論文ID: CJ-17-0959
論文ID: CJ-17-0959
Background: The clinical characteristics associated with elevated right atrial pressure (RAP) in hypertrophic cardiomyopathy (HCM) are unknown. Few data exist as to whether elevated RAP has prognostic implications in patients with HCM. This study investigated the clinical correlates and prognostic value of elevated RAP in HCM.
Methods and Results: This retrospective cohort study was performed on 180 patients with HCM who underwent right heart catheterization between 1997 and 2014. Elevated RAP was defined as >8 mmHg. Baseline characteristics, mean pulmonary artery pressure, and mean pulmonary capillary wedge pressure (PCWP) were assessed for association with elevated RAP. The predictive value of elevated RAP for all-cause mortality and the development of atrial fibrillation (AF), ventricular tachycardia/fibrillation (VT/VF), and stroke was evaluated. Elevated RAP was associated with higher New York Heart Association class, dyspnea on exertion, orthopnea, edema, jugular venous distention, larger left atrial size, right ventricular hypertrophy, higher pulmonary artery pressure, and higher PCWP. RAP independently predicted all-cause mortality (adjusted hazard ratio [aHR] 2.18 per 5-mmHg increase, 95% confidence interval [CI] 1.05–4.50, P=0.04) and incident AF (aHR 1.85 per 5-mmHg increase, 95% CI 1.20–2.85, P=0.005). Elevated RAP did not predict VT/VF (P=0.36) or stroke (P=0.28).
Conclusions: Elevated RAP in patients with HCM is associated with left-sided heart failure and is an independent predictor of all-cause mortality and new-onset AF.
Hypertrophic cardiomyopathy (HCM) is one of the most common genetic cardiac diseases, affecting approximately 1 in 500 individuals.1 Manifestations of the disease include heart failure (HF) associated with left ventricular diastolic dysfunction, atrial fibrillation (AF), ventricular tachycardia/fibrillation (VT/VF), stroke, and sudden cardiac death. HCM is the most common cause of sudden death in the young population.2 It is well known that morphological and functional alternations of the left ventricle are associated with adverse outcomes in the HCM population.3 It has been reported that patients with HCM may have diffusely increased right ventricular wall thickness and a greater prevalence of right ventricular systolic dysfunction than healthy controls.4–6 Evidence of right ventricular diastolic dysfunction, such as impaired relaxation and delayed filling, may also be present in patients with HCM.7–9 In non-HCM HF populations, it is well known that right ventricular dysfunction is associated with increased mortality and AF burden.10–14 Unlike in other forms of HF, in which the most common cause of elevated right atrial pressure (RAP) is known to be left-sided HF,15,16 few data exist with regard to the clinical correlates of elevated RAP in the HCM population. Furthermore, little is known as to whether the presence of elevated RAP predicts unfavorable outcomes in this patient population. Therefore, the aims of the present study were to identify clinical characteristics associated with elevated RAP and to determine the predictive value of elevated RAP for all-cause mortality and development of morbidity related to HCM (e.g., AF, VT/VF, and stroke).
The present single-center retrospective cohort study was performed using data from patients with HCM followed in the Hypertrophic Cardiomyopathy Program of Massachusetts General Hospital who underwent right heart catheterization between 1997 and 2014. The diagnosis of HCM was established on the basis of echocardiographic evidence of left ventricular hypertrophy (left ventricular wall thickness ≥15 mm) out of proportion to the degree of systemic loading condition, a non-dilated left ventricle, and no other disease capable of producing similar findings (i.e., HCM phenocopies, such as Fabry disease and cardiac amyloidosis).17 If an HCM phenocopy was suspected, appropriate tests, such as cardiac magnetic resonance imaging and/or genetic testing, were performed.17
Data Collection at BaselineData were obtained on baseline patient characteristics, including age, sex, symptoms, and comorbidities, at the time of right heart catheterization by chart review. New York Heart Association (NYHA) class and specific symptoms, including dyspnea on exertion, paroxysmal nocturnal dyspnea, orthopnea, edema, chest pain, palpitations, dizziness, and syncope, were tabulated. Medical comorbidities were collected at the time of right heart catheterization and included hypertension, diabetes mellitus, coronary artery disease, end-stage renal disease requiring hemodialysis, chronic obstructive pulmonary disease (COPD) or asthma, sleep apnea, other intrinsic lung disease, AF (defined as either atrial fibrillation or atrial flutter), VT/VF, and stroke. Baseline objective data included N-terminal pro B-type natriuretic peptide (NT-proBNP), the presence of pulmonary edema on the most recent chest X-ray, left atrial diameter on transthoracic echocardiography, and the presence of right ventricular hypertrophy (wall thickness >5 mm) on transthoracic echocardiography or cardiac magnetic resonance imaging. Cardiac catheterization data were reviewed to determine each patient’s mean RAP, mean pulmonary artery pressure (mPAP), pulmonary capillary wedge pressure (PCWP), and cardiac index. Patients with RAP >8 mmHg were considered to have elevated RAP.18
Clinical Management and Right Heart CatheterizationPatients presenting with HCM and symptoms were managed according to a standard algorithm published previously.19,20 The indication for right heart catheterization was determined according to clinical necessity (e.g., determining cardiac vs. pulmonary cause of dyspnea, assessment of ventricular filling pressures and pulmonary artery pressures). Right heart catheterization was performed under conscious sedation and local anesthesia in the cardiac catheterization laboratory. A 7-Fr introducer was inserted into either the right internal jugular or femoral vein. A 7-Fr balloon-tipped catheter was introduced via the venous sheath, the balloon was inflated, the catheter was advanced through the right heart chambers to the pulmonary capillary wedge position, and pressures were measured. Cardiac output was measured using the thermodilution technique. Data obtained through right heart catheterization were used to optimize treatment (e.g., titration of diuretic).
Outcome MeasuresThe primary endpoint was death from any cause. The secondary endpoints were incident AF, VT/VF, and stroke among patients without a prior history of the endpoint at baseline. Development of new-onset AF and VT/VF was determined by 12-lead or continuous electrocardiogram, device interrogation, or telemetry monitoring. Follow-up was performed at routine visits in the office supplemented by intermittent telephone interviews. Outcome data were extracted by an investigator (Y.J.S.) blinded to baseline characteristics and cardiac catheterization data.
Statistical AnalysisContinuous values are presented as the mean±SD if normally distributed, and as the median [interquartile range] if not. Unpaired Student’s t-test assuming unequal variances was used for continuous variables, whereas the Chi-squared test or Fisher’s exact test was used for categorical variables. Patients with elevated RAP were compared to those without elevated RAP using the log-rank test for the development of the primary and secondary endpoints. All potential predictors listed in Table 1 were tested for univariable association with the endpoints using the Cox proportional hazards model. Time 0 for the survival analysis was the time of right heart catheterization. All univariable predictors with a P value <0.05 were included in the multivariable model and analyzed in a backward stepwise fashion with a significance level for removal from the model of 0.05. This threshold was set low in order to limit the number of variables included in the final multivariable model to address the possibility of overfitting. Additional analyses were performed by forcing PCWP in and excluding RAP from the model. To investigate whether right ventricular hypertrophy is due to a direct effect of HCM or elevated mPAP, another analysis was performed to compare mPAP between patients with and without right ventricular hypertrophy. Stata Statistical Software Release 12 (StataCorp LP, College Station, TX, USA) was used for the analysis and P<0.05 was considered significant; all confidence intervals (CIs) are reported as 2-sided values with a confidence level of 95%.
| Characteristics | Elevated RAP (n=51) |
No elevated RAP (n=129) |
P value |
|---|---|---|---|
| Age (years) | 64±15 | 68±14 | 0.13 |
| Females | 17 (33) | 56 (43) | 0.22 |
| NYHA class | 3 [2–3] | 2 [2–3] | 0.002 |
| Dyspnea on exertion | 47 (92) | 91 (71) | 0.002 |
| Paroxysmal nocturnal dyspnea | 4 (8) | 5 (4) | 0.27 |
| Orthopnea | 10 (20) | 5 (4) | 0.001 |
| Edema | 21 (41) | 31 (24) | 0.02 |
| Chest pain | 27 (53) | 54 (42) | 0.18 |
| Palpitations | 9 (18) | 25 (19) | 0.79 |
| Dizziness | 12 (24) | 43 (33) | 0.20 |
| Syncope | 5 (10) | 9 (7) | 0.52 |
| Hypertension | 38 (75) | 84 (65) | 0.16 |
| Diabetes mellitus | 9 (18) | 23 (18) | 0.98 |
| Coronary artery disease | 32 (63) | 80 (62) | 0.81 |
| COPD or asthma | 11 (22) | 18 (14) | 0.26 |
| Sleep apnea | 5 (10) | 17 (13) | 0.62 |
| Other intrinsic lung disease* | 5 (10) | 7 (5) | 0.33 |
| Prior AF | 23 (45) | 39 (30) | 0.06 |
| Prior VT/VF | 11 (22) | 28 (22) | 0.98 |
| Prior stroke | 5 (10) | 8 (6) | 0.40 |
| β-blockers | 44 (86) | 108 (84) | 0.47 |
| Non-dihydropyridine calcium channel blocker | 12 (24) | 23 (18) | 0.35 |
| Disopyramide | 4 (8) | 19 (15) | 0.23 |
| Diuretic | 27 (53) | 49 (38) | 0.052 |
| Antiarrhythmic | 8 (16) | 10 (8) | 0.10 |
| JVD | 13 (25) | 16 (12) | 0.03 |
| NT-proBNP (pg/mL) | 2,108 [691–3,777] | 1,805 [512–3,919] | 0.84 |
| AST (U/L) | 46±50 | 34±27 | 0.007 |
| ALT (U/L) | 72±290 | 35±73 | 0.37 |
| Pulmonary edema on chest X-ray | 16 (31) | 23 (18) | 0.06 |
| Left atrial size (mm) | 48±8 | 44±7 | 0.006 |
| Interventricular septum thickness (mm) | 19±5 | 18±4 | 0.29 |
| Posterior wall thickness (mm) | 13±3 | 13±3 | 0.37 |
| Maximum wall thickness (mm) | 19±5 | 19±4 | 0.35 |
| LV outflow tract gradient at rest (mmHg) | 50 [14–80] | 49 [11–85] | 0.96 |
| Degree of mitral regurgitation** | 2.5 [1.5–3] | 2.5 [2–3] | 0.93 |
| RVH | 14 (27) | 15 (12) | 0.009 |
| RAP (mmHg) | 12±4 | 5±2 | <0.001 |
| PAP (mmHg) | 34±10 | 23±9 | <0.001 |
| PCWP (mmHg) | 21±6 | 14±6 | <0.001 |
| Cardiac index (L·min−1·m−2) | 2.6±0.6 | 2.6±0.7 | 0.51 |
Data are given as the mean±SD, n (%), or as the median [interquartile range]. *Includes restrictive lung disease (n=1), bronchiolitis obliterans organizing pneumonia (n=1), interstitial lung disease (n=4), and status after lung resection and/or radiation therapy (n=6). **Degree of mitral regurgitation was converted to numerical values according to the following rule: trace=1, trace to mild=1.5, mild=2, mild to moderate=2.5, moderate=3, moderate to severe=3.5, severe=4. AF, atrial fibrillation; ALT, alanine transaminase; AST, aspartate transaminase; COPD, chronic obstructive pulmonary disease; JVD, jugular venous distension; LV, left ventricular; NT-proBNP, N-terminal pro B-type natriuretic peptide; NYHA, New York Heart Association; PAP, pulmonary artery pressure; PCWP, pulmonary capillary wedge pressure; RAP, right atrial pressure; RVH, right ventricular hypertrophy; VT/VF, ventricular tachycardia/fibrillation.
Overall, 180 patients who underwent right heart catheterization were identified, with median follow-up of 7.0 years. Mean age was 67±14 years and 41% of patients were women (Table 1). Of the 180 patients, 51 (28%) had elevated RAP. The distribution of RAP is shown in Figure 1. Urgency of right heart catheterization was categorized as elective in 124 patients (69%), urgent in 23 patients (13%), and emergent in 33 patients (18%). One patient had decreased right ventricular systolic function and 7 had a dilated right ventricle. With regard to the use of antiarrhythmic agents, 23 patients (13%) were on disopyramide, 16 (9%) were on amiodarone, 1 was on dronedarone, and 1 was on sotalol at the time of right heart catheterization.
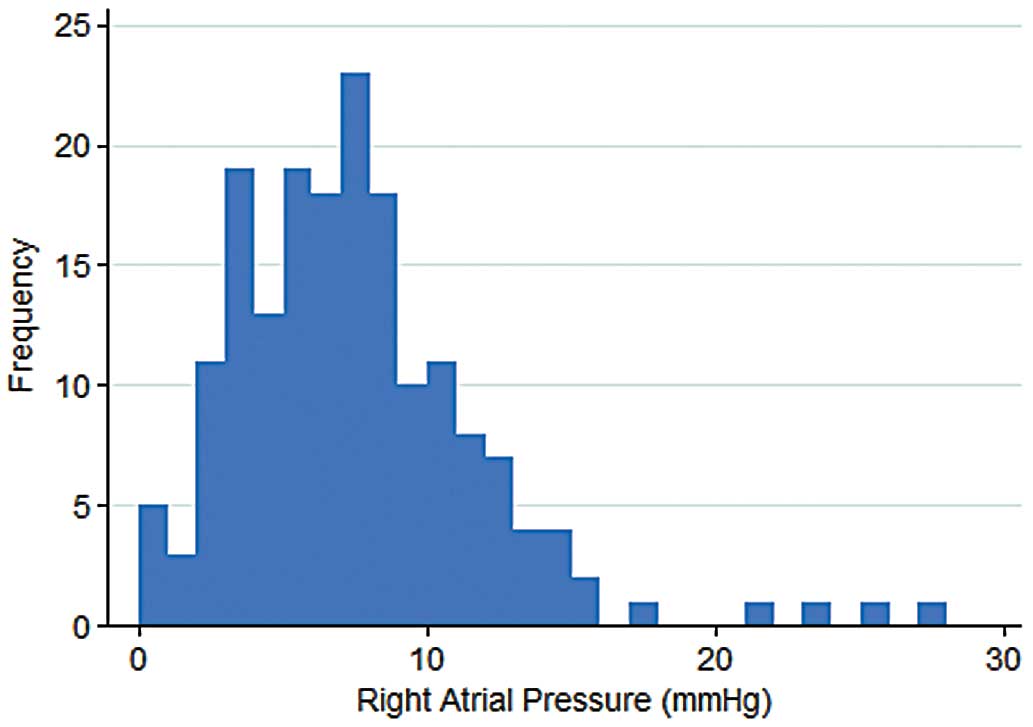
Distribution of right atrial pressure in patients with hypertrophic cardiomyopathy who underwent right heart catheterization.
Patients with elevated RAP had higher NYHA class and were more likely to have dyspnea on exertion, orthopnea, edema, and jugular venous distention than patients without elevated RAP (Table 1). There were no significant differences in the proportion of patients on disopyramide or other antiarrhythmic agents between the 2 groups (Table 1). Patients with elevated RAP had larger left atrial size (48±8 vs. 44±7 mm; P=0.006), higher mPAP (34±10 vs. 23±9 mmHg; P<0.001), and higher PCWP (21±6 vs. 14±6 mmHg; P<0.001) than patients without elevated RAP (Table 1). Right ventricular hypertrophy was present more frequently in patients with elevated RAP (27% vs. 12%; P=0.009) and was associated with higher mPAP (31±10 vs. 26±10 mmHg; P=0.01). There was a trend towards a higher prevalence of AF at baseline among patients with elevated RAP (45% vs. 30%; P=0.06; Table 1). The prevalence of prior VT/VF and prior stroke was similar in patients with and without elevated RAP (Table 1).
MortalityFrom the time of right heart catheterization onward, 13 patients experienced the primary endpoint of death from any cause. Of the patients with elevated RAP, 6 died (12%): 2 from HF, 2 from multisystem organ failure, 1 from an intracranial hemorrhage, and 1 from pulseless electrical activity without a known precipitating factor. Of patients without elevated RAP, 7 died (5.4%): 3 from HF, 2 from multisystem organ failure, 1 from sepsis due to cholecystitis, and 1 with an unknown cause of death. Elevated RAP predicted all-cause mortality (P=0.046, log-rank test; Figure 2). Univariable predictors of mortality were age, COPD or asthma, antiarrhythmic therapy, jugular venous distension, NT-proBNP, aspartate transaminase, pulmonary edema on chest X-ray, left atrial size, mPAP, PCWP, and RAP (hazard ratio [HR] 2.24 per 5-mmHg increase, 95% CI 1.50–3.36, P<0.001; Table 2). In multivariable analysis, NT-proBNP, pulmonary edema on chest X-ray, and RAP (adjusted HR 2.18 per 5-mmHg increase, 95% CI 1.05–4.50, adjusted P=0.04) independently predicted all-cause mortality (Table 2). In contrast, in the additional analysis forcing PCWP into the model and excluding RAP, the association between all-cause mortality and PCWP was not significant after adjusting for other factors that remained through the stepwise elimination method (adjusted HR 1.36 per 5-mmHg increase in PCWP, 95% CI 0.83–2.24, adjusted P=0.22).
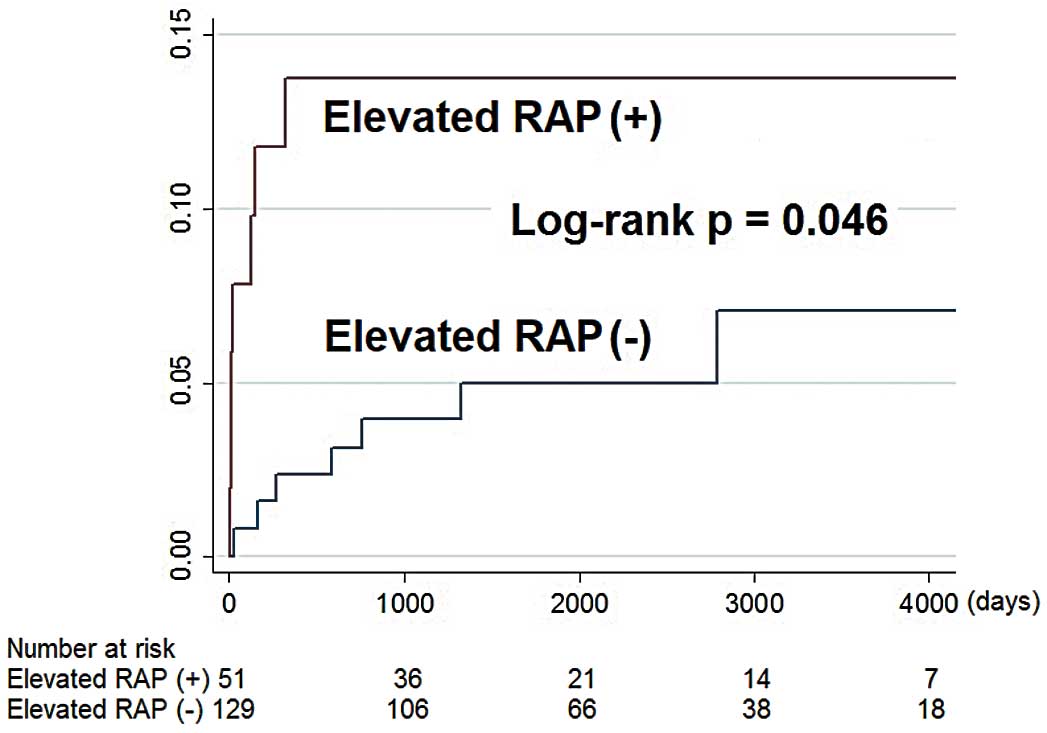
All-cause mortality in patients with and without right-sided heart failure. RAP, right atrial pressure.
| Variable | Univariable analysis | Multivariable analysis | ||||
|---|---|---|---|---|---|---|
| HR | 95% CI | P value | Adjusted HR |
95% CI | P value | |
| Age (per 1-year increase) | 1.05 | 1.005–1.10 | 0.03 | * | ||
| Female | 1.93 | 0.67–5.6 | 0.22 | |||
| NYHA class | 1.63 | 0.86–3.1 | 0.14 | |||
| Dyspnea on exertion | 4.2 | 0.55–32 | 0.17 | |||
| Paroxysmal nocturnal dyspnea | 4.0 | 0.89–18 | 0.07 | |||
| Orthopnea | 2.1 | 0.47–9.5 | 0.33 | |||
| Edema | 1.94 | 0.67–5.6 | 0.22 | |||
| Chest pain | 0.94 | 0.33–2.7 | 0.91 | |||
| Palpitations | 1.19 | 0.33–4.3 | 0.79 | |||
| Dizziness | 1.31 | 0.44–3.9 | 0.63 | |||
| Syncope | 0.96 | 0.13–7.3 | 0.97 | |||
| Hypertension | 2.5 | 0.55–11 | 0.24 | |||
| Diabetes mellitus | 1.40 | 0.38–5.1 | 0.61 | |||
| Coronary artery disease | 1.03 | 0.34–3.1 | 0.96 | |||
| COPD or asthma | 3.2 | 1.07–9.6 | 0.04 | * | ||
| Sleep apnea | 2.8 | 0.89–9.0 | 0.08 | |||
| Prior AF | 2.8 | 0.96–8.0 | 0.06 | |||
| Prior VT/VF | 2.2 | 0.74–6.6 | 0.15 | |||
| Prior stroke | 2.3 | 0.51–10 | 0.28 | |||
| β-blockers | 0.58 | 0.16–2.1 | 0.41 | |||
| Non-dihydropyridine calcium channel blocker | 1.85 | 0.57–6.0 | 0.31 | |||
| Disopyramide | 0.53 | 0.07–4.1 | 0.55 | |||
| Diuretic | 1.53 | 0.51–4.6 | 0.44 | |||
| Antiarrhythmic | 4.3 | 1.32–14 | 0.02 | * | ||
| JVD | 3.0 | 1.02–9.1 | 0.046 | * | ||
| NT-proBNP (per 1,000-pg/mL increase) | 1.10 | 1.04–1.16 | 0.001 | 1.09 | 1.02–1.17 | 0.01 |
| AST (per 10-U/L increase) | 1.11 | 1.03–1.19 | 0.006 | * | ||
| ALT (per 10-U/L increase) | 1.002 | 0.98–1.03 | 0.87 | |||
| Pulmonary edema on chest X-ray | 8.9 | 2.8–28 | <0.001 | 16.5 | 2.3–116 | 0.005 |
| Left atrial size (per 1-mm increase) | 1.08 | 1.01–1.16 | 0.03 | * | ||
| Interventricular septum thickness (per 1-mm increase) | 1.02 | 0.92–1.14 | 0.68 | |||
| Posterior wall thickness (per 1-mm increase) | 1.08 | 0.90–1.28 | 0.41 | |||
| Maximum wall thickness (per 1-mm increase) | 1.02 | 0.91–1.14 | 0.75 | |||
| LV outflow tract gradient at rest (per 10-mmHg increase) | 1.09 | 0.96–1.24 | 0.17 | |||
| Degree of mitral regurgitation | 1.04 | 0.55–1.94 | 0.91 | |||
| RVH | 0.41 | 0.05–3.2 | 0.40 | |||
| RAP (per 5-mmHg increase) | 2.24 | 1.50–3.36 | <0.001 | 2.18 | 1.05–4.50 | 0.04 |
| PAP (per 5-mmHg increase) | 1.41 | 1.15–1.73 | 0.001 | * | ||
| PCWP (per 5-mmHg increase) | 1.62 | 1.12–2.36 | 0.01 | * | ||
| Cardiac index (per 1-L·min−1·m−2 increase) | 1.31 | 0.62–2.8 | 0.47 | |||
*Eliminated through the stepwise method. CI, confidence interval; HR, hazard ratio. Other abbreviations as in Table 1.
Among the 118 patients without a prior history of AF at baseline, 37 developed new-onset AF during follow-up. Elevated RAP was associated with an increased risk of developing new AF (log-rank P=0.002; Figure 3). In univariable analysis, RAP was positively related to incident AF (HR 1.87 per 5-mmHg increase, 95% CI 1.25–2.81, P=0.002; Table 3). Other univariable predictors of incident AF were prior syncope, diuretic use, treatment with an antiarrhythmic agent, pulmonary edema on chest X-ray, left atrial size, mPAP, and PCWP. Multivariable predictors of new-onset AF were antiarrhythmic treatment, left atrial size, and RAP (adjusted HR 1.44 per 5-mmHg increase, 95% CI 1.20–2.85, adjusted P=0.005). In the additional analysis forcibly keeping PCWP in the model and removing RAP, PCWP did not independently predict new-onset AF (adjusted HR 1.19 per 5-mmHg increase, 95% CI 0.92–1.53, adjusted P=0.18). Elevated RAP was not associated with the development of VT/VF (log-rank P=0.36) or stroke (log-rank P=0.28).

Incident atrial fibrillation in patients with and without right-sided heart failure. RAP, right atrial pressure.
| Variable | Univariable analysis | Multivariable analysis | ||||
|---|---|---|---|---|---|---|
| HR | 95% CI | P value | Adjusted HR |
95% CI | P value | |
| Age (per 1-year increase) | 0.99 | 0.97–1.02 | 0.66 | |||
| Female | 1.22 | 0.63–2.3 | 0.56 | |||
| NYHA class | 1.08 | 0.73–1.61 | 0.69 | |||
| Dyspnea on exertion | 1.33 | 0.61–2.9 | 0.47 | |||
| Paroxysmal nocturnal dyspnea | 2.8 | 0.68–12 | 0.15 | |||
| Orthopnea | 3.1 | 0.94–10 | 0.06 | |||
| Edema | 1.19 | 0.59–2.4 | 0.63 | |||
| Chest pain | 0.62 | 0.32–1.20 | 0.16 | |||
| Palpitations | 1.19 | 0.56–2.5 | 0.65 | |||
| Dizziness | 1.35 | 0.69–2.6 | 0.38 | |||
| Syncope | 3.1 | 1.08–8.7 | 0.04 | * | ||
| Hypertension | 1.08 | 0.54–2.1 | 0.83 | |||
| Diabetes mellitus | 1.70 | 0.77–3.7 | 0.19 | |||
| Coronary artery disease | 1.46 | 0.73–2.9 | 0.28 | |||
| COPD or asthma | 1.36 | 0.59–3.1 | 0.47 | |||
| Sleep apnea | 0.90 | 0.28–2.9 | 0.86 | |||
| Prior VT/VF | 1.32 | 0.55–3.2 | 0.54 | |||
| Prior stroke | 0.56 | 0.08–4.1 | 0.57 | |||
| β-blockers | 0.87 | 0.36–2.1 | 0.76 | |||
| Non-dihydropyridine calcium channel blocker | 0.84 | 0.35–2.0 | 0.70 | |||
| Disopyramide | 1.25 | 0.52–3.0 | 0.61 | |||
| Diuretic | 2.1 | 1.11–4.0 | 0.02 | * | ||
| Antiarrhythmic | 4.0 | 1.74–9.0 | 0.001 | 2.6 | 1.14–6.1 | 0.02 |
| JVD | 1.32 | 0.51–3.4 | 0.57 | |||
| NT-proBNP (per 1,000-pg/mL increase) | 1.08 | 0.97–1.19 | 0.15 | |||
| AST (per 10-U/L increase) | 1.07 | 0.997–1.14 | 0.06 | |||
| ALT (per 10-U/L increase) | 1.01 | 0.998–1.02 | 0.12 | |||
| Pulmonary edema on chest X-ray | 2.2 | 1.10–4.6 | 0.03 | * | ||
| Left atrial size (per 1-mm increase) | 1.11 | 1.06–1.16 | <0.001 | 1.10 | 1.05–1.16 | <0.001 |
| Interventricular septum thickness (per 1-mm increase) | 0.97 | 0.90–1.06 | 0.54 | |||
| Posterior wall thickness (per 1-mm increase) | 0.89 | 0.78–1.03 | 0.13 | |||
| Maximum wall thickness (per 1-mm increase) | 0.97 | 0.89–1.05 | 0.46 | |||
| LV outflow tract gradient at rest (per 10-mmHg increase) | 0.97 | 0.89–1.06 | 0.52 | |||
| Degree of mitral regurgitation | 1.08 | 0.74–1.56 | 0.69 | |||
| RVH | 1.54 | 0.67–3.5 | 0.31 | |||
| RAP (per 5-mmHg increase) | 1.87 | 1.25–2.81 | 0.002 | 1.85 | 1.20–2.85 | 0.005 |
| PAP (per 5-mmHg increase) | 1.32 | 1.14–1.53 | <0.001 | * | ||
| PCWP (per 5-mmHg increase) | 1.44 | 1.13–1.84 | 0.003 | * | ||
| Cardiac index (per 1-L·min−1·m−2 increase) | 0.59 | 0.33–1.04 | 0.07 | |||
*Eliminated through stepwise method. Abbreviations as in Tables 1,2.
In the present single-center retrospective cohort study, we characterized the clinical correlates and prognostic effect of elevated RAP in a contemporary HCM referral population. The present study is the first using invasive hemodynamic values to characterize and analyze prognosis related to elevated RAP in patients with HCM. We observed that in HCM, as in other cardiac diseases, elevated RAP is associated with symptoms and signs of left-sided HF, such as higher NYHA class, dyspnea on exertion, orthopnea, left atrial enlargement, and elevated PCWP. Mortality was higher in patients with elevated RAP at baseline than those without, and RAP remained a significant predictor of all-cause death after adjusting for possible confounding factors. RAP was also independently associated with incident AF. These findings provide further insights into the pathophysiology and prognostic value of elevated RAP in this patient population.
In the present study, patients with elevated RAP were more likely to have higher NYHA class, dyspnea on exertion, and orthopnea than were patients without elevated RAP. In addition, objective evidence of left-sided HF, in the form of left atrial enlargement and elevated PCWP, was more common in patients with elevated RAP than in those without elevated RAP. These findings in our cohort of patients with HCM are consistent with the observation in cardiac patients in general that the most common cause of right-sided HF is left-sided HF.15,16 We also found that patients with elevated RAP more frequently had right ventricular hypertrophy than patients without elevated RAP, suggesting that diastolic dysfunction associated with right ventricular hypertrophy may play a role in the development of elevated RAP in the setting of HCM. An important clinical question is whether the presence of right ventricular hypertrophy in HCM is a direct effect of a sarcomeric gene mutation or it results from higher right ventricular afterload. In the present study, mPAP was higher in patients with right ventricular hypertrophy than in those without, suggesting that right ventricular hypertrophy observed in our HCM population was likely due, at least in part, to elevated mPAP.
Non-HCM patients with left-sided HF have a worse prognosis if there is coexisting right-sided HF than patients with left-sided HF without right-sided HF.10–13 However, in patients with HCM, few data are available with regard to whether right-sided HF is associated with increased mortality. A prospective cohort study of patients with HCM showed that right ventricular systolic dysfunction, as assessed by tricuspid annular plane systolic excursion, was independently associated with an increased likelihood of death or transplantation.6 A more recent study reported that pulmonary hypertension, as measured with Doppler echocardiography, predicted HCM-related death.21 The present study reports, for the first time, that elevated RAP is associated with increased risk of death in HCM patients. The association remained significant after adjusting for possible confounding factors. In contrast, PCWP was not an independent predictor of mortality.
Detection of AF in patients with HCM carries significant clinical importance, inasmuch as the combination of HCM and AF is associated with increased risk of HF-related mortality, stroke, and severe functional disability and is a Class I indication for anticoagulation.22 Among non-HCM patient populations, such as those with dilated or ischemic cardiomyopathy, HF patients with right ventricular dysfunction have a higher prevalence of AF than patients without right ventricular dysfunction.13,14 There was a trend towards higher baseline prevalence of AF among our HCM patients with elevated RAP, which is in agreement with the prior observations reported in the literature.
In terms of incident AF, it has been reported that increased left atrial volume and impaired left atrial function predict the development of AF among patients with HCM.23,24 This is consistent with our observation that left atrial size was an independent predictor of incident AF. It has also been shown that age and congestive symptoms are independent predictors of AF occurrence.22 However, few data exist as to whether elevated RAP is associated with a higher incidence of new-onset AF in patients with HCM.25 The present study is the first to demonstrate that elevated RAP is a significant and independent predictor of the development of new AF among patients with HCM, whereas PCWP was not a multivariable predictor of incident AF. This finding indicates that there may be a need for more intensive surveillance for the development of AF among patients with HCM and elevated RAP.
The present study has several limitations. Being observational, the study does not establish a causal link between elevated RAP and mortality or incident AF, and thus cannot inform us as to whether specific therapies for elevated RAP would improve patient outcomes. The present study is a single-center experience with patients at a tertiary care facility with clinical indications for right heart catheterization, so that the results may not be generalizable to patient populations with less severe HCM. The number of subjects who died was low (n=13), which may make our multivariable analysis less statistically robust or overfitted.
The present study is the first to assess clinical characteristics associated with elevated RAP and to determine the prognostic value of elevated RAP in patients with HCM. We demonstrated associations of elevated RAP with overall mortality and the development of new AF. These associations remained valid after adjusting for possible confounding factors. These findings may aid in refining risk stratification to identify patients with a severe form of HCM who are at higher risk of dying from any cause or developing AF.
Y.J.S. was supported, in part, by unrestricted grants from the American Heart Association National Clinical and Population Research Award, Honjo International Scholarship Foundation, and Massachusetts General Hospital Executive Committee on Research Fund for Medical Discovery Award. The funding organizations did not have any role in the study design, collection, analysis, or interpretation of data, in writing of the manuscript, or in the decision to submit the article for publication. The researchers were independent from the funding organizations.
M.A.F. is a consultant to and scientific advisory board member of MyoKardia. The other authors have no relationships relevant to the content of this paper to disclose.