論文ID: CJ-17-1446
論文ID: CJ-17-1446
Background: Peripheral artery disease (PAD) is a risk factor for the development of cardiovascular disease and death. Surfactant protein-D (SP-D) is a 43-kDa protein secreted from type II pneumocytes in the lungs. Recent studies have demonstrated that circulating SP-D plays a key role in the development of atherosclerosis and is related to clinical outcomes in patients with ischemic heart disease. However, it remains unclear whether circulating SP-D is associated with clinical outcomes in patients with PAD.
Methods and Results: We enrolled 364 patients with PAD who underwent endovascular therapy. We measured serum levels of SP-D and Krebs von den Lungen-6 (KL-6). During a median follow-up period of 974 days, there were 69 major adverse cardiovascular and leg events (MACLE), including 48 major adverse cardiovascular events (MACE). Kaplan-Meier analysis demonstrated that patients with high SP-D (≥110 ng/mL) had higher rates of MACE and MACLE than those with low SP-D. Multivariate Cox proportional hazard regression analysis demonstrated that SP-D, but not KL-6, was an independent predictor of MACE and MACLE. The addition of SP-D to known risk factors significantly improved the C index and net reclassification index. The circulating SP-D level was affected by sex, diabetes mellitus, and cilostazol prescription.
Conclusions: Circulating SP-D was associated with clinical outcomes in patients with PAD, suggesting that it may be a new therapeutic target in these patients.
Peripheral artery disease (PAD) is an occlusive disease of the lower limb arteries that is associated with increased morbidity. Despite advances in endovascular therapy (EVT), PAD remains an important medical issue with an increasing prevalence and high all-cause and cardiovascular mortality rates.1–4 Therefore, it is increasingly important to identify patients with PAD at an early stage and, furthermore, patients with PAD at high risk should be stratified according to their risk.
Surfactant protein-D (SP-D) is a 43-kDa protein located in the pulmonary endothelium. It belongs to the collectin family of collagen-like lectin.5 Circulating SP-D has been considered as a biomarker for acute and chronic inflammatory lung injury, such as chronic obstructive lung disease, smoking-induced lung injury, and acute respiratory distress syndrome, because it is secreted from type II pneumocytes in the lungs and plays an important role in pulmonary immune responses through direct microbial interaction and modulation of host cell effect.6 Hitherto, it has been used as a diagnostic marker for interstitial pneumonia, together with Krebs von den Lungen-6 (KL-6).7
Recently, SP-D was reported to be expressed in vascular endothelial cells and coronary smooth muscular cells.8,9 Thus, the focus on SP-D shifted towards its role in atherosclerosis and subsequent atherothrombosis. Contrary to its favorable action of removing antigens from the bronchial space in the lungs, SP-D in the arterial wall was reported to play an atherogenic function and predict cardiovascular morbidity and mortality in patients with ischemic heart disease (IHD).10,11 Based on these reports, circulating SP-D is increasingly being recognized as a pro-atherogenic protein. However, the effect of circulating SP-D on the clinical outcomes in patients with PAD remains unclear.
The aim of the present study was to examine whether circulating SP-D is associated with the severity and clinical outcomes in patients with PAD.
This was a prospective study of 364 patients who were admitted to hospital for their first PAD treatment. PAD was diagnosed in accordance with the ankle-brachial index (ABI) and CT angiography findings. Experienced cardiologists performed EVT according to the recommendations of the Trans-Atlantic Inter-Society Consensus II (TASC II) guidelines.12 Optimized medical therapy was independently administered by physicians on the basis of symptom improvement. The exclusion criteria were acute coronary syndrome within 3 months preceding admission, and malignant disease, including lung cancer, and interstitial pneumonia. In addition, patients with collagen diseases were excluded because steroid medications reduce the circulating SP-D level.13 Demographic and clinical data, including age, sex, smoking history, cardiovascular risk factors, ABI, and medications were collected from the patients’ medical records and by interviews.
Ethics StatementThe study protocol was approved by the Ethics Committee of Yamagata University School of Medicine (No. 395), and all participants provided written informed consent. All procedures were performed in accordance with the Helsinki Declaration principles.
MeasurementsHypertension was defined as a systolic blood pressure ≥140 mmHg, diastolic blood pressure ≥90 mmHg, or use of antihypertensive medication. Hyperlipidemia was defined as a total cholesterol level ≥220 mg/dL, triglyceride level ≥150 mg/dL, or use of antihyperlipidemic medication. Diabetes mellitus (DM) was defined as a fasting blood sugar level ≥126 mg/dL and glycosylated hemoglobin A1c level ≥6.5% (National Glycohemoglobin Standardization Program).
Biochemical MarkersBlood samples were obtained early in the morning before the first EVT. Circulating SP-D and KL-6 levels were measured using enzyme immunoassay and latex agglutination method, respectively. An abnormal level of SP-D was defined as >110 ng/mL, which is the diagnostic value of interstitial pneumonia based on the manufacturer’s instruction.
The serum creatinine levels of PAD patients tend to be underestimated they have muscular atrophy from both decreased physical activity and amputation. Therefore, we measured the cystatin C-based estimated glomerular filtration rate (eGFRcys)14 using the following equations: eGFRcys in men=(104×cystatin C−1.019×0.996Age)−8; eGFRcys in women=(104×cystatin C−1.019×0.996Age×0.929)−8.15
Severity of PADThe severity of PAD was determined using the Fontaine class according to the TASC II guidelines.12 Briefly, Fontaine classes II, III, and IV were defined as intermittent claudication, rest pain, and critical limb ischemia (CLI), respectively.
Endpoints and Follow-upAll participants were prospectively followed up twice yearly using telephone interviews or medical records for a median period of 974 days (interquartile range, 564–1,563 days; longest follow-up, 2,000 days). The primary endpoints were major adverse cardiovascular events (MACEs), including cardiovascular death, and rehospitalization because of cardiovascular diseases, such as non-fatal acute myocardial infarction, unstable angina, or heart failure (HF). The secondary endpoint was major adverse cardiovascular and leg events (MACLEs), including MACE, and amputation.
Statistical AnalysisContinuous data are expressed as mean±standard deviation or median. Continuous and categorical variables were compared using t-tests and chi-square tests, respectively. Data that were not normally distributed were compared using the Mann-Whitney U-test. Survival curves were constructed using the Kaplan-Meier method and compared using the log-rank test. A Cox proportional hazard analysis was performed to identify the independent predictors of MACE and MACLE. A multivariate analysis was performed to evaluate the independent predictors of MACEs and MACLEs. P<0.05 was considered statistically significant.
Receiver-operating characteristics curves for MACEs and MACLEs were illustrated and C indexes of the baseline model and those with SP-D were calculated. We also calculated the net reclassification index (NRI) to measure the quality of improvement for correct reclassification following the addition of the SP-D to the current model. We preformed propensity score matching to examine whether cilostazol was related to the circulating SP-D level. All statistical analyses were performed using a standard program package (JMP version 12; SAS Institute Inc., Cary. NC, USA and R 3.2.4 with additional packages, including Rcmdr, Epi, pROC, and PredictABEL).
The patients’ baseline characteristics are shown in Table 1. There were 288 (79%) male patients. Hypertension, DM and hyperlipidemia were identified in 296 (81%), 138 (38%) and 251 (69%) patients, respectively. There were 118 (32%) current smokers, 115 (32%) previous IHD patients, 67 (18%) cerebrovascular disease (CVD) patients, and 71 (20%) hemodialysis (HD) patients. CLI was identified in 52 (14%) of the PAD patients. The median SP-D and KL-6 levels were 47 ng/mL and 275 pg/mL, respectively. Distribution of SP-D is shown in Figure 1. Abnormal SP-D was identified in 41 (11%) PAD patients. The success rate of first EVT was 95%, especially in CLI patients, which was 89%.
| Variables | All patients (n=364) |
|---|---|
| Age (years) | 74±9 |
| Men, n (%) | 288 (79) |
| Hypertension, n (%) | 296 (81) |
| DM, n (%) | 138 (38) |
| Hyperlipidemia, n (%) | 251 (69) |
| Smoking, n (%) | 118 (32) |
| Previous IHD, n (%) | 115 (32) |
| Previous CVD, n (%) | 67 (18) |
| Hemodialysis, n (%) | 71 (20) |
| Fontaine II/III/IV | 273/39/52 |
| Endovascular therapy data | |
| Iliac artery, n (%) | 220 (60) |
| Femoropopliteal artery, n (%) | 211 (58) |
| Tibial or peroneal artery, n (%) | 62 (17) |
| Stent, n (%) | 323 (90) |
| Pre ABI | 0.59±0.17 |
| Post ABI | 0.86±0.19 |
| Biochemical data | |
| eGFRcys (mg/dL) | 52±30 |
| KL-6 (pg/mL) | 275 (203–375) |
| SP-D (ng/mL) | 47 (27–79) |
| TC (mg/dL) | 173±41 |
| TG (mg/dL) | 130±104 |
| LDL-C (mg/dL) | 102±34 |
| HDL-C (mg/dL) | 51±16 |
| HbA1c (%) | 6.1±1.1 |
| FBS (mg/dL) | 120±42 |
| Medications | |
| Aspirin, n (%) | 258 (71) |
| Clopidogrel, n (%) | 240 (66) |
| Cilostazol, n (%) | 113 (31) |
| Other antiplatelet drugs, n (%) | 55 (15) |
| ACEI and/or ARB, n (%) | 220 (60) |
| Statins, n (%) | 192 (53) |
Data are expressed as mean±SD, number (percentage) or median (interquartile range). ABI, ankle-brachial index; ACEI, angiotensin-converting enzyme inhibitor; ARB, angiotensin II recepter blocker; CLI, critical limb ischemia; CVD, cerebrovascular disease; DM, diabetes mellitus; eGFRcys, cystatin C-based estimated glomerular filtration rate; FBS, fasting blood sugar; HbA1c, glycosylated hemoglobin A1c; HDL-C, high-density lipoprotein cholesterol; IHD, ischemic heart disease; KL-6, Krebvon den Lungen-6; LDL-C, low-density lipoprotein cholesterol; SP-D, surfactant protein D; TC, total cholesterol; TG, triglyceride.
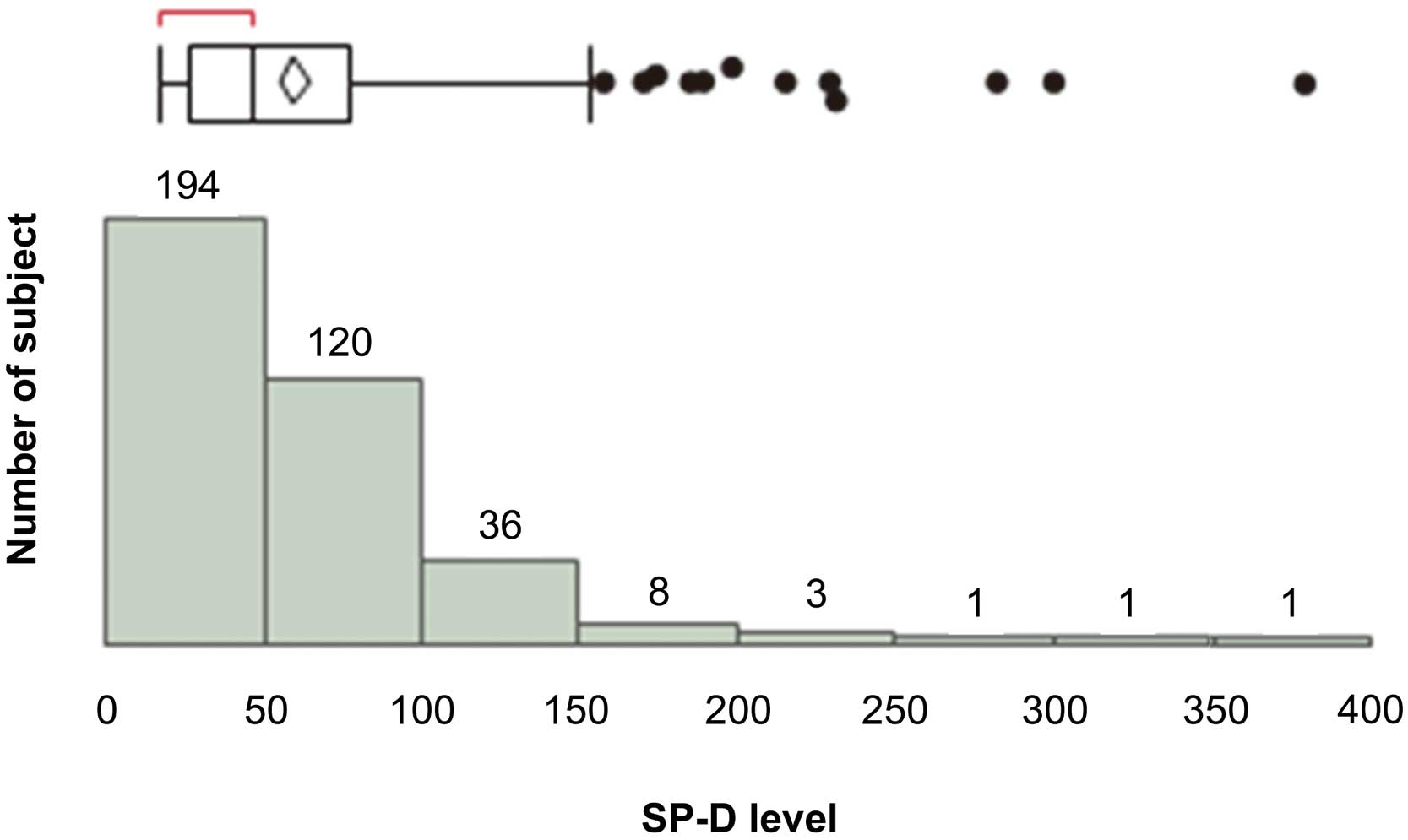
Distribution of circulating SP-D levels in patients with peripheral artery disease. SP-D, surfactant protein-D.
The patients with high SP-D levels had more severe disease as identified using the Fontaine class and had a higher prevalence rate of DM, tibial or peroneal artery stenosis, or occlusion compared with those with low SP-D (Table 2). Also, fasting blood sugar was higher in patients with high SP-D than in those with low SP-D. There were no significant differences in age, sex, prevalence of hypertension, hyperlipidemia, current smoking status, previous IHD, previous CVD, HD, eGFRcys, KL-6, low-density lipoprotein cholesterol, triglycerides, total cholesterol, high-density lipoprotein cholesterol, glycosylated hemoglobin A1c or medication use other than cilostazol between patients with high and low SP-D levels.
| Variables | Low SP-D (<110ng/mL) (n=323) |
High SP-D (≥110ng/mL) (n=41) |
P value |
|---|---|---|---|
| Age (years) | 73±9 | 76±8 | 0.1007 |
| Men, n (%) | 253 (78) | 35 (85) | 0.2784 |
| Hypertension, n (%) | 265 (82) | 31 (76) | 0.3341 |
| DM, n (%) | 115 (36) | 23 (56) | 0.0122 |
| Hyperlipidemia, n (%) | 221 (68) | 30 (73) | 0.5309 |
| Smoking, n (%) | 109 (34) | 9 (22) | 0.4979 |
| Previous IHD, n (%) | 98 (30) | 17 (41) | 0.1573 |
| Previous CVD, n (%) | 61 (19) | 6 (15) | 0.4973 |
| Hemodialysis, n (%) | 61 (19) | 10 (24) | 0.4137 |
| Fontaine II/III/IV | 248/34/41 | 25/5/11 | 0.0409 |
| Endovascular therapy data | |||
| Iliac artery, n (%) | 197 (61) | 23 (56) | 0.5481 |
| Femoropopliteal artery, n (%) | 183 (57) | 28 (68) | 0.1495 |
| Tibial or peroneal artery, n (%) | 50 (15) | 12 (29) | 0.0380 |
| Stent, n (%) | 290 (91) | 33 (80) | 0.0580 |
| Pre ABI | 0.59±0.17 | 0.53±0.19 | 0.0305 |
| Post ABI | 0.87±0.18 | 0.82±0.19 | 0.2572 |
| Biochemical data | |||
| eGFRcys (mg/1.73 mL/m2) | 52±30 | 46±28 | 0.2596 |
| KL-6 (pg/mL) | 271 (202–369) | 323 (230–516) | 0.0515 |
| TC (mg/dL) | 174±42 | 163±62 | 0.1038 |
| TG (mg/dL) | 130±107 | 128±67 | 0.9042 |
| LDL (mg/dL) | 103±35 | 95±26 | 0.1831 |
| HDL (mg/dL) | 51±16 | 49±21 | 0.4214 |
| HbA1c (%) | 6.1±1.1 | 6.4±1.1 | 0.1296 |
| FBS (mg/dL) | 118±35 | 136±66 | 0.0127 |
| Medications | |||
| Aspirin, n (%) | 229 (71) | 29 (71) | 0.9824 |
| Clopidogrel, n (%) | 211 (65) | 29 (71) | 0.4866 |
| Cilostazol, n (%) | 108 (33) | 5 (12) | 0.0028 |
| Other antiplatelet drugs, n (%) | 48 (15) | 7 (17) | 0.7135 |
| ACEI and/or ARB, n (%) | 195 (60) | 25 (60) | 0.9406 |
| Statins, n (%) | 168 (52) | 24 (59) | 0.4292 |
| Cause of hospitalization | |||
| MACE, n (%) | 36 (11) | 12 (29) | 0.0036 |
| HF, n (%) | 20 (6) | 7 (17) | 0.0264 |
| MALE, n (%) | 15 (5) | 6 (15) | 0.0266 |
| MACLE, n (%) | 51 (16) | 18 (44) | <0.0001 |
Data are expressed as mean±SD, number (percentage) or median (interquartile range). MACE, major adverse cardiovascular event; MACLE, major adverse cardiovascular and leg event; MALE, major adverse leg event. Other abbreviationsa as in Table 1.
During the follow-up period, there were 48 MACEs, 21 major adverse leg events, and 69 MACLEs. The Kaplan-Meier analysis showed that patients with high SP-D had higher rates of MACEs and MACLEs compared with those with low SP-D (Figure 2A,B).
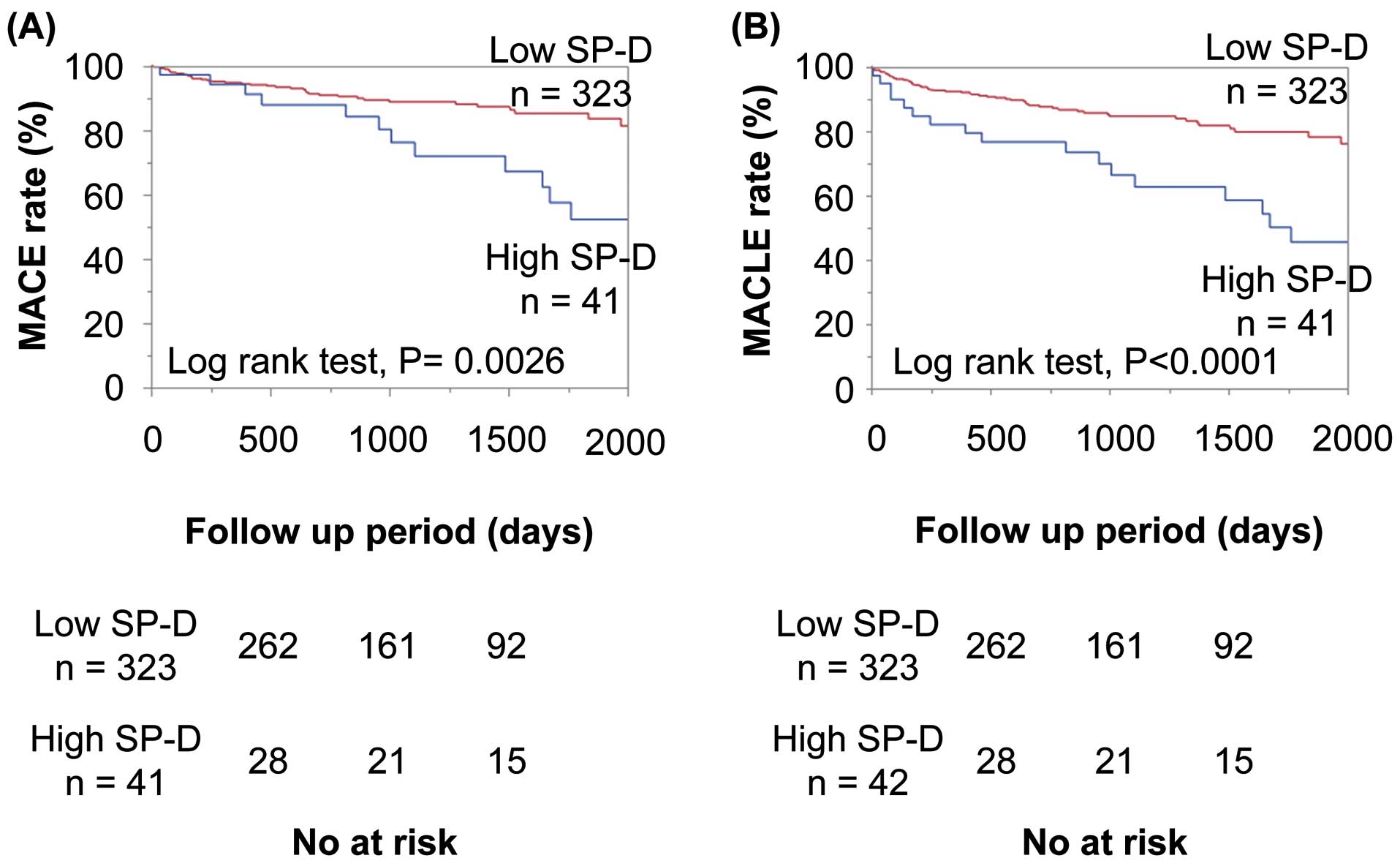
Kaplan-Meier analysis of circulating SP-D for (A) MACE and (B) MACLE in patients with high and low SP-D levels. MACE, major adverse cardiovascular events; MACLE, major adverse cardiovascular and leg events; SP-D, surfactant protein-D.
We performed univariate and multivariate Cox proportional hazard regression analyses to examine the effect of SP-D on MACEs and MACLEs in patients with PAD. The univariate Cox proportional hazard regression analysis demonstrated that DM, previous IHD, HD, CLI, post ABI, eGFRcys, and SP-D were significantly associated with MACEs and MACLEs (Table 3). The multivariate Cox proportional hazard regression analysis demonstrated that a high SP-D level was an independent predictor of both MACEs and MACLEs after adjusting for age, male sex, DM, previous IHD, CLI, and eGFRcys (Table 3).
| Variables | Univariate analysis | Multivariate analysis | ||||
|---|---|---|---|---|---|---|
| HR | 95% CI | P value | HR | 95% CI | P value | |
| MACE | ||||||
| Age (per 1-year increase) | 1.013 | 0.982–1.048 | 0.4093 | 1.016 | 0.983–1.051 | 0.3532 |
| Sex male vs. female | 1.327 | 0.422–2.516 | 0.4224 | 1.273 | 0.609–2.459 | 0.4929 |
| Hypertension | 1.169 | 0.550–2.254 | 0.6659 | |||
| DM | 1.855 | 1.044–3.392 | 0.0349 | 1.285 | 0.702–2.316 | 0.4075 |
| Hyperlipidemia | 1.017 | 0.563–1.928 | 0.9559 | |||
| Smoking | 1.510 | 0.802–2.735 | 0.1953 | |||
| Previous IHD | 2.645 | 1.498–4.693 | 0.0009 | 2.424 | 1.352–4.365 | 0.0029 |
| Previous CVD | 1.736 | 0.756–5.023 | 0.2096 | |||
| Hemodialysis | 2.178 | 1.147–3.946 | 0.0185 | |||
| CLI | 2.699 | 1.266–5.238 | 0.0121 | 2.144 | 0.978–4.296 | 0.0561 |
| Pre ABI (per 1-SD increase) | 0.955 | 0.708–1.310 | 0.7725 | |||
| Post ABI (per 1-SD increase) | 0.676 | 0.508–0.917 | 0.0092 | |||
| eGFRcys (per 1-SD increase) | 0.573 | 0.426–0.765 | 0.0002 | 0.625 | 0.459–0.852 | 0.0029 |
| SP-D (>110 ng/mL) | 2.639 | 1.314–4.937 | 0.0079 | 2.077 | 1.010–4.003 | 0.0359 |
| KL-6 (>500 pg/mL) | 1.117 | 0.512–2.931 | 0.7969 | |||
| MACLE | ||||||
| Age (per 1-year increase) | 1.010 | 0.983–1.038 | 0.4616 | 1.011 | 0.984–1.040 | 0.4319 |
| Sex male vs. female | 1.224 | 0.666–2.113 | 0.4973 | 1.170 | 0.633–2.036 | 0.5948 |
| Hypertension | 1.341 | 0.742–2.290 | 0.3167 | |||
| DM | 2.178 | 1.356–3.516 | 0.0013 | 1.816 | 1.107–2.991 | 0.0182 |
| Hyperlipidemia | 1.163 | 0.697–1.891 | 0.5538 | |||
| Smoking | 1.531 | 0.913–2.501 | 0.1045 | |||
| Previous IHD | 2.331 | 1.449–3.746 | 0.0006 | 2.123 | 1.305–3.456 | 0.0027 |
| Previous CVD | 1.204 | 0.645–2.503 | 0.5798 | |||
| Hemodialysis | 2.364 | 1.407–3.863 | 0.0015 | |||
| CLI | 4.922 | 2.919–8.075 | <0.0001 | 3.898 | 2.286–6.649 | <0.0001 |
| Pre ABI (per 1-SD increase) | 0.142 | 0.036–0.586 | 0.0060 | |||
| Post ABI (per 1-SD increase) | 0.093 | 0.025–0.372 | 0.0005 | |||
| eGFRcys (per 1-SD increase) | 0.559 | 0.438–0.713 | <0.0001 | 0.656 | 0.506–0.850 | 0.0014 |
| SP-D (>110 ng/mL) | 2.836 | 1.611–4.766 | 0.0005 | 1.969 | 1.125–3.445 | 0.0177 |
| KL-6 (>500 pg/mL) | 1.045 | 0.503–1.952 | 0.8979 | |||
CI, confidence interval; HR, hazard ratio. Other abbreviationsa as in Tables 1,2.
We evaluated the improvement in the NRI to examine whether prediction capacity was improved by the addition of SP-D to the basic predictors, such as age, DM, previous IHD, CLI, eGFRcys, and cilostazol prescription. The NRI significantly improved following the addition of SP-D to the basic predictors (Table 4). Receiver-operating characteristics curves for MACEs and MACLEs are shown in Figure 3, where it can be seen that the C index in the baseline model with SP-D was significantly greater than that in the baseline model alone. The abnormal cutoff value of circulating SP-D in patients with PAD was 84.7 ng/mL. To confirm the usefulness of this value, we performed a Kaplan-Meier analysis, which demonstrated that patients with SP-D ≥84.7 ng/mL had a significantly higher rate of MACE and MACLE than those with SP-D <84.7 ng/mL (Figure S1).
| C index (P value) | NRI (95% CI, P value) | |
|---|---|---|
| MACE | ||
| Baseline model | 0.714 | Ref. |
| +SP-D | 0.750 (P=0.0471) | 0.3671 (0.0662–0.6680, P=0.0168) |
| MACLE | ||
| Baseline model | 0.766 | Ref. |
| +SP-D | 0.798 (P=0.0147) | 0.4265 (0.1683–0.6848, P=0.0012) |
Baseline model includes age, sex, DM, IHD, CLI, eGFRcys and cilostazol prescription. NRI, net reclassification index. Other abbreviationsa as in Tables 1–3.
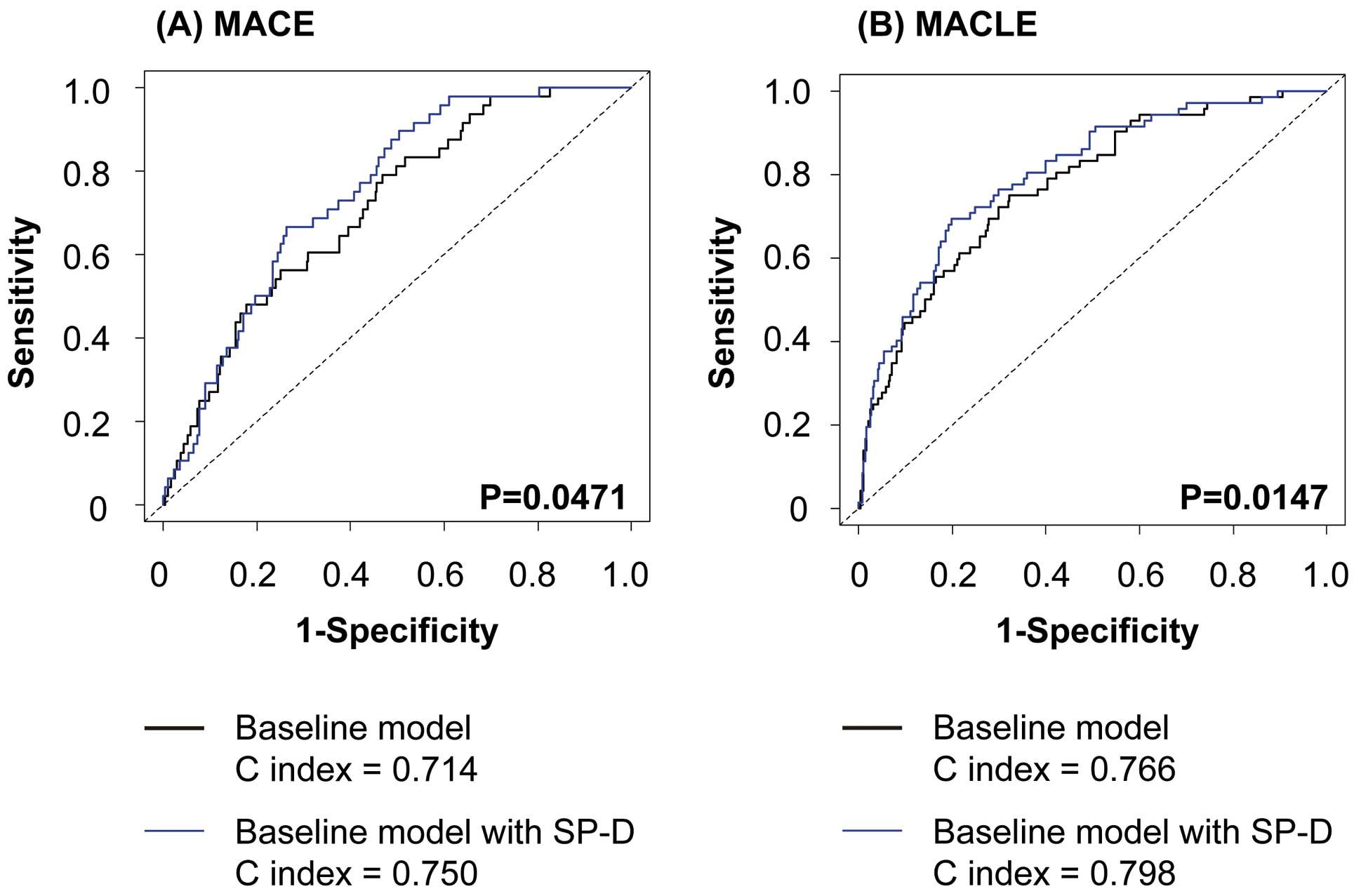
Receiver-operating characteristics curve for (A) MACE and (B) MACLE. MACE, major adverse cardiovascular events; MACLE, major adverse cardiovascular and leg events.
Finally, we examined the association of SP-D with sex, DM, HD, and cilostazol prescription with poor prognosis in patients with PAD. As shown in Figure 4, SP-D levels were elevated in male and DM patients. SP-D levels were significantly lower in patients with cilostazol prescription than in those without. Propensity scores were developed using a logistic model, which included age, sex, DM, previous IHD, and eGFRcys, to examine whether cilostazol was related to circulating SP-D. As shown in Figure S2, the circulating SP-D level was lower in patients with cilostazol prescription than in those without. In contrast, previous IHD, cerebral artery disease, and HD were not associated with SP-D elevation.
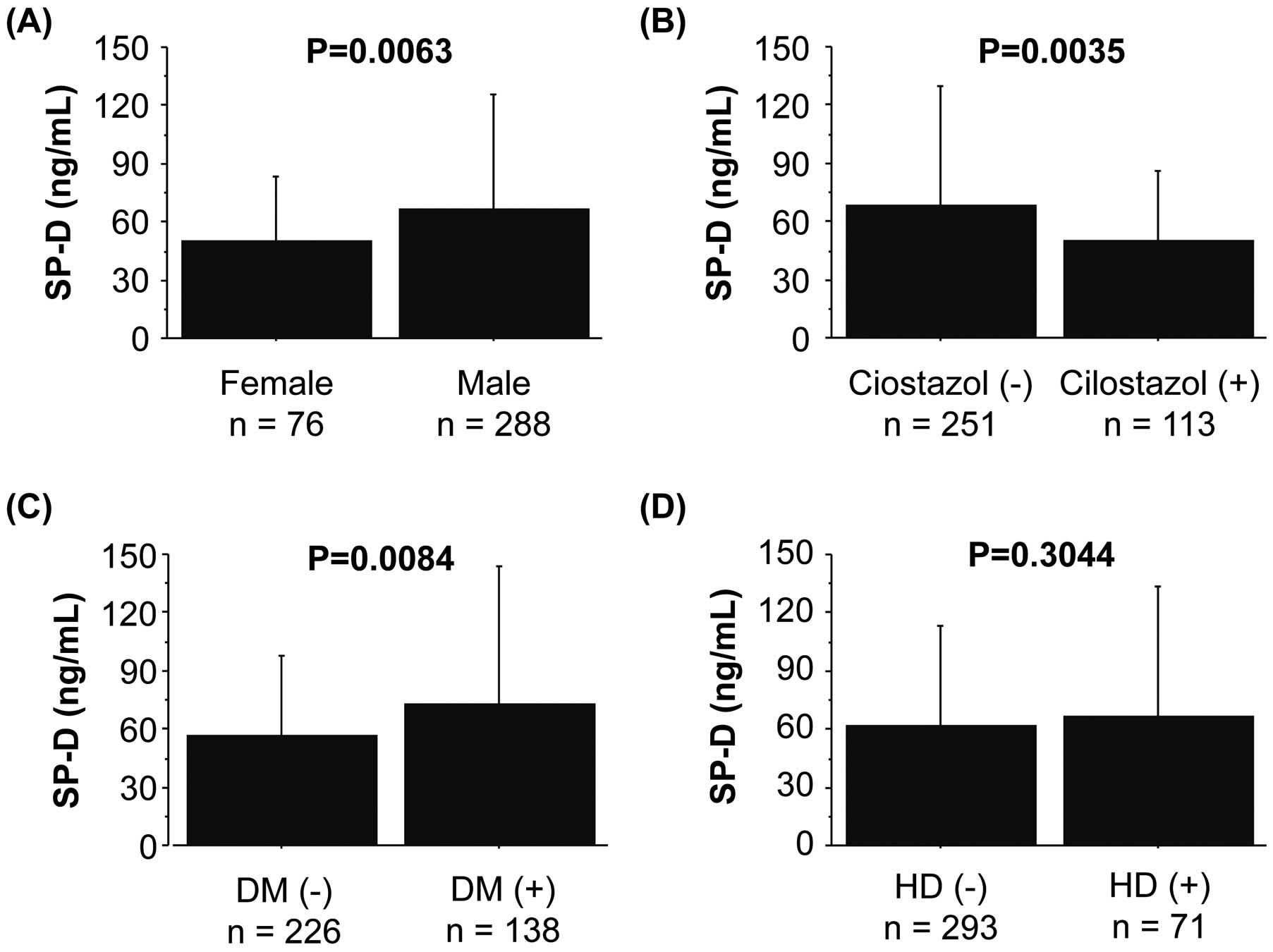
Comparison of circulating SP-D levels as affected by different clinical factors, such as sex, DM, HD, and cilostazol prescription. DM, diabetes mellitus; HD, hemodialysis; SP-D, surfactant protein-D.
The main findings from the present study were (1) abnormal levels of SP-D were identified in 41 (11%) PAD patients; (2) Kaplan-Meier analysis demonstrated that patients with high SP-D had higher rates of MACEs and MACLEs compared with those with low SP-D level; (3) multivariate analysis demonstrated that circulating SP-D, but not KL-6, was an independent predictor for MACEs and MACLEs after adjusting for confounding risk factors; (4) the C index and NRI were significantly improved by the addition of SP-D to the established risk factors; and (5) circulating SP-D levels were affected by sex, DM, and cilostazol use.
Atherosclerosis and SP-DAtherothrombosis is an increasingly common health problem and is the most common cause of death worldwide. Atherothrombosis includes IHD, stroke, and PAD. It is important to identify common therapeutic targets and useful markers for poor clinical outcomes in patients with atherothrombosis. The clinical significance of circulating SP-D has been established in patients with IHD and carotid artery disease.10,16 We have shown the clinical importance of circulating SP-D in patients with PAD.
KL-6 was not related to clinical outcomes in the patients with PAD, although interstitial pneumonia was reported to be related to cardiovascular death.17 Thus, it is possible that circulating SP-D itself carries a risk for poor clinical outcomes in patients with PAD rather than interstitial pneumonia. The circulating SP-D is considered to be a product of translocation from the lungs because injured lungs become permeable.18 However, Sorensen et al raised the possibility that circulating SP-D levels are affected by leakage from atherosclerotic artery walls.11 Inflammation dependent on tumor necrosis factor α (TNFα) induces endothelial SP-D expression during atherogenesis. Circulating SP-D conversely induces TNFα in monocytes through osteoclast-associated receptor signaling, which is the SP-D receptor.19 SP-D knockout mice had lower levels of plasma proinflammatory cytokine TNFα and did not develop diet-induced atherosclerosis.8 Similarly, SP-D and Apo-E double knockout mice had attenuated atherosclerosis because of the reduction in macrophage accumulation and increased smooth muscle cell coverage.20 It was reported that TNFα induces atherosclerosis through efferocytosis,21 and patients with collagen disease who took TNFα-inhibiting antibodies were protected from myocardial infarction.22 These findings suggest that SP-D interacts with TNFα and causes progression of atherosclerosis. In the present study, patients with high SP-D levels were associated with more severe Fontaine classes than those with low SP-D, suggesting that circulating SP-D also worsens atherosclerosis in the peripheral arteries.
Association of Circulating SP-D With Smoking, Exercise, DM, and CilostazolSingle-nucleotide polymorphism of SP-D has been related to insulin resistance and DM.23 Of note, circulating SP-D was reported to be improved by exercise training and was related to aerobic tolerance in patients with DM.24 In the present study, patients with DM had higher levels of circulating SP-D than those without. Because almost all PAD patients had muscle sarcopenia, rehabilitation may potentially be beneficial for DM patients with PAD.
Several reports have demonstrated the usefulness of cilostazol administration in patients with PAD.25,26 Surprisingly, the present patients with cilostazol prescriptions had significantly lower SP-D levels than those without it, even after performing propensity score matching (Figure S2). It was beyond the scope of this study to determine the mechanism by which cilostazol reduced the circulating SP-D level because this was a prospective observational study. However, cilostazol may inhibit atherosclerosis by reducing the circulating SP-D level.
Although there was no significant relationship between circulating SP-D and current smoking in the present study, it was reported that the circulating SP-D level is affected by smoking. Interestingly, smoking cessation was reported to reduce circulating SP-D level by 40%.13,27 Considering the role of SP-D in atherosclerosis, our data reinforce the therapeutic importance of DM management, rehabilitation, smoking cessation, and cilostazol administration in patients with PAD.
Clinical Outcomes and SP-DPAD increases the risk for cardiovascular disease and death. The American College of Cardiology/American Heart Association guidelines regard PAD as stage A HF.28 Our results demonstrated that circulating SP-D was closely associated with MACE in patients with PAD and served as a means of identifying high-risk patients. Circulating SP-D was also related to acute coronary syndrome in patients with PAD in accordance with past studies.10,29 However, other mechanisms should be proposed to explain the association between SP-D and the development of HF. It was reported that surfactant protein reflects alveolar membrane damage in patients with HF. Notably, SP-D levels positively correlated with B-type natriuretic peptide levels, indicating an association between SP-D and left ventricular volume overload.30 Therefore, circulating SP-D may be caused in part by translocation from the lungs because of alveolar damage secondary to HF.
Circulating SP-D is associated with death in several lung diseases such as lung cancer, idiopathic pulmonary fibrosis, and acute respiratory distress syndrome.31,32 Furthermore, the association of SP-D with death was reported in elderly women.33 Nybo et al demonstrated that circulating SP-D predicted the development of dementia and augmented mortality.34 Importantly, we showed that both the C index and the NRI were improved by the addition of circulating SP-D, indicating that it could provide additional information to existing confounding risk factors. Therefore, circulating SP-D is a feasible marker for MACE and MACLE in patients with PAD.
Study LimitationsFirst, the precise mechanism by which SP-D initiates or accelerates atherosclerosis was not revealed because this was a prospective, observational study. Secondly, the circulating SP-D level was significantly lower in patients who took cilostazol than in those who did not among the propensity score-matched patients. Although the result was statistically significant, prospective interventional studies of cilostazol are required. Third, the smoking index was not measured in this study. Almost all PAD patients had a smoking history. Because we only checked for current smoking at admission, the period of smoking cessation was not assessed. This may affect the result of a lack of difference in circulating SP-D levels between patients with and without current smoking. Finally, the study population was small, so further studies with larger populations are needed to determine the abnormal cutoff value of SP-D for atherosclerosis.
We demonstrated for the first time that the circulating SP-D level was associated with MACE and MACLE in patients with PAD. Circulating SP-D could potentially be a therapeutic target and a useful marker for clinical outcomes, specifically for tracking atherosclerotic health status in patients with PAD.
None.
Supplementary File 1
Figure S1. Kaplan-Meier analysis for (A) MACE and (B) MACLE between patients with greater than the abnormal cutoff value of SP-D (≥84.7ng/mL) and those with less than the abnormal cutoff value (SP-D <84.7 ng/mL).
Figure S2. Comparison of circulating SP-D level between propensity score-matched patients with and without cilostazol.
Please find supplementary file(s);
http://dx.doi.org/10.1253/circj.CJ-17-1446