Abstract
Background:
Despite the well-established benefits in patients with nonvalvular atrial fibrillation (NVAF), anticoagulants have been underused in elderly patients. The All Nippon AF In the Elderly (ANAFIE) Registry is a multicenter, prospective, observational study with 2-year follow-up of Japanese patients aged ≥75 years with a definitive diagnosis of NVAF, aiming to collect detailed information on clinical status and therapeutic challenges in this patient population.
Methods and Results:
Patients were enrolled from October 2016 to January 2018. A total of 32,726 patients (57.2% male) were included. The average age, CHADS2
score, and creatinine clearance were 81.5±4.8 years (26.2% of patients were aged ≥85 years), 2.9±1.2, and 48.4±21.8 mL/min, respectively. Paroxysmal AF was the most common clinical AF type (42.0%), and most patients (97.2%) had comorbidities. Most patients (91.9%) were receiving anticoagulant therapy; of these, 27.8% and 72.2% were treated with warfarin and direct oral anticoagulants, respectively. The average number of concomitant drugs used was 6.6±3.2, including anticoagulants.
Conclusions:
The ANAFIE Registry is the largest prospective registry study of elderly Japanese patients with NVAF to date. Baseline data indicate that patients in this age group are treated in a manner similar to their younger counterparts.
Nonvalvular atrial fibrillation (NVAF) increases in prevalence with advancing age and is associated with an increased risk of thromboembolic events, including stroke and systemic embolism.1–3
To both prevent stroke in patients with AF and minimize the negative consequences of stroke, clinical guidelines recommend the use of oral anticoagulant therapies, which include vitamin K antagonists, such as warfarin, and direct oral anticoagulants (DOACs), including dabigatran, rivaroxaban, apixaban, and edoxaban.4–6
However, to date, patients with AF aged ≥75 years have been undertreated with oral anticoagulants,7,8
for reasons that may include the following. First, most randomized controlled clinical trials aiming to establish the clinical benefits of oral anticoagulants have not enrolled sufficient numbers of patients aged ≥75 years, therefore clinical data, including efficacy and safety data, are not available to determine adequate treatment in this patient population.9–12
Second, age is a risk factor for antithrombotic drug-related bleeding.1–3
Finally, multiple comorbidities and concomitant polypharmacy also increase the bleeding risk in elderly patients with AF.13
A recent age-stratified subanalysis of the PREFER in AF (PREvention oF thromboembolic events-European Registry in Atrial Fibrillation) study demonstrated that the absolute benefit of oral anticoagulants was higher in patients with AF aged ≥85 years than in those aged <85 years.14
Furthermore, an age-stratified subanalysis of a Fushimi AF Registry demonstrated that Japanese patients with AF aged ≥85 years had a higher incidence of stroke but similar major bleeding risk, as compared with a younger population with AF.15
It is therefore reasonable to propose that elderly patients with NVAF should be treated with oral anticoagulants in the same way as younger patients. Indeed, current guidelines for the management of NVAF have doubled the score of the age factor for patients aged ≥75 years (CHA2DS2-VASc), and recommend initiation of anticoagulant therapy for this age group.4–6
To disseminate and implement this recommendation in clinical practice, surveys and registries are needed to verify whether real-world clinical practice is in keeping with current guidelines. The aims of the present study, the All Nippon AF In the Elderly (ANAFIE) Registry,16
is therefore to collect information regarding the actual clinical status of elderly patients with NVAF aged ≥75 years, to elucidate the current status of anticoagulant therapy and its clinical outcomes, to identify risk factors for death, thromboembolism, and major bleeding, as well as their interrelationships, and to establish a database for this specific patient population to aid in the development of therapeutic strategies. The analysis of these data may provide information on what is required or recommended in clinical practice when treating elderly patients with NVAF, which involves not only of cardiologists, but also of clinicians of various specialties.
Here we describe the baseline demographic and clinical characteristics of the elderly patients with NVAF and their medical histories and medications at the time of their enrollment in the ANAFIE Registry.
Methods
Study Design
The ANAFIE Registry is a multicenter, prospective observational study of elderly men and women in Japan aged ≥75 years with AF, which started in 2016 with the aim of enrolling 30,000 patients within 2 years, and in which a minimum 2-year follow-up will be conducted for each patient.
The study design and ethical considerations have been reported previously.16
Briefly, patients eligible for the study included those aged ≥75 years with a definitive diagnosis of NVAF based on ECG. Patients who were currently participating or planning to participate in an interventional study, those with a definitive diagnosis of mitral stenosis or with an artificial heart valve and those with a very recent history of a cardiovascular event were excluded.
The analysis set comprised all participants enrolled in the study, excluding those for whom either of the following significant protocol deviations was observed at the time of written consent or later in the study: not meeting all of the inclusion criteria or meeting any of the exclusion criteria.
A frequency table was created for categorical variables, and summary statistics (n, mean, standard deviation (SD), minimum value, median, and maximum value) were calculated for continuous variables.
Results
A total of 33,115 patients were enrolled between October 2016 and January 2018 (this was achieved in 15 months, 9 months shorter than the planned 2-year period). As shown in
Figure 1, a total of 389 patients were excluded, resulting in an analysis set of 32,726 patients.
Demographic Characteristics
Table 1
summarizes the demographic backgrounds of the enrolled patients. The age distribution of patients with NVAF is shown in
Figure 2, which was similar to that of the general population in Japan,17
particularly between the ages of 75 and 88 years (≈0.2% of the general population). However, the proportion of patients decreased linearly after age 89 years. The average age of patients was 81.5±4.8 years. Men numbered 18,733 (57.2%) in the analysis set. Average height and body weight were 157.2±9.5 cm and 57.8±11.2 kg, respectively (Table 1), and 52.3% of patients had body weight <60 kg, 38.8% ≥60 kg, and 8.9% had no body weight data (Table 1).
Table 1.
Baseline Patient Demographics
| Analysis set |
n=32,726 |
| Sex |
| Male |
18,733 (57.2) |
| Age (on the day written informed consent was obtained), years |
| Mean±SD |
81.5±4.8 |
| Minimum |
75 |
| Q1 |
77 |
| Median |
81 |
| Q3 |
85 |
| Maximum |
103 |
| ≥75 to <80 |
13,059 (39.9) |
| ≥80 to <85 |
11,103 (33.9) |
| ≥85 to <90 |
6,401 (19.6) |
| ≥90 to <95 |
1,877 (5.7) |
| ≥95 to <100 |
275 (0.8) |
| ≥100 |
11 (0.0) |
| Height, cm (n=28,393) |
157.2±9.5 |
| Body weight, kg (n=29,824) |
57.8±11.2 |
| Systolic blood pressure, mmHg (n=30,076) |
127±17 |
| Diastolic blood pressure, mmHg |
71±12 |
| Creatinine clearance, mL/min (n=26,498)* |
| Mean±SD |
48.4±21.8 |
| <15 or on dialysis |
428 (1.3) |
| ≥15 to <30 |
3,516 (10.7) |
| ≥30 to <50 |
10,886 (33.3) |
| ≥50 to <80 |
10,577 (32.3) |
| ≥80 |
1,122 (3.4) |
| eGFR (mL/min/1.73 m2) (n=28,723) |
54.0±29.2 |
Data are presented as n (%) except for mean±SD, minimum, Q1, median, Q2 and maximum. *Calculated using the Cockcroft–Gault equation. eGFR, estimated glomerular filtration rate; SD, standard deviation.
Table 2
summarizes patients’ baseline clinical characteristics, morbidity, and medical histories. Among the patients in the analysis set, 42.0% were categorized as having paroxysmal AF, 16.6% and 13.5% of patients as having persistent AF and long-term persistent AF, respectively, and 27.9% of patients as having permanent AF. Patients with a history of nonpharmacological therapy accounted for 17.5% of the analysis set: 9.2% with catheter ablation, 2.2% with electrical defibrillation, 0.5% with implantable cardioverter defibrillator, and 7.3% with implanted pacemaker.
Table 2.
Baseline Clinical Characteristics, Morbidity, and Medical History
| Type of atrial fibrillation |
n=32,726 |
| Paroxysmal |
13,751 (42.0) |
| Persistent |
5,423 (16.6) |
| Long-term persistent |
4,427 (13.5) |
| Permanent |
9,125 (27.9) |
| History of major bleeding |
1,265 (3.9) |
| Intracranial bleeding |
649 (2.0) |
| Upper gastrointestinal bleeding |
274 (0.8) |
| Lower gastrointestinal bleeding |
309 (0.9) |
| Other bleeding |
251 (0.8) |
| Morbidity and medical history |
31,826 (97.2) |
| Hypertension |
24,615 (75.2) |
| Diabetes mellitus |
8,833 (27.0) |
| Dyslipidemia |
13,887 (42.4) |
| Hyperuricemia |
7,402 (22.6) |
| Kidney diseases |
7,642 (23.4) |
| Severe hepatic dysfunction |
299 (0.9) |
| Respiratory diseases |
4,194 (12.8) |
| Cardiac diseases |
19,253 (58.8) |
| Myocardial infarction |
1,874 (5.7) |
| Angina pectoris |
5,600 (17.1) |
| Heart failure* |
12,262 (37.5) |
| Left ventricular systolic dysfunction |
689 (2.1) |
| Valvular disease (including post-surgery valvular disease) |
4,006 (12.2) |
| Cardiomyopathy |
1,203 (3.7) |
| Cerebrovascular diseases |
7,410 (22.6) |
| Atherosclerotic cerebral infarction |
672 (2.1) |
| Cardiogenic cerebral infarction |
2,412 (7.4) |
| Lacunar cerebral infarction |
1,456 (4.4) |
| Hemorrhagic stroke |
370 (1.1) |
| Other vascular diseases |
1,740 (5.3) |
| Thromboembolism |
2,809 (8.6) |
| VTE: pulmonary embolism+deep vein thrombosis |
417 (1.3) |
| Pulmonary embolism |
117 (0.4) |
| Deep vein thrombosis |
340 (1.0) |
| Internal carotid artery stenosis |
767 (2.3) |
| Hyperthyroidism |
482 (1.5) |
| Malignant tumor (primary tumor-bearing) |
3,589 (11.0) |
| Dementia |
2,560 (7.8) |
| Falling (within 1 year prior to giving written informed consent) |
2,379 (7.3) |
Data are n (%). *Non-congestive or controlled heart failure. VTE, venous thromboembolism.
Most patients (97.2%) had the following comorbidities or medical history: 75.2% had hypertension, 58.8% cardiac disease, 42.4% dyslipidemia, 27.0% diabetes mellitus, 22.6% cerebrovascular disorder, 22.6% hyperuricemia, and 12.8% had respiratory disease (Table 2).
Among the 19,253 patients with cardiac disease, 5.7% had myocardial infarction, 17.1% had angina pectoris, 37.5% had heart failure, and 12.2% had valvular disease, including post-surgery valvular disease (Table 2). Patients with kidney disease comprised 23.4% of the analysis set, most of whom had chronic kidney disease (Table 2).
Among the 7,410 patients with a cerebrovascular disorder, 2,412 (32.6%) had cardiogenic cerebral infarction, and 370 (5.0%) had hemorrhagic stroke (Table 2). Thromboembolism-related diseases were found in 2,809 patients; among these patients, 4.2% had pulmonary embolism, 12.1% had deep vein thrombosis, and 27.3% had internal carotid artery stenosis (Table 2).
History of Major Bleeding
Among participants in the analysis set, 3.9% had a history of major bleeding (Table 2), including intracranial bleeding (2.0%), upper gastrointestinal bleeding (0.8%), and lower gastrointestinal bleeding (0.9%).
Creatinine Clearance (CrCL)
CrCL and estimated glomerular filtration rate (eGFR) are summarized in
Table 1. Average CrCL was 48.4±21.8 mL/min; 1.3% of patients had renal failure (CrCL <15 mL/min or were on dialysis), and 10.7% had severely reduced renal function (CrCL ≥15 to <30 mL/min).
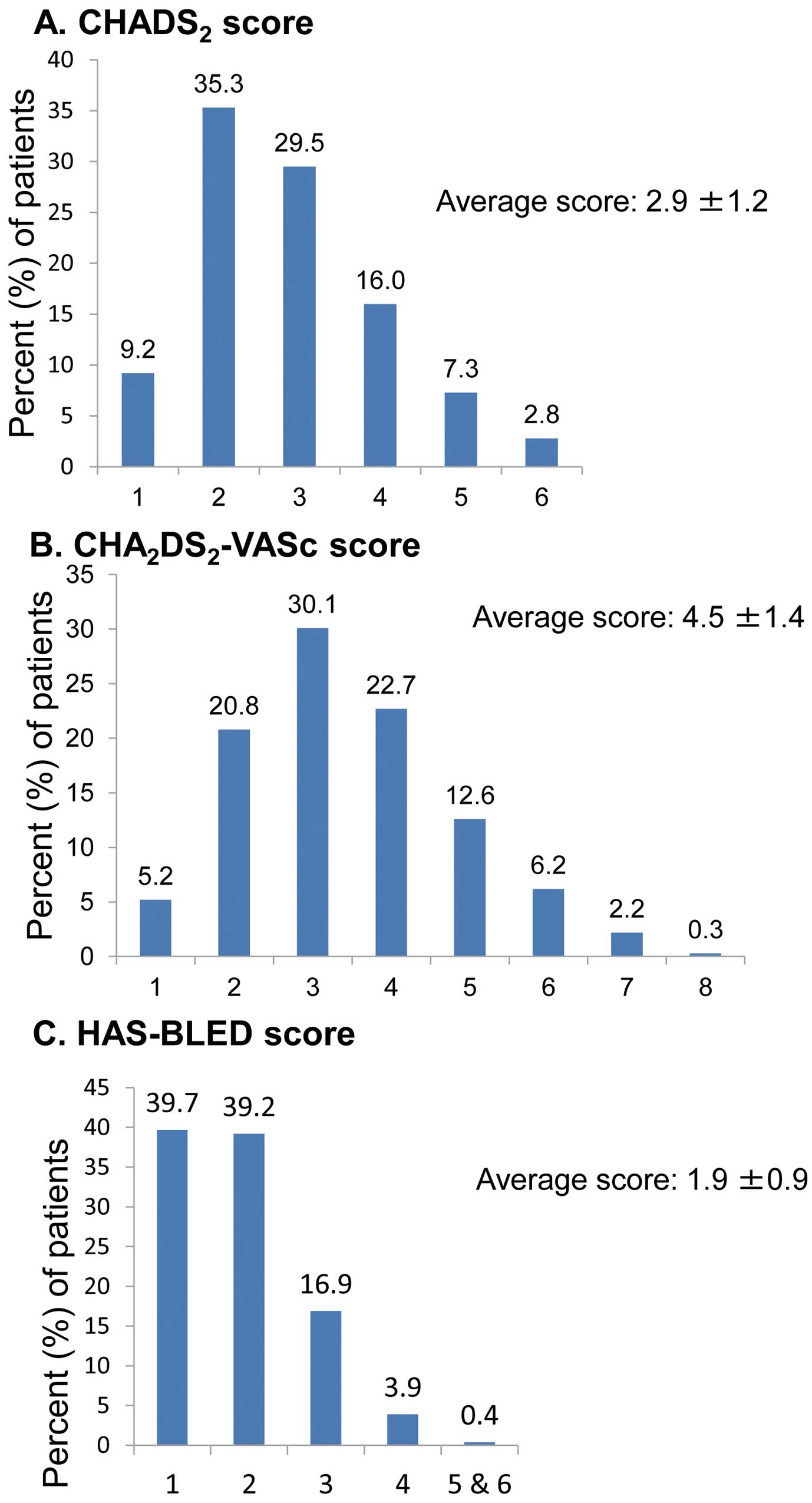
Risk Scores
Three risk scores and their distributions are shown in
Figure 3. The average CHADS2
score was 2.9±1.2, with a median score of 3 (range 1–6), and 55.6% of patients had a score ≥3 (Figure 3).
The average CHA2DS2-VASc score was 4.5±1.4, with a median of 4 (range, 2–9); 52.8% of patients had a score of 4 or 5 and 21.2% had a score of 6–9 (Figure 3). The average HAS-BLED score was 1.9±0.9, with a median score of 2 (range, 1–6); 78.9% of patients had a score of 1 or 2 and 21.1% had a score of 3–6 (Figure 3).
Anticoagulants
Over 90% (91.9%) of patients had been treated with anticoagulants prior to study participation. The anticoagulants received were warfarin in 27.8% of patients, apixaban in 26.9%, rivaroxaban in 21.5%, edoxaban in 16.0%, dabigatran in 7.8%, and <0.1% received a parenteral anticoagulant. Daily dose distributions of the DOACs are illustrated in
Figure 4. Among patients who received warfarin, the average prothrombin time (PT)–international normalized ratio (INR) was 1.97±0.36, and 326 (3.9%) patients had PT-INR ≥2.6. The average time in therapeutic range (TTR) was 75.3±29.9% (Figure 5).
Concomitant Medications Other Than Anticoagulants
Approximately 95% of patients received concomitant therapy with drugs other than anticoagulants Antiarrhythmic agents were used in 59.2% of patients, 43.4% of patients received rate control therapy, and 20.5% received rhythm control therapy. Antiplatelet agents included aspirin in 11.4% of patients, P2Y12 inhibitor (P2Y12-I) in 4.5% of patients, and other antiplatelet agents in 4.2% of patients. Dual antiplatelet therapy (concomitant use of aspirin and P2Y12-1) was received by 0.8% of patients (Supplementary Table). Other medications for the treatment of comorbidities, including hypertension, dyslipidemia, diabetes mellitus, dementia, cancer, and psychotic disorder, are also summarized in
Supplementary Table.
As shown in
Figure 6, the average number of drugs per patient was 6.6±3.2, with a median of 6 (range, 0–27). More than half (54.2%) of the patients were being treated with 5–9 drugs, followed by 23.9% with 2–4 drugs, and 16.9% with ≥10 drugs.
Discussion
The ANAFIE Registry is a prospective observational study in which a total of 33,115 Japanese patients with NVAF aged ≥75 years were enrolled, with a planned follow-up period of 2 years. This is the first registry study in a large population of elderly patients with NVAF in real-world clinical settings.
In the present study, we showed that the age distribution of patients with NVAF was similar to that of the general population in Japan, particularly the age group 75–88 years. However, the proportion decreased linearly in patients aged over 89 years. This may be attributable to (1) a decline in the survival of patients with NVAF beginning even earlier than 89 years of age, given that the prevalence of AF increases with advancing age, or (2) a shift of these patients from home to nursing homes, which may have made it difficult for the hospitals and clinics to enroll the patients.
Because this registry included elderly patients aged ≥75 years, the average patient age was markedly higher than in other registries, such as the Fushimi AF Registries15,18
and the PREFER in AF Registries14,19
(81.5±4.8 years vs. 73.6±11.018
and 71.514
years, respectively). As expected, patients in our registry had multiple comorbidities, including cardiovascular disease, hypertension, vascular disease, diabetes mellitus, kidney disease, and thrombosis/embolism-related diseases. In line with this, the CHADS2
and CHA2DS2-VASc scores were also higher in our patients compared with those in other registries: CHADS2, 2.9±1.2 vs. 2.014
and 2.03±1.3315; CHA2DS2-VASc, 4.5±1.4 vs. 3.4,19
3.4±1.7,15
and 3.36±1.70.15
The HAS-BLED score was 1.9±0.9, which is comparable to that of the PREFER in AF Registry19
(2.0) and slightly higher than that of the Fushimi AF Registry18
(1.6±0.9), demonstrating that bleeding risk did not differ significantly among patients in these 3 registries.
Regarding kidney function, the eGFR in the present study (54.0 mL/min/1.73 m2) was slightly higher than in the most recent Fushimi AF Registry18
(51.1 mL/min/1.73 m2) and lower than in the previous Fushimi AF Registry15
(61.0 mL/min/1.73 m2). The prevalence of patients on dialysis or with CrCL <15 mL/min in the present study was 1.3%, compared with 2.4% and 2.5% in 2 Fushimi AF Registries, respectively, and 0.2% (patients with eGFR <15 mL/min/1.73 m2) in the PREFER in AF Registry.19
Based on the demonstrated benefit of oral anticoagulants for preventing stroke and systemic embolism in patients with AF, current guidelines recommend anticoagulation therapy for patients with a CHADS2
score ≥1 but a CHA2DS2-Vasc score ≥2.4–6
The European Society of Cardiology guidelines recommend consideration of anticoagulation therapy even for those with CHA2DS2-VASc=1.4
Despite this recommendation, there has been a trend towards underuse of oral anticoagulants in elderly patients with AF, a trend that has continued despite the development of newer drugs (DOACs) with anticoagulation effects similar to warfarin but with a reduced risk of bleeding.20
A recent study that included Fushimi AF Registry data demonstrated that only 53% of patients aged 73.7±10.9 years were on anticoagulant therapy.15
In another recent study using data from the Fushimi AF Registry, 64% of patients aged 73.6±11.0 years18
were on anticoagulant therapy. The latter study also demonstrated a gradual (year-by-year) increase in the proportion of patients on anticoagulant therapy for each CHADS2
score from 0 to 6. However, only 70% of patients with a CHADS2
score of 5 or 6 were prescribed anticoagulants.
In contrast, 91.9% of patients with AF aged 81.5±4.8 years were receiving anticoagulant therapy in the present registry study. This seems an extremely high rate, even considering that the higher average CHA2DS2-VASc score (4.5±1.4) of included patients was comparable to that in the most recent PREFER in AF Registry.14
In that registry, the CHA2DS2-VASc score in patients aged ≥85 years and <85 years was 4.7±1.4 and 3.3±1.3, respectively, and the rate of patients on anticoagulant therapy was 77.8% and 83.2%, respectively.14
In that registry,14
the authors demonstrated that patients aged ≥85 years received more benefit from anticoagulant therapy than those aged <85 years. Based on these observations, the elderly patients in the present study appeared to be adequately treated for prevention of thromboembolic events, with no apparent underuse of oral anticoagulants in patients with AF, even among those aged ≥75 years. The very high rate of patients on anticoagulant therapy in the present study may partly be attributable to recent guidelines recommending anticoagulant therapy in elderly patients and/or an era difference. Selection bias may have also contributed to the high rate of anticoagulation therapy in the present study, because physicians may have tended to choose patients who appeared to be able to complete the 2-year observation study.
In addition to the comorbidities described, in the present study we collected data on dementia and history of falling prior to enrollment. The prevalence of dementia and prior falling was 7.8% and 7.3%, respectively. The association between AF and dementia is well documented,21–23
and oral anticoagulant therapy is known to reduce the risk of developing dementia in patients with AF.24,25
Patients with AF at high risk of falling are presumed to be at increased risk of intracranial hemorrhage, and a high risk of falling is cited as a contraindication of anticoagulation therapy.13,26
However, recent studies have demonstrated that DOACs and warfarin are beneficial for patients with AF who have a high risk of falling.27,28
The 2-year follow-up data from the present study may provide additional evidence in this regard.
Concomitant use of multiple drugs, or polypharmacy, can produce negative consequences associated with an increased risk of adverse drug events, drug–drug interactions, medication nonadherence, reduced functional capacity, and multiple geriatric syndromes.29
With respect to anticoagulant therapy for patients with NVAF, major bleeding events are of the greatest concern.30,31
Patients in the present study were taking more than 6 drugs on average at baseline; the 2-year follow-up data will provide information regarding the efficacy and safety of anticoagulant therapy in elderly populations.
Conclusions
The ANAFIE Registry consists of 33,115 Japanese patients with NVAF aged ≥75 years, the largest elderly patient cohort of individuals affected by AF within a single ethnic group, representing the real-world clinical status of NVAF treatment among elderly patients in Japan. Taking well-documented global observations into account, the baseline data indicated that patients in this age group were being treated in a similar manner to their younger counterparts, indicating the appropriate implementation of current guidelines with respect to the rate of anticoagulant therapy.
The 2-year follow-up observation and planned subcohort studies,16
including data on biomarkers, ECG, heart rate, hypertension, cognitive function, frailty, and treatment adherence, will further illuminate real-world clinical practices for the treatment of NVAF in the era of DOAC therapy.
Acknowledgments
We would like to thank all centers that participated in this registry, and all patients who gave their consent to participate. We also thank ASCA Corporation for their assistance in writing and editing the manuscript. This research was supported in part by IQVIA Services Japan and EP-CRSU.
Sources of Funding
The ANAFIE Registry is sponsored by Daiichi Sankyo.
Disclosures
Y.K. received remuneration from Daiichi Sankyo, Bayer, and Nippon Boehringer Ingelheim. T. Yamashita received research funding from Bristol-Myers Squibb, Bayer, and Daiichi Sankyo, manuscript fees from Daiichi Sankyo, and Bristol-Myers Squibb, and remuneration from Daiichi Sankyo, Bayer, Pfizer Japan, Bristol-Myers Squibb, and Ono Pharmaceutical. M.A. received research funding from Bayer, and Daiichi Sankyo, and remuneration from Bristol-Myers Squibb, Nippon Boehringer Ingelheim, Bayer, and Daiichi Sankyo. H.A. received remuneration from Daiichi Sankyo. T.I. received research funding from Daiichi Sankyo and Bayer, and remuneration from Daiichi Sankyo, Bayer, Nippon Boehringer Ingelheim, and Bristol-Myers Squibb. K.O. received remuneration from Nippon Boehringer Ingelheim, Daiichi Sankyo, Johnson & Johnson, and Medtronic. W.S. received research funding from Bristol-Myers Squibb, Daiichi Sankyo, and Nippon Boehringer Ingelheim, and patent royalties/licensing fees from Daiichi Sankyo, Pfizer Japan, Bristol-Myers Squibb, Bayer, and Nippon Boehringer Ingelheim. H.T. received research funding from Daiichi Sankyo, Mitsubishi Tanabe Pharma, Nippon Boehringer Ingelheim, and IQVA services Japan, remuneration from Daiichi Sankyo, Bayer, Nippon Boehringer Ingelheim, Pfizer Japan, Otsuka Pharmaceutical, and Mitsubishi Tanabe Pharma, scholarship funding from Daiichi Sankyo, Mitsubishi Tanabe Pharma, and Teijin Pharma, and consultancy fee from Novartis Pharma, Pfizer Japan, Bayer, Nippon Boehringer Ingelheim and Ono Pharmaceutical. K.T. received remuneration from Daiichi Sankyo, Bayer, Bristol-Myers Squibb, and Nippon Boehringer Ingelheim. A.H. participated in a course endowed by Boston Scientific Japan, and has received research funding from Daiichi Sankyo and Bayer, and remuneration from Bayer, Daiichi Sankyo, Bristol-Myers Squibb, Nippon Boehringer Ingelheim, Sanofi, Astellas Pharma, Sumitomo Dainippon Pharma, Amgen Astellas BioPharma, and AstraZeneca, and patent royalties/licensing fees from Toa Eiyo. M.Y. received research funding from Nippon Boehringer Ingelheim, and remuneration from Nippon Boehringer Ingelheim, Daiichi Sankyo, Bayer, Bristol-Myers Squibb, Pfizer Japan, and CSL Behring. T. Yamaguchi acted as an Advisory Board member of Daiichi Sankyo, and received remuneration from Daiichi Sankyo, and Bristol-Myers Squibb. S.T. received research funding from Nippon Boehringer Ingelheim and remuneration from Daiichi Sankyo. T.K. has stock, and is an employee of Daiichi Sankyo. J.K., A.T. are employees of Daiichi Sankyo. H.I. received remuneration from Daiichi Sankyo, Bayer, Bristol-Myers Squibb, and Nippon Boehringer Ingelheim.
Supplementary Files
Please find supplementary file(s);
http://dx.doi.org/10.1253/circj.CJ-19-0094
References
- 1.
Marinigh R, Lip GYH, Fiotti N, Giansante C, Lane DA. Age as a risk factor for stroke in atrial fibrillation patients: Implication for thromboprophylaxis. J Am Coll Cardiol 2010; 56: 827–837.
- 2.
Andreotti F, Rocca B, Husted S, Ajjan RA, ten Berg J, Cattaneo M, et al; on behalf of the EAC Thrombosis Working Group. Antithrombotic therapy in the elderly: Expert Position Paper of the European Society of Cardiology Working Group on Thrombosis. Eur Heart J 2015; 34: 3238–3249.
- 3.
Edholm K, Ragle N, Rondina MT. Antithrombotic management of atrial fibrillation in the elderly. Med Clin North Am 2015; 99: 417–430.
- 4.
Kirchhof P, Benussi S, Kotecha D, Ahlsson A, Atar D, Casadei B, et al. 2016 ESC guidelines for the management of atrial fibrillation developed in collaboration with EACTS. Eur J Cardiothorac Surg 2016; 50: e1–e88.
- 5.
January CT, Wann JS, Calkins H, Cigarroa JE, Cleveland JC, Conti JB, et al. 2014 AHA/ACC/HRS guidelines for the management of patients with atrial fibrillation: Executive summary: A report of the American College of Cardiology/American Heart Association Task Force on Practice Guidelines and the Heart Rhythm Society developed in collaboration with the Society of Thoracic Surgeons. J Am Coll Cardiol 2014; 64: 2246–2280.
- 6.
JCS Joint Working Group. Guidelines for pharmacotherapy of atrial fibrillation (JCS 2013): Digest version. Circ J 2014; 78: 1997–2021.
- 7.
Monte S, Macchia A, Pellegrini F, Romero M, Repore V, D’Ettorre A, et al. Antithrombotic treatment is strongly underused despite reducing overall mortality among high-risk elderly patients hospitalized with atrial fibrillation. Eur Heart J 2006; 27: 2217–2223.
- 8.
Ogilvie IM, Newton N, Welner SA, Cowell W, Lip GY. Underuse of oral anticoagulants in atrial fibrillation: A systematic review. Am J Med 2010; 123: 638–645.
- 9.
Granger CB, Alexander JH, MucMurray JJ, Lopes RD, Hylek EM, Hanna M, et al. Apixaban versus warfarin in patients with atrial fibrillation. N Engl J Med 2011; 365: 981–992.
- 10.
Connolly SJ, Ezekowitz MD, Yusuf S, Eikelboom J, Oldgren J, Parekh A, et al. Dabigatran versus warfarin in patients with atrial fibrillation. N Engl J Med 2009; 361: 1139–1151.
- 11.
Patel MR, Mahaffey KW, Garg J, Pan G, Singer DE, Hacke W, et al. Rivaroxaban versus warfarin in patients with atrial fibrillation. N Engl J Med 2011; 365: 883–891.
- 12.
Giugliano RP, Ruff CT, Braunwald E, Murphy SA, Wicott SD, Halperin JL, et al. Edoxaban versus warfarin in patients with atrial fibrillation. N Engl J Med 2013; 369: 2093–2104.
- 13.
Turagam MK, Velagapudi P, Flaker GC. Stroke prevention in the elderly atrial fibrillation patient with comorbid conditions: Focus on non-vitamin K antagonist oral anticoagulants. Clin Interv Aging 2015; 10: 1431–1444.
- 14.
Patti G, Lucerna M, Pecen L, Siller-Matula JM, Cavallari L, Kirchhof P, et al. Thromboembolic risk, bleeding outcomes and effect of different antithrombotic strategies in very elderly patients with atrial fibrillation: A sub-analysis from the PREFER in AF (PREvention oF thromboembolic Events – European Registry in Atrial Fibrillation). J Am Heart Assoc 2017; 6: e005657.
- 15.
Yamashita Y, Hamatani Y, Esato M, Chun YH, Tsuji H, Wada H, et al. Clinical characteristics and outcomes in extreme elderly (age ≥85 years) Japanese patients with atrial fibrillation. Chest 2015; 149: 401–412.
- 16.
Inoue H, Yamashita T, Akao M, Atarashi H, Ikeda T, Okumura K, et al. Prospective observational study in elderly patients with non-valvular atrial fibrillation: Rationale and design of the All Nippon AF In the Elderly (ANAFIE) Registry. J Cardiol 2018; 72: 300–306.
- 17.
Statistics of Japan. Table 1: Population by age (single year), sex and sex ratio – Total population, Japanese population December 16, 2016 (Portal site of official statistics of Japan). https://www.e-stat.go.jp/en/stat-search/file-download?statInfId=000031473235&fileKind=1 (accessed December 27, 2018) (in Japanese).
- 18.
Yamashita Y, Uozumi R, Hamatani Y, Esato M, Chun Y, Tsuji H, et al. Current status and outcomes of direct oral anticoagulant use in real-world atrial fibrillation patients: Fushimi AF Registry. Circ J 2017; 81: 1278–1285.
- 19.
Kirchhof P, Ammentorp B, Darius H, De Caterina R, Heuzey JYL, Scilling RJ, et al. Management of atrial fibrillation in seven European countries after the publication of the 2010 ESC guidelines on atrial fibrillation: Primary results of the PREvention oF thromboembolic events – European Registry in Atrial Fibrillation (PREFER in AF). Europace 2014; 14: 6–14.
- 20.
Ruff CT, Giugliano RP, Braunwald E, Hoffman EB, Deenadayalu N, Ezekowitz MD, et al. Comparison of the efficacy and safety of new oral anticoagulants with warfarin in patients with atrial fibrillation: A meta-analysis of randomized trials. Lancet 2014; 383: 955–962.
- 21.
Santangeli P, Biase LD, Bai R, Mohanty S, Pump A, Brantes MC, et al. Atrial fibrillation and the risk of incident dementia: A meta-analysis. Heart Rhythm 2012; 9: 1761–1768.
- 22.
de Bruijn RF, Heeringa J, Wolters FJ, Franco OH, Stricker BHC, Hofman A, et al. Association between atrial fibrillation and dementia in the general population. JAMA Neurol 2015; 72: 1288–1294.
- 23.
Chen LI, Norby FL, Gottesman RF, Mosley TH, Soliman EZ, Agaval SK, et al. Association of atrial fibrillation with cognitive decline and dementia over 20 years: The ARIC-NCS (Atherosclerosis Risk in Communities Neurocognitive Study). J Am Heart Assoc 2018; 7: e007301.
- 24.
Friberg L, Rosenqvist M. Less dementia with oral anticoagulation in atrial fibrillation. Eur Heart J 2018; 39: 453–560.
- 25.
Viscogliosi G, Ettorre E, Chiriac IM. Dementia correlates with anticoagulation underuse in older patients with atrial fibrillation. Arch Gerontol Geriatr 2017; 72: 108–112.
- 26.
Fumagatti S, Potpara TS, Larsen TB, Haugaa KH, Dobreanu D, Proclemer A, et al. Frailty syndrome: An emerging clinical problem in the everyday management of clinical arrhythmias [The results of the European Heart Rhythm Association survey]. Europace 2017; 8: 1–7.
- 27.
Martinez BK, Sood NA, Bunz TJ, Coleman GI. Effectiveness and safety of apixaban, dabigatran, and rivaroxaban versus warfarin in frail patients with nonvalvular atrial fibrillation. J Am Heart Assoc 2018; 7: e008643.
- 28.
Steffel J, Giuliano RF, Braunwald E, Murphy SA, Mercuri M, Choi Y, et al. Edoxaban versus warfarin in atrial fibrillation patients at risk of falling: ENGAGE AF-TIMI 48 analysis. J Am Coll Cardiol 2016; 68: 1169–1178.
- 29.
Maher R Jr, Hanlon JT, Hajjer ER. Clinical consequences of polypharmacy in elderly. Expert Opin Drug Saf 2014; 13: 57–65.
- 30.
Chan SH, Chou IJ, Yeh YH, Chiou MJ, Wen MS, Kuo CT, et al. Association between use of non-vitamin K oral anticoagulants with and without concurrent medications and risk of major bleeding in nonvalvular atrial fibrillation. JAMA 2017; 318: 1250–1259.
- 31.
Focks JJ, Brouwer MA, Wojdyla DM, Thomas L, Lopes RD, Washam JB, et al. Polypharmacy and effects of apixaban versus warfarin in patients with atrial fibrillation: Post hoc analysis of the ARISTOTLE trial. BMJ 2016; 353: i2868.