSE in prediction of the CE in a preselected population with TAPSE ≤20 mm.
| Parameter | Hazard ratio, 95% CI |
P value |
|---|---|---|
| TRPG/TAPSE | 11.4, 2.8–45.6 | <0.0006 |
| TAPSE | 0.07, 0.01–0.35 | 0.0012 |
| TRPG | 2.8, 0.4–22.9 | 0.33 |
| RV/LV | 1.4, 0.2–2.7 | 0.6 |
CI, confidence interval. Other abbreviations as in Table 2.
In the multivariable proportional hazard Cox regression model we included TRPG, RV/LV, TAPSE and TRPG/TAPSE using the cut-off values from the univariable analysis. In the multivariable proportional hazard Cox model only TAPSE was significant and TRPG/TAPSE was borderline significant (Table 5).
| Parameter | Hazard ratio, 95% CI |
P value |
|---|---|---|
| TRPG/TAPSE | 4.438, 0.981–20.065 | 0.052 |
| TAPSE | 0.118, 0.02–0.676 | 0.016 |
Abbreviations as in Tables 2,4.
Among normotensive APE patients with TAPSE ≤20 mm, TRPG/TAPSE >4.5 mmHg/mm was associated with 21.1% risk of APE-related death or rescue thrombolysis. Figure 3 shows the Kaplan-Meier curves according to TRPG/TAPSE in 207 patients with TAPSE ≤20 mm.

Kaplan-Meier curves according to TRPG/TAPSE in 207 patients with TAPSE ≤20 mm. Abbreviations as in Figure 1.
Non-high-risk patients with APE are a very heterogeneous group. Some of them demonstrate rapid improvement, and can be discharged home early; in others, hemodynamic collapse occurs and they need treatment escalation.14,16 Echocardiography should be performed in all intermediate-risk APE patients for risk stratification, and it can be done in low-risk patients.4 In our study, we tried to perform risk stratification in normotensive APE patients according to TAPSE and a newly proposed echocardiographic parameter TRPG/TAPSE, first assessed in the APE population. Previously, Guazzi et al evaluated a similar TAPSE/PASP (pulmonary arterial systolic pressure) parameter in 293 patients with heart failure, which showed that the TAPSE/PASP relationship shifted downward in nonsurvivors.17 In a group of 1,663 patients with heart failure, Ghio et al demonstrated that TAPSE/PASP was a powerful predictor of prognosis.18 Other studies have confirmed the prognostic significance of TAPSE/PASP parameter in patients with heart failure.19,20
TAPSE is a well-known, quick, and easily measurable echocardiographic parameter that does not require sophisticated equipment or prolonged image analysis.21 It predominantly reflects RV longitudinal function. TAPSE has shown good correlation with parameters estimating global RV systolic function, such as the radionuclide-derived RV ejection fraction or on cardiac magnetic resonance.21–24
In a study by Schmid et al that included 81 patients with APE who underwent pulmonary embolectomy, TAPSE <18 mm was an independent predictor of intraoperative cardiopulmonary resuscitation and death.25
According to the recent Recommendations for Cardiac Chamber Quantification by Echocardiography in Adults, TAPSE <17 mm is highly suggestive of RV systolic dysfunction.12 Our group has already reported that TAPSE is a useful parameter for risk stratification in normotensive APE patients, and TAPSE >20 mm can be used for identification of very low-risk patients.8,11
In the current study we tried to more precisely risk-stratify normotensive APE patients using a stepwise echocardiographic approach. As the first step, we propose TAPSE assessment. Among 400 non-high-risk APE patients, 193 had TAPSE >20 mm, and in this group the CE did not occur. All cases of the CE were observed in patients with TAPSE ≤20 mm. Subsequently, we attempted to further differentiate the risk in these patients using TRPG/TAPSE, a new echo parameter.
The method of choice for noninvasive estimation of systolic pulmonary artery pressure is based on continuous wave Doppler measurement of TRPG. This method is based on the Bernoulli equation and has a strong pathophysiologic rationale.4,26,27 In APE patients with RV overload, an increase in TRPG and a decrease in TAPSE should be observed. ROC analysis showed significantly higher AUC for TRPG/TAPSE than for RV/LV and TRPG alone (0.74 vs. 0.41, P=0.009, 0.74 vs. 0.57, P<0.0001) in 207 APE patients with TAPSE ≤20 mm. Therefore, we used TRPG/TAPSE for assessing RV overload in normotensive APE patients and for risk stratification.
We identified that the NPV and PPV for TRPG/TAPSE >4.5 mmHg/mm were 0.98 and 0.2, respectively. Four cases of the CE (21.1%) occurred in this group of patients whereas in patients with TRPG/TAPSE ≤4.5 mmHg/mm the frequency of the CE was significantly lower (4, 2.1%, P=0.0005). Therefore, we proposed a new, simple echocardiographic approach to risk stratification in normotensive APE patients. Patients with TAPSE >20 mm are a very low risk group and thus can be quickly discharged or even treated at home. In contrast, if TAPSE is <20 mm, TRPG/TAPSE >4.5 mmHg/mm identifies patients at a higher risk who should be monitored for hemodynamic deterioration. Thus the model of TRPG/TAPSE was better for prediction of the CE than TAPSE in a preselected population with TAPSE <20 mm.
We also considered factors that could influence our results. It is well known that age and comorbidities, particularly malignant diseases, affect the outcome in patients with APE.28,29 In our study, patients in the CE group were older than the others, which was consistent with results in the literature.30,31 On the other hand, the sPESI score was similar between CE patients and the good prognosis group. The all-cause mortality rate in our patients was 3.75% (15 patients). Beside APE, the causes of death were comorbidities, especially cancers. All study patients were hemodynamically stable at admission, so all of them were initially treated with anticoagulation.
Clinical ImplicationsWe think that a simple echocardiographic approach to risk stratification in non-high-risk APE patients has potential clinical value. As already known, patients with TAPSE >20 mm have a benign clinical course. On the other hand, approximately 50% of patient have TAPSE ≤20 mm, and in this group TRPG/TAPSE, a novel echocardiographic parameter, can help identify patients with approximately 20% risk of death or hemodynamic deterioration. These patients should be closely monitored and may need treatment escalation.
Study LimitationsThis was a single-center, retrospective study, so our conclusions should be interpreted with caution. Our findings need validation in an external cohort of APE patients, especially the usefulness of the cut-off value 4.5 mmHg/mm for TRPG/TASPE. Moreover, the number of cases of the CE was small, and causes of death were not adjudicated.
TRPG/TAPSE, a novel echocardiographic parameter, may be useful for stepwise echocardiographic risk stratification of normotensive patients with APE, and it identifies patients with a poor prognosis.
None.