Abstract
Background:
Plasma renin activity (PRA) is associated with cardiovascular events in patients with heart failure (HF), but its prognostic role in acute myocardial infarction (AMI) is unclear.
Methods and Results:
A total of 878 patients with information on baseline PRA on admission were selected from 1,055 AMI patients who underwent emergency coronary angiography between 2007 and 2016. The patients were divided into 2 groups according to their median PRA (2.0 ng/mL/h). The primary endpoint was major adverse cardiac events (MACE), defined as a composite of cardiovascular death and hospitalization because of HF. During follow-up (median 4.5±3.1 years), MACE occurred in 108 patients. Kaplan-Meier analysis showed that the high PRA group had significantly lower MACE-free survival than the low PRA group (log-rank P=0.0009). By multivariate analysis, high PRA was an independent predictor of MACE (hazard ratio (HR) 1.573; 95% confidence interval (CI) 1.049–2.396, P=0.0282). Similarly, among 580 patients who had not been previously treated with renin-angiotensin system inhibitors or β-blockers on admission, high PRA was an independent predictor of MACE (HR 1.732; 95% CI 1.010–3.047, P=0.0460).
Conclusions:
In the studied AMI patients, elevated levels of PRA were independently associated with poor prognosis.
Acute myocardial infarction (AMI) is one of the most important causes of cardiovascular death or heart failure (HF). Several prognostic biomarkers have been identified as independent predictors for death in AMI patients. Previous studies demonstrated that plasma B-type natriuretic peptide (BNP) predicts death in patients with acute coronary syndrome1
or AMI.2,3
On the other hand, plasma renin activity (PRA), known as a key regulator of the renin-angiotensin system (RAS), is also associated with cardiovascular outcomes in hypertensive populations,4
HF patients,5
diabetic patients,6
general population,7
and coronary artery disease (CAD) patients without a history of AMI.8
We hypothesized that PRA might be associated with clinical outcomes in AMI patients, so this study investigated whether PRA can predict cardiovascular events in patients with AMI.
Editorial p ????
Methods
Study Participants
Between 2007 and 2016, 1,055 AMI patients who underwent emergency coronary angiography at Nara Medical University were enrolled as the Nara Registry and Analysis for Myocardial Infarction II (NARA-MI II) study. After excluding 58 patients who were admitted >48 h after the onset of AMI, 43 patients who died in hospital, 18 patients who were lost to follow-up, and 58 patients without baseline PRA data, 878 patients were finally included in this study. AMI was diagnosed by a history of chest pain, typical ECG changes suggesting AMI, and increased creatinine kinase (CK) levels of twice the upper normal limit.9
Data Collection and Endpoints
PRA was measured within 48 h of admission and patients were divided into 2 groups according to their median PRA value. Coronary angiography and coronary revascularization were performed using standard techniques with the femoral or radial approach. Revascularization procedures, such as thrombectomy, distal protection, predilatation, stenting, and postdilatation, were selected at each operator’s discretion. The primary endpoint was major adverse cardiac event (MACE), defined as a composite of cardiovascular death and HF requiring hospitalization. Cardiovascular death was defined as death from AMI, sudden cardiac death, stroke, HF, or vascular disease. The diagnosis of HF was based on the Framingham criteria for HF.10
Research assistants recorded data using individual chart review. When this information was unavailable, they telephoned the patients or their families. After having confirmed the results, R.K. and M.W. adjudicated every endpoint. The study protocol was approved by the Ethics Committee of Nara Medical University (ID no. #1625), and was performed in accordance with the 1975 Declaration of Helsinki guidelines for clinical research protocols. Informed consent was given by all patients.
Statistical Analysis
Continuous variables are presented as mean±SD. The differences between the groups in clinical characteristics and laboratory data were analyzed using the unpaired t-test or Wilcoxon rank sum test. We constructed Kaplan-Meier curves for patients in the high PRA or low PRA group to the primary endpoint and the difference between the groups was assessed by the log-rank test in each group. Univariate and multivariate analyses of event-free survival were performed using Cox proportional hazards models. A P-value <0.05 was considered statistically significant. All data were analyzed using JMP version 12.2 for Windows (SAS Institute, Cary, NC, USA).
Results
Baseline Clinical Characteristics
Baseline clinical characteristics of the study population are shown in
Table 1. The mean age was 67.8±11.9 years (77.0% were male). The median value of PRA was 2.0 ng/mL/h (interquartile range: 1.0–4.5). We divided patients into 2 groups according to the median PRA: high PRA group (n=448) and low PRA group (n=430).
Table 1
shows the baseline clinical characteristics, Killip class, laboratory data, ejection fraction (EF) by echocardiography, maximum (Max) CK levels, procedural characteristics, and medications at discharge compared between the high and low PRA groups. The high PRA group had more male patients, and those with diabetes mellitus and hypertension than the low PRA group. On the other hand, age, prevalence of smoking, dyslipidemia, and end-stage renal disease requiring hemodialysis were similar between groups. Laboratory examination revealed that the estimated glomerular filtration rate (eGFR) and low-density lipoprotein cholesterol levels were significantly lower, and hemoglobin was significantly higher in the high PRA group than in the low PRA group. BNP was similar in both groups. The patients in the high PRA group had significantly lower LVEF and higher max. CK compared with the low PRA group. The left main trunk was more commonly documented as the culprit vessel in the high PRA group. The final TIMI-3 flow was similarly achieved in both groups. Aspirin and β-blockers were more frequently used in the high PRA group, but angiotensin-converting enzyme inhibitors (ACEIs), angiotensin-receptor blockers (ARBs), and statins were similarly used in the 2 groups.
Table 1.
Baseline Clinical, Lesion Characteristics and Medications of Study Patients With AMI
| |
Total
(n=878) |
High PRA
(n=448) |
Low PRA
(n=430) |
P value |
| Age (years) |
67.8±11.9 |
67.3±12.5 |
68.2±11.1 |
0.4926 |
| Male sex, n (%) |
676 (77.0) |
366 (81.7) |
310 (72.1) |
0.007 |
| Medical history |
| Smoking, n (%) |
601 (68.5) |
319 (71.2) |
282 (65.6) |
0.0730 |
| Diabetes mellitus, n (%) |
293 (33.4) |
173 (38.6) |
120 (27.9) |
0.0007 |
| Dyslipidemia, n (%) |
469 (53.4) |
247 (55.1) |
222 (51.6) |
0.2978 |
| Hypertension, n (%) |
560 (63.8) |
304 (67.9) |
256 (59.5) |
0.0103 |
| Dialysis, n (%) |
23 (2.6) |
13 (2.9) |
10 (2.3) |
0.5924 |
| Killip class |
| IV, n (%) |
66 (7.5) |
40 (8.9) |
26 (6.1) |
0.1040 |
| Laboratory data on admission |
| Hb (g/dL) |
13.9±2.0 |
14.1±2.1 |
13.7±2.0 |
0.0340 |
| eGFR (mL/min/1.73 m2) |
67.5±25.5 |
65.4±26.8 |
69.6±23.9 |
0.0067 |
| HbA1c (%) |
6.1±1.3 |
6.3±1.5 |
6.0±1.0 |
0.0680 |
| LDL-C (mg/dL) |
113.3±37.5 |
111.4±39.4 |
115.2±35.3 |
0.0365 |
| BNP (pg/mL) |
137.4 (62.4–285.0) |
139.6 (61.8–308.9) |
134.0 (63.1–263.8) |
0.3043 |
| PRA (ng/mL/h) |
2.0 (1.0–4.5) |
4.4 (2.7–8.6) |
1.0 (0.6–1.4) |
– |
| Aldosterone (pg/mL) |
73.6 (46.4–127.2) |
75.8 (49.7–131) |
71.7 (43.5–123.6) |
0.0631 |
| Max CK (IU/I) |
2,962±2,661 |
3,346±2,966 |
2,561±2,232 |
<0.0001 |
| EF (%) |
58.0±10.9 |
56.8±11.2 |
59.3±10.5 |
0.0008 |
| Culprit vessel |
| RCA, n (%) |
333 (37.9) |
163 (36.4) |
170 (39.5) |
0.3361 |
| LAD, n (%) |
418 (47.6) |
227 (50.7) |
191 (44.4) |
0.0636 |
| LCX, n (%) |
104 (11.9) |
44 (9.8) |
60 (14.0) |
0.0579 |
| LMT, n (%) |
10 (1.1) |
9 (2.0) |
1 (0.2) |
0.0077 |
| Final TIMI flow grade |
| 3, n (%) |
834 (95.0) |
424 (94.6) |
410 (95.4) |
0.6314 |
| Medications at discharge |
| Aspirin, n (%) |
856 (97.5) |
443 (98.9) |
413 (96.1) |
0.0058 |
| ACEIs or ARBs, n (%) |
855 (97.4) |
437 (97.5) |
418 (97.2) |
0.7558 |
| ACEIs, n (%) |
753 (85.8) |
386 (86.2) |
367 (85.4) |
0.7308 |
| ARBs, n (%) |
125 (14.2) |
62 (13.8) |
63 (14.7) |
0.7308 |
| β-blockers, n (%) |
575 (65.6) |
308 (68.9) |
267 (62.1) |
0.0338 |
| MR blockers, n (%) |
118 (13.4) |
70 (15.6) |
48 (11.2) |
0.0519 |
| Statins, n (%) |
618 (70.4) |
315 (70.3) |
303 (70.5) |
0.9605 |
Data presented as n (%), mean±SD, or median (25th–75th percentile). ACEI, angiotensin-converting enzyme inhibitor; AMI, acute myocardial infarction; ARB, angiotensin-receptor blocker; BNP, B-type natriuretic peptide; EF, ejection fraction; eGFR, estimated glomerular filtration rate; Hb, hemoglobin; LAD, left anterior descending artery; LCX, left circumflex artery; LDL-C , low-density lipoprotein cholesterol; LMT, left main trunk; Max CK, maximum creatinine kinase; MR, mineralocorticoid receptor; PRA, plasma renin activity; RCA, right coronary artery; TIMI, Thrombolysis in Myocardial infarction.
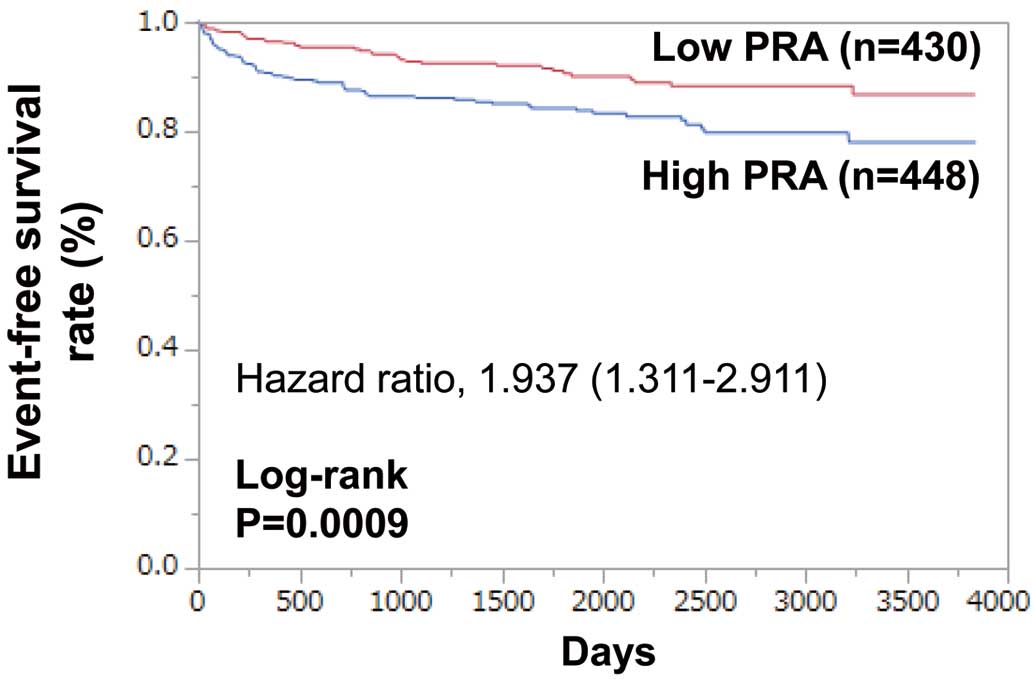
During follow-up (median 4.5±3.1 years), MACE occurred in 108 patients: 71 (15.9%) in the high PRA group and 37 (8.6%) in the low PRA group. Kaplan-Meier curves show that MACE-free survival was significantly lower in the high PRA group than in the low PRA group (log-rank P=0.0009;
Figure 1). High PRA was significantly associated with MACE (hazard ratio (HR) 1.937; 95% confidence interval (CI) 1.311–2.911, P=0.0008). High PRA was also significantly associated with cardiovascular death (HR 2.255; 95% CI 1.316–4.020, P=0.0027), hospitalization for HF (HR 1.841; 95% CI 1.155–3.004, P=0.0100), and sudden cardiac death (HR 7.396; 95% CI 2.086–46.93, P=0.0009). However, there were no statistically significant differences in the incidence of death from AMI, stroke, HF or vascular disease.
The results of the univariate and multivariate analyses are shown in
Table 2. High PRA, age, sex, BNP, LVEF, Hb, eGFR, Max CK, and Killip IV were predictors of MACE. By multivariate analysis, high PRA (HR 1.573; 95% CI 1.049–2.396, P=0.0282), age (HR 1.055; 95% CI 1.032–1.080, P<0.0001), BNP (HR 1.055; 95% CI 1.020–1.087, P=0.0027), Max CK (HR 1.009; 95% CI 1.003–1.015, P=0.0039), and LVEF (HR 0.969; 95% CI 0.953–0.985, P=0.0002) were independent predictors of MACE. Following adjustment for covariates including age, sex, BNP, LVEF, Hb, eGFR, Max CK and Killip IV, a high PRA remained as an independent predictor of MACE (HR 1.573; 95% CI 1.049–2.396, P=0.0282).
Table 2.
Univariate and Multivariate Analyses of Predictors of MACE
| Variable |
Univariate analysis |
Multivariate analysis |
| HR (95% CI) |
P value |
HR (95% CI) |
P value |
| High PRA |
1.937 (1.311–2.911) |
0.0008 |
1.573 (1.049–2.396) |
0.0282 |
| Age (years) |
1.081 (1.060–1.103) |
<0.0001 |
1.055 (1.032–1.080) |
<0.0001 |
| Male sex |
0.574 (0.387–0.865) |
0.0086 |
0.988 (0.622–1.588) |
0.9610 |
| Diabetes mellitus |
1.402 (0.948–2.054) |
0.0898 |
|
|
| Hypertension |
1.167 (0.786–1.765) |
0.4495 |
|
|
| Killip class IV |
3.071 (1.816–4.925) |
<0.0001 |
1.157 (0.636–2.004) |
0.6202 |
| Hb (g/dL) |
0.759 (0.698–0.827) |
<0.0001 |
0.924 (0.823–1.040) |
0.1892 |
| eGFR (mL/min/1.73 m2) |
0.972 (0.997–0.979) |
<0.0001 |
0.990 (0.980–1.000) |
0.0608 |
| HbA1c (%) |
0.996 (0.846–1.146) |
0.9620 |
|
|
| BNP (100 pg/mL) |
1.111 (1.087–1.132) |
<0.0001 |
1.055 (1.020–1.087) |
0.0027 |
| Max CK (100 IU/L) |
1.008 (1.001–1.013) |
0.0178 |
1.009 (1.003–1.015) |
0.0039 |
| EF (%) |
0.945 (0.931–0.960) |
<0.0001 |
0.969 (0.953–0.985) |
0.0002 |
| Final TIMI flow grade 3 |
0.621 (0.322–1.387) |
0.2246 |
|
|
CI, confidence interval; HR, hazard ratio; MACE, major adverse cardiac event. Other Abbreviations as in Table 1.
For the sensitivity analysis, we also divided the patients into 3 groups. For patients in the highest tertile for baseline PRA compared with those in the lowest tertile, the unadjusted HR for MACE was 2.132 (95% CI 1.337–3.491, P=0.0013). After adjustment for covariates including age, BNP, LVEF, Hb, and eGFR, the adjusted HR for MACE was 1.781 (95% CI 1.091–2.979, P=0.0207). This means that higher PRA is a predictor of poor outcome in AMI patients.
Because PRA is strongly affected by the use of RAS inhibitors, we further assessed the results of a similar analysis in 603 patients who had not been previously treated with RAS inhibitors. They were divided into 2 groups according to their PRA values (2.0 ng/mL/h): high PRA group (n=281) and low PRA group (n=322). During follow-up (median 4.8±3.1 years), MACE occurred in 66 patients: 43 (15.3%) in the high PRA group and 23 (7.1%) in the low PRA group. Kaplan-Meier curves show that MACE-free survival was significantly lower in the high PRA group than in the low PRA group (log-rank P=0.0022;
Figure 2). High PRA was associated with MACE (HR 2.166; 95% CI 1.319–3.651, P=0.0021). After the adjustment for covariates including age, BNP, LVEF, Hb, and eGFR, a high PRA remained as an independent predictor of MACE (HR 1.701; 95% CI 1.010–2.927, P=0.0459) (Table 3).
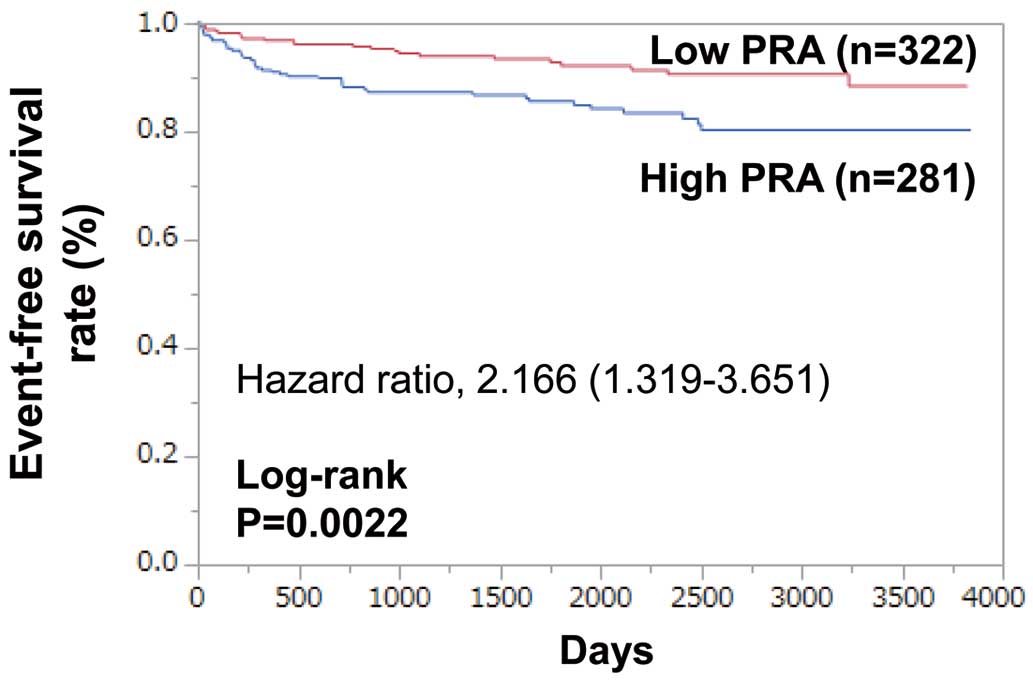
Table 3.
Cox Regression Analysis of PRA for MACE in Patients Not Previously Treated With RAS Inhibitors
| |
MACE |
| Low PRA |
High PRA |
P value |
| Unadjusted HR |
1 |
2.166 (1.319–3.651) |
0.0021 |
| Adjusted HR |
1 |
1.701 (1.010–2.927) |
0.0459 |
Cox proportional hazards model adjusted for the following covariates: age, BNP, left ventricular ejection fraction, hemoglobin and eGFR. RAS, renin-angiotensin system. Other abbreviations as in Tables 1,2.
Furthermore, to exclude the effect of β-blockers on PRA, 580 patients who had not been treated with RAS inhibitors or β-blockers on admission were analyzed. They were again divided into 2 groups according to their PRA (2.0 ng/mL/h): high PRA group (n=272) and low PRA group (n=308). During follow-up (median 4.8±3.1 years), MACE occurred in 61 patients: 40 (14.7%) in the high PRA group and 21 (6.8%) in the low PRA group. Kaplan-Meier curves show that MACE-free survival was significantly lower in the high PRA group than in the low PRA group (log-rank P=0.0032;
Figure 3). High PRA was associated with MACE (HR 2.168; 95% CI 1.293–3.743, P=0.0031). After adjusting for covariates including age, BNP, LVEF, Hb, eGFR, a high PRA remained as an independent predictor of MACE (HR 1.732; 95% CI 1.010–3.047, P=0.0460) (Table 4).
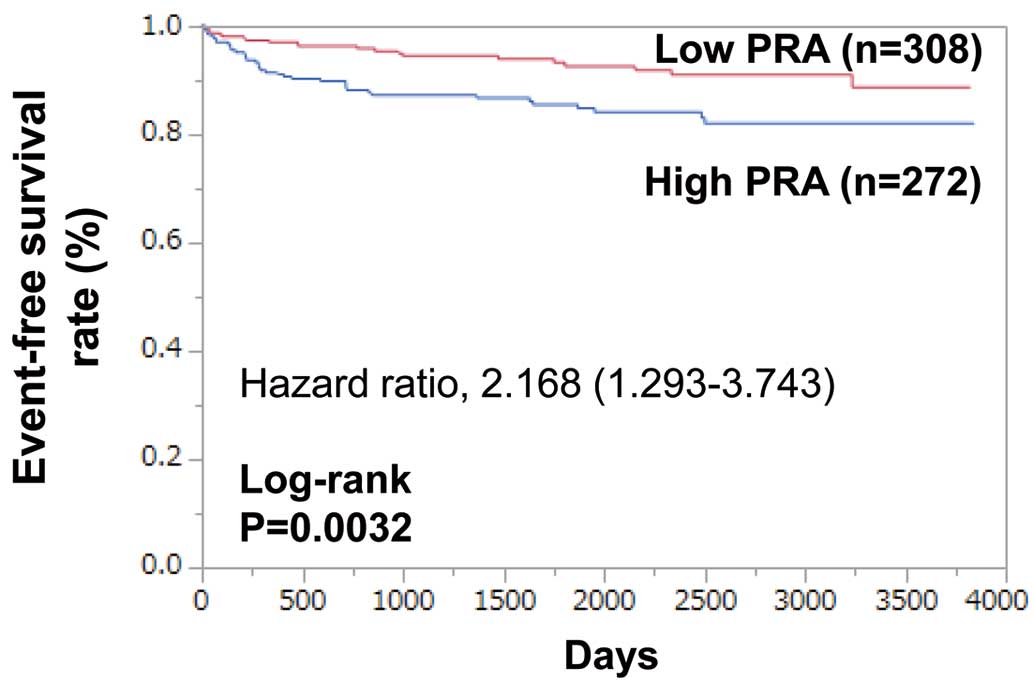
Table 4.
Cox Regression Analysis of PRA for MACE in Patients Not Previously Treated With RAS Inhibitors or β-blockers
| |
MACE |
| Low PRA |
High PRA |
P value |
| Unadjusted HR |
1 |
2.168 (1.293–3.743) |
0.0031 |
| Adjusted HR |
1 |
1.732 (1.010–3.047) |
0.0460 |
Cox proportional hazards model adjusted for the following covariates: age, BNP, left ventricular ejection fraction, hemoglobin and eGFR. Other abbreviations as in Tables 1–3.
To avoid the acute effect of the emergency use of diuretic medications on PRA, we excluded 85 patients who were treated with diuretics in the acute phase before blood sampling for PRA measurement. The remaining 793 patients were divided into 2 groups according to their PRA values (2.0 ng/mL/h): high PRA group (n=395) and low PRA group (n=398). During follow-up (median 4.7±3.0 years), MACE occurred in 77 patients: 50 patients (12.7%) in the high PRA group and 27 patients (6.8%) in the low PRA group. High PRA was associated with MACE (HR 1.943; 95% CI 1.227–3.144, P=0.0044). After the adjustment for covariates including age, BNP, LVEF, Hb, eGFR, a high PRA remained as an independent predictor of MACE (HR 1.759; 95% CI 1.091–2.894, P=0.0202).
Discussion
The principal finding of our present study was that higher PRA was associated with poor prognosis in patients with AMI who underwent emergency coronary angiography independent of previously known predictors such as BNP and EF. Muhlestein et al showed that in 1,165 patients with proven CAD with normal left ventricular function and no previous history of MI or HF, elevated baseline PRA was associated with cardiac morbidity and mortality.8
Verma et al reported that in patients with stable vascular disease, baseline PRA predicted cardiovascular death and HF.6
Our results from AMI patients were quite concordant with these previous studies of stable CAD patients.
Renin is a member of the family of aspartic proteases.11
PRA is influenced by medication, such as ACEIs, ARBs, and β-adrenergic receptor blockers. ACEIs and ARBs increase PRA.12
Conversely, β-blockers may lower PRA in patients treated with ACEIs and/or diuretics.13,14
Therefore, we also analyzed in patients who were not treated with these drugs before the onset of AMI. The results were similar to those obtained in the entire group of patients. In the analyses of the entire group of patients, the high PRA group took more β-blockers, which was discordant with previous reports. Beta-blockers are more strongly recommended in MI patients with reduced EF than in those with preserved EF. Therefore, it is possible that β-blockers were prescribed because of lower EF in the high PRA group. An earlier study showed that PRA was associated with an increased risk of all-cause and cardiovascular death in acute decompensated HF (ADHF) patients already receiving RAS inhibitors.15
In the present study of AMI patients, PRA independently predicted prognosis, even in RAS-inhibitor naive patients, and in both RAS-inhibitor and β-blocker naive patients. Therefore, a high PRA in AMI patients may be useful for predicting cardiovascular events regardless of previous use of RAS inhibitors or β-blockers.
Although several previous studies have shown the association of PRA with cardiovascular diseases, the mechanisms of the relationship between high PRA and clinical outcome remains unclear. Renin is the initial and rate-limiting step of the RAS16
and may exert direct effects augmenting pro-inflammatory pathways.17
Therefore, it is hypothesized that the pharmacological targeting of PRA may suppress the inflammatory process, stop the progression of the disease, and thereby improve the clinical outcome of AMI patients. Alternatively, aldosterone breakthrough may be related to increased risk in the high PRA group. Aldosterone breakthrough is the phenomenon in which aldosterone rises to normal levels despite treatment with RAS inhibitors and this occurs in 10% of patients treated with RAS inhibitors over 6 months, and in >50 % over 1 year, leading to adverse cardiovascular effects.18
In our study, plasma aldosterone tended to be higher in the high PRA group compared with the low PRA group, suggesting a possible effect of aldosterone breakthrough.
Study Limitations
First, this was a retrospective, single-center study, so the data might have a selection bias. Second, it is generally recommended that PRA is measured while in the patient is supine for >30 min, but the supine position might exacerbate AMI in the acute stage. Therefore, most blood samples were not obtained after 30 min at rest. In addition, because of being in the acute phase of AMI, some patients were vomiting and so we could not get sufficient information on the amount of any meals. Furthermore, we could not evaluate accurately the last meal time because the hemodynamics on admission of AMI are sometimes unstable and we could not get enough information from the patient’s interview. Therefore, we could not compare the influence of meal state after admission of AMI between the 2 groups. Third, residual confounders might exist. Fourth, we used only baseline PRA values, so it is unknown if serial changes in PRA values are associated with prognosis. Similarly, it is uncertain if some medications that lower the PRA could improve clinical outcomes in AMI patients.
Conclusions
Elevated levels of PRA were independently associated with long-term prognosis in AMI patients regardless of previous use of RAS inhibitors or β-blockers.
Acknowledgments
We thank Yoko Wada, Yuki Kamada, Rika Nagao, and Ikuyo Yoshida for their support with the data collection process.
Disclosures
There are no conflicts of interest to declare regarding this study.
References
- 1.
de Lemos JA, Morrow DA, Bentley JH, Omland T, Sabatine MS, McCabe CH, et al. The prognostic value of B-type natriuretic peptide in patients with acute coronary syndromes. N Engl J Med 2001; 345: 1014–1021.
- 2.
Richards AM, Nicholls MG, Espiner EA, Lainchbury JG, Troughton RW, Elliott J, et al. B-type natriuretic peptides and ejection fraction for prognosis after myocardial infarction. Circulation 2003; 107: 2786–2792.
- 3.
Suzuki S, Yoshimura M, Nakayama M, Mizuno Y, Harada E, Ito T, et al. Plasma level of B-type natriuretic peptide as a prognostic marker after acute myocardial infarction: A long-term follow-up analysis. Circulation 2004; 110: 1387–1391.
- 4.
Alderman MH, Madhavan S, Ooi WL, Cohen H, Sealey JE, Laragh JH. Association of the renin-sodium profile with the risk of myocardial infarction in patients with hypertension. N Engl J Med 1991; 324: 1098–1104.
- 5.
Vergaro G, Emdin M, Iervasi A, Zyw L, Gabutti A, Poletti R, et al. Prognostic value of plasma renin activity in heart failure. Am J Cardiol 2011; 108: 246–251.
- 6.
Verma S, Gupta M, Holmes DT, Xu L, Teoh H, Gupta S, et al. Plasma renin activity predicts cardiovascular mortality in the Heart Outcomes Prevention Evaluation (HOPE) study. Eur Heart J 2011; 32: 2135–2142.
- 7.
Parikh NI, Gona P, Larson MG, Wang TJ, Newton-Cheh C, Levy D, et al. Plasma renin and risk of cardiovascular disease and mortality: The Framingham Heart Study. Eur Heart J 2007; 28: 2644–2652.
- 8.
Muhlestein JB, May HT, Bair TL, Prescott MF, Horne BD, White R, et al. Relation of elevated plasma renin activity at baseline to cardiac events in patients with angiographically proven coronary artery disease. Am J Cardiol 2010; 106: 764–769.
- 9.
Nomenclature and criteria for diagnosis of ischemic heart disease. Report of the Joint International Society and Federation of Cardiology/World Health Organization task force on standardization of clinical nomenclature. Circulation 1979; 59: 607–609.
- 10.
Ho KK, Pinsky JL, Kannel WB, Levy D. The epidemiology of heart failure: The Framingham Study. J Am Coll Cardiol 1993; 22: 6A–13A.
- 11.
Krop M, Lu X, Danser AH, Meima ME. The (pro)renin receptor. Pflugers Arch 2013; 465: 87–97.
- 12.
Schroten NF, Gaillard CA, van Veldhuisen DJ, Szymanski MK, Hillege HL, de Boer RA. New roles for renin and prorenin in heart failure and cardiorenal crosstalk. Heart Fail Rev 2012; 17: 191–201.
- 13.
Holmer SR, Hense HW, Danser AH, Mayer B, Riegger GA, Schunkert H. β Adrenergic blockers lower renin in patients treated with ACE inhibitors and diuretics. Heart 1998; 80: 45–48.
- 14.
Holmer SR, Hengstenberg C, Mayer B, Engel S, Löwel H, Riegger GA, et al. Marked suppression of renin levels by beta-receptor blocker in patients treated with standard heart failure therapy: A potential mechanism of benefit from beta-blockade. J Intern Med 2001; 249: 167–172.
- 15.
Ueda T, Kawakami R, Nishida T, Onoue K, Soeda T, Okayama S, et al. Plasma renin activity is a strong and independent prognostic indicator in patients with acute decompensated heart failure treated with renin-angiotensin system inhibitors. Circ J 2015; 79: 1307–1314.
- 16.
de Boer RA, Schroten NF, Bakker SJ, Mahmud H, Szymanski MK, van der Harst P, et al. Plasma renin and outcome in the community: Data from PREVEND. Eur Heart J 2012; 33: 2351–2359.
- 17.
Campbell DJ. Critical review of prorenin and (pro)renin receptor research. Hypertension 2008; 51: 1259–1264.
- 18.
Bomback AS, Klemmer PJ. The incidence and implications of aldosterone breakthrough. Nat Clin Pract Nephrol 2007; 3: 486–492.




