論文ID: CJ-19-0145
論文ID: CJ-19-0145
Background: Antiplatelet therapy is the corner stone of treatment following acute myocardial infarction (AMI). Prasugrel, a new and potent antiplatelet agent, was recently introduced to clinical practice. We compared the clinical outcomes of patients with AMI treated with prasugrel with those treated with clopidogrel in real-world clinical practice in Japan.
Methods and Results: The Japan AMI Registry (JAMIR) is a multicenter, nationwide, prospective registry enrolling patients with AMI from 50 institutes. Between December 2015 and May 2017, a total of 3,411 patients were enrolled. Among them, 3,069 patients were treated with either prasugrel (n=2,607) or clopidogrel (n=462) during hospitalization. Median follow-up period was 12 months. Prasugrel-treated patients were predominantly male, younger, more often showed ST-elevation AMI, and had fewer comorbidities. After adjustment using inverse probability of treatment weighting, the primary endpoint, defined as a composite of cardiovascular death, non-fatal MI and non-fatal stroke, was comparable between the prasugrel and clopidogrel groups (adjusted hazard ratio [HR] 1.07, 95% confidence interval [CI] 0.67–1.72), whereas the risk of major bleeding (BARC type 3 or 5 bleeding) was significantly lower in the prasugrel group (adjusted HR 0.62, 95% CI 0.39–0.99).
Conclusions: The present real-world database of the JAMIR demonstrated that the potent P2Y12-inhibitor prasugrel showed comparable rates of 1-year ischemic events to clopidogrel, but the risk of bleeding was lower with prasugrel than with clopidogrel.
Dual antiplatelet therapy (DAPT) with a P2Y12 inhibitor and aspirin is the mainstay for reducing ischemic events following acute myocardial infarction (AMI). For more than a decade, clopidogrel has been widely used as the P2Y12 inhibitor of choice in DAPT. However, clopidogrel has important limitations, including modest antiplatelet effects, with significant inter-patient variability, mainly because of genetic variations in cytochrome P450 (CYP) 2C19.1 Of note, a decreased response to clopidogrel is common among Asians because of the high prevalence of CYP2C19 loss-of-function alleles.2–4 Recently, new P2Y12 inhibitors, such as prasugrel and ticagrelor, have been developed. Prasugrel has a more consistent, rapid and pronounced inhibition of platelet activity than clopidogrel.5,6 In the TRITON-TIMI 38 (Therapeutic Outcomes by Optimizing Platelet Inhibition with Prasugrel-Thrombolysis in Myocardial Infarction 38) trial, which was conducted in 30 Western and South American countries, but no Asian countries, prasugrel reduced the incidence of recurrent cardiovascular events in patients with acute coronary syndrome (ACS) undergoing percutaneous coronary intervention (PCI) compared with clopidogrel.7 However, the use of prasugrel increased bleeding risk, offsetting the clinical benefits. Indeed, use of prasugrel in patients with a history of cerebrovascular disease, the elderly, and those with low body weight was associated with less clinical efficacy and greater risk of bleeding, resulting in reduced net clinical benefit or clinical harm. As Asians are reported to show a higher bleeding risk than Western populations,8 the PRASFIT ACS (PRASugrel compared with clopidogrel For Japanese patIenTs with ACS undergoing PCI) study examined an adjusted dose of prasugrel (loading dose of 20 mg and maintenance dose of 3.75 mg) and demonstrated efficacy and safety in Japanese ACS patients.9 As a result, an adjusted dose of prasugrel was approved in 2014 in Japan. However, a paucity of data exists on contemporary patterns of antiplatelet therapy and the incidences of ischemic and bleeding events in Japanese AMI patients. We therefore undertook a prospective observational multicenter study to examine the use of antiplatelet therapy in real-world clinical practice and outcomes in a large population of Japanese AMI patients treated with prasugrel or clopidogrel, who were enrolled into the nationwide Japan AMI Registry (JAMIR).
The design of the JAMIR has been published elsewhere.10 Briefly, consecutive patients presenting with spontaneous onset of AMI were enrolled between December 2015 and May 2017 at 50 institutions. AMI was diagnosed on the basis of the universal definition, with allowance of the MONICA criteria according to the institutional setting.11,12 We excluded patients who were admitted to the hospital ≥24 h after onset, with no return of spontaneous circulation on admission after out-of-hospital cardiopulmonary arrest, or who had AMI as a complication of PCI or coronary artery bypass grafting (CABG). Patient management, including the choice of antiplatelet drugs, was at the discretion of treating physician. Primary data collection was derived from the medical records of the patients. Information was collected on patient demographics, medical history, ambulance use, details about coronary angiography and invasive therapy, cardiac medications, and outcomes. Investigators, clinical research coordinators, or local data managers at each study site registered the data using the JAMIR registration system. A follow-up study of patients was performed 1 year after the onset of AMI based on the medical information available at each study site. A letter requesting follow-up was sent to patients for whom medical information was unavailable at the study sites after 1 year because of hospital transfer or other reasons.
This study was conducted in accordance with the ethical guidelines for medical research on humans laid out in the Declaration of Helsinki. This research protocol was approved by the Institutional Review Board of the National Cerebral and Cardiovascular Center and local ethics committees or local institutional review board at each study site. Although informed consent was not obtained, because of the observational nature of this registry, details about the study were posted on a website and at the study sites (opt-out) to inform the subjects of the content and timeline of the study and to ensure they had the opportunity to refuse inclusion in this registry. In addition, the research secretariat confirmed compliance with opt-out procedures at each study site. This study was registered with the Japanese UMIN Clinical Trials Registry (UMIN000019479).
Study EndpointsThe primary endpoint of the study was a composite of cardiovascular death, non-fatal MI, and non-fatal stroke. Major safety endpoints included major bleeding based on Thrombolysis in Myocardial Infarction (TIMI) criteria and type 3 or 5 bleeding based on Bleeding Academic Research Consortium (BARC) criteria.13,14
Secondary endpoints included a composite of ischemic events (cardiovascular death, non-fatal MI, and non-fatal cerebral infarction) and bleeding events (major bleeding based on TIMI criteria); individual components of ischemic events; all-cause death; stent thrombosis; major and minor bleeding based on TIMI criteria; and type 2, 3, or 5 bleeding based on BARC criteria. Stent thrombosis was defined as definite or probable stent thrombosis according to the Academic Research Consortium definition.15
Statistical AnalysisBaseline continuous variables are presented as mean±SD or median and interquartile range, according to the distribution of the data. Categorical variables are presented as percentages. T-test and the Mann-Whitney U test were used to compare continuous variables, and the χ2 test was used to compare dichotomous variables. Multivariate Cox proportional hazard models were used to estimate the hazard ratios (HRs) and 95% confidence intervals (CIs) of antiplatelet therapy on the primary outcome (composite of cardiovascular death, non-fatal MI, and non-fatal stroke), major bleeding based on TIMI criteria, and type 3 or 5 bleeding based on BARC criteria. Age (≥75 years), sex, body weight (≤50 kg), clinical presentation (ST-elevation MI [STEMI] or non-STEMI), use of anticoagulants, history of cerebrovascular disease, use of primary PCI, transradial approach, Killip class ≥2, estimated glomerular filtration rate (eGFR) <30 mL/min/1.73 m2, history of previous MI or PCI, and history of malignancy were included in the models as confounders. To further account for significant differences in the baseline characteristics of the prasugrel and clopidogrel groups, we conducted a propensity analysis. Propensity scores for all patients were first estimated using multivariable logistic regression models, with the dependent variable of prasugrel use at enrolment, and baseline characteristics were entered as covariates. Propensity analysis was then conducted using inverse probability of treatment weights (IPTW), where individuals are weighted by the inverse probability of receiving the treatment that they actually received.16 Adjusted survival curves were constructed using IPTW-adjusted Kaplan-Meier estimates at time to the first event.17 For subgroup analyses, a new IPTW was calculated in each subgroup and the adjusted HRs were estimated using Cox regression models adjusted by the IPTW. The levels of significance were set as P<0.05. All statistical analyses were performed using SAS version 9.4 (SAS Institute, Cary, NC, USA).
A total of 3,411 patients were registered to the JAMIR from the 50 sites. All institutes except 1 used the universal definition for the diagnosis of AMI, and 99% of registered patients were diagnosed on the basis of the universal definition. The current study excluded the following subjects: 61 patients who did not have data regarding the use of antiplatelet therapy; 139 patients who did not receive either prasugrel or clopidogrel during the hospitalization; 17 patients with triple antiplatelet therapy (aspirin, prasugrel and clopidogrel); and 125 patients who underwent switching between prasugrel and clopidogrel during hospitalization. Finally, 3,069 patients were included in the current analysis (Figure 1). Of these, 2,607 patients were treated with prasugrel and 462 patients were treated with clopidogrel during hospitalization. Patients’ characteristics were compared between prasugrel and clopidogrel (Table 1). Patients receiving prasugrel were younger, more likely to be male, experienced STEMI and be a current smoker, presented to hospital earlier, and were less likely to have comorbidities, including hypertension, diabetes, previous history of MI, PCI, CABG, cerebrovascular disease, malignancy, peripheral artery disease or atrial fibrillation, compared with those receiving clopidogrel. The frequency of patients with Killip class ≥2 did not differ significantly between the groups. The majority of prasugrel-treated patients (97.6%) received 3.75 mg of prasugrel as a maintenance dose. In terms of concomitant medications during hospitalization, use of cardioprotective drugs such as angiotensin-converting enzyme inhibitors (ACEI), angiotensin II receptor blockers (ARBs), β-blockers and statins was comparable between groups, but the use of oral anticoagulants was more frequent in the clopidogrel group. Of note, patients with a history of cerebrovascular disease (21.9% vs. 7.1%), older age (≥75 years: 50.7% vs. 31.0%) or low body weight (≤50 kg: 19.4% vs. 14.8%) were more common in the clopidogrel group compared with the prasugrel group. Table 2 shows the angiographic and interventional characteristics. Prasugrel-treated patients were more likely to undergo emergency coronary angiography and primary PCI with a higher rate of transradial access, and showed a shorter door-to-balloon time. AMI from the left main coronary artery was more frequent in the clopidogrel group. After PCI, the rate of patients with TIMI flow grade 3 was comparable between groups. Peak creatinine kinase (CK), peak CK-MB, eGFR, and hemoglobin levels on admission were significantly higher in the prasugrel group than in the clopidogrel group. Patients treated with clopidogrel were more likely to undergo CABG than those treated with prasugrel.

Study flow chart. JAMIR, Japan AMI Registry.
| Overall (n=3,069) |
Prasugrel (n=2,607) |
Clopidogrel (n=462) |
P value | |
|---|---|---|---|---|
| Age (years) | 68.0±13.1 | 67.2±13.1 | 72.6±11.9 | <0.001 |
| Female | 22.9 | 22.0 | 28.1 | 0.004 |
| BMI (kg/m2) | 23.9±3.9 | 24.0±4.0 | 23.4±3.7 | 0.005 |
| Use of ambulance | 81.9 | 82.1 | 80.7 | 0.49 |
| Time from onset to admission (min) | 135 (63, 308) | 130 (61, 300) | 174 (80, 367) | <0.001 |
| STEMI | 77.9 | 81.0 | 60.4 | <0.001 |
| Killip class ≥2 | 21.8 | 21.3 | 24.5 | 0.13 |
| Hypertension | 72.4 | 71.4 | 77.9 | 0.004 |
| Diabetes | 34.9 | 34.3 | 38.1 | 0.114 |
| Dyslipidemia | 69.7 | 70.6 | 64.3 | 0.006 |
| Previous MI | 9.1 | 7.8 | 16.7 | <0.001 |
| Previous PCI | 11.1 | 9.1 | 22.7 | <0.001 |
| Previous CABG | 2.4 | 1.5 | 7.1 | <0.001 |
| Previous cerebrovascular disease | 9.3 | 7.1 | 21.9 | <0.001 |
| Peripheral artery disease | 4.0 | 2.7 | 11.9 | <0.001 |
| Malignancy | 8.3 | 7.3 | 14.3 | <0.001 |
| Atrial fibrillation | 6.3 | 5.6 | 10.0 | <0.001 |
| Current smoking | 40.7 | 42.4 | 31.2 | <0.001 |
| eGFR (mL/min/1.73 m2) | 65.3±24.4 | 66.6±23.7 | 57.6±26.9 | <0.001 |
| Hemoglobin (g/dL) | 13.9±2.2 | 14.0±2.1 | 13.0±2.1 | <0.001 |
| Peak CK (IU/L) | 2,423±2,691 | 2,544±2,744 | 1,741±2,254 | <0.001 |
| Peak CK-MB (IU/L) | 230±248 | 240±253 | 176±211 | <0.001 |
| LVEF (%) | 52.0±12.3 | 52.2±12.3 | 51.0±12.3 | 0.08 |
| Loading dose of P2Y12 inhibitor* | ||||
| Prasugrel | ||||
| 20 mg | – | 82.1 | – | – |
| 3.75 mg | – | 16.9 | – | – |
| Other | – | 1.0 | – | – |
| Clopidogrel | ||||
| 300 mg | – | – | 44.8 | – |
| 75 mg | – | – | 51.9 | – |
| Other | – | – | 3.3 | – |
| P2Y12 inhibitor during hospitalization | ||||
| Prasugrel | ||||
| 3.75 mg | – | 97.6 | 0 | – |
| Other | – | 2.4 | 0 | – |
| Clopidgrel | ||||
| 75 mg | – | 0 | 95.5 | – |
| Other | – | 0 | 4.5 | – |
| Other medications during hospitalization | ||||
| Aspirin | 99.2 | 99.9 | 95.2 | <0.001 |
| ACEI | 52.6 | 52.2 | 54.6 | 0.35 |
| ARB | 27.5 | 27.3 | 28.4 | 0.64 |
| β-blocker | 64.9 | 64.8 | 65.2 | 0.89 |
| Statin | 90.9 | 91.3 | 88.5 | 0.06 |
| Oral anticoagulant | 12.1 | 10.9 | 18.8 | <0.001 |
| Proton pump inhibitor | 92.0 | 92.2 | 90.9 | 0.34 |
Data are given as mean±standard deviation, median [interquartile range] or percent. *Among patients who did not receive P2Y12 inhibitor before admission. ACEI, angiotensin-converting enzyme inhibitor; ARB, angiotensin II receptor blocker; BMI, body mass index; CABG, coronary artery bypass grafting; CK, creatinine kinase; eGFR, estimated glomerular filtration rate; LVEF, left ventricular ejection fraction; PCI, percutaneous coronary intervention; STEMI, ST-elevation myocardial infarction.
| Overall (n=3,069) |
Prasugrel (n=2,607) |
Clopidogrel (n=462) |
P value | |
|---|---|---|---|---|
| Emergency CAG | 98.1 | 99.1 | 92.9 | <0.001 |
| Puncture site | 0.04 | |||
| Radial | 65.8 | 66.5 | 61.5 | |
| Femoral | 32.4 | 31.9 | 35.4 | |
| Brachial | 1.9 | 1.7 | 3.0 | |
| Culprit lesion | ||||
| Left main coronary artery | 2.0 | 1.7 | 3.9 | 0.001 |
| Left anterior descending artery | 47.3 | 48.0 | 43.5 | 0.08 |
| Left circumflex artery | 14.9 | 14.4 | 17.3 | 0.11 |
| Right coronary artery | 36.3 | 37.0 | 32.5 | 0.06 |
| None | 0.7 | 0.5 | 1.3 | 0.06 |
| No. of diseased vessels | <0.001 | |||
| 0 | 0.3 | 0.3 | 0.5 | |
| 1 | 56.4 | 58.6 | 42.8 | |
| 2 | 26.4 | 25.5 | 32.1 | |
| 3 | 16.9 | 15.6 | 24.7 | |
| Mean no. of diseased vessels | 1.6±0.8 | 1.6±0.8 | 1.8±0.8 | <0.001 |
| Thrombolysis | 0.6 | 0.6 | 0.5 | 0.70 |
| Primary PCI | 95.8 | 96.9 | 89.3 | <0.001 |
| Door-to-balloon time, (min) | 70 [51, 103] | 68 [50, 99] | 80 [59, 130] | <0.001 |
| Stent use* | 92.4 | 93.3 | 86.2 | <0.001 |
| DES use† | 97.5 | 97.9 | 94.5 | <0.001 |
| Final TIMI flow | 0.005 | |||
| 0 | 1.9 | 1.6 | 3.5 | |
| 1 | 1.5 | 1.6 | 0.9 | |
| 2 | 5.4 | 5.8 | 3.0 | |
| 3 | 91.3 | 91.1 | 92.5 | |
| Concomitant PCI in nonculprit lesion | 5.5 | 5.5 | 5.6 | 0.91 |
| Use of IABP | 12.5 | 12.0 | 15.2 | 0.06 |
| Use of V-A ECMO | 1.7 | 1.6 | 2.6 | 0.12 |
| Use of CABG | 1.6 | 1.3 | 3.5 | <0.001 |
Data are given as median [interquartile range] or percent. *Among patients treated with primary PCI. †Among patients undergoing stent deployment. CABG, coronary artery bypass grafting; CAG, coronary angiography; DES, drug-eluting stent; ECMO, extracorporeal membrane oxygenation; IABP, intra-aortic balloon pumping; PCI, percutaneous coronary intervention; TIMI, Thrombolysis in Myocardial Infarction.
Median follow-up was 12 months (interquartile range, 9–13 months). A total of 9.3% of patients with prasugrel were switched to clopidogrel and 2.2% of patients with clopidogrel were switched to prasugrel after discharge. The proportion of patients who discontinued P2Y12 inhibitor was 13.9% at 6 months and 29.1% at 12 months in the prasugrel group, compared with 10.5% at 6 months and 17.5% at 12 months in the clopidogrel group (P=0.03 for 6 months, P<0.001 for 12 months). Table 3 shows the unadjusted incidence of the primary composite outcome and major bleeding events between patients treated with prasugrel and those treated with clopidogrel. Kaplan-Meier curves are presented in Figure 2A–C. The unadjusted incidence of the primary outcomes was significantly lower in the prasugrel group compared with the clopidogrel group (6.8% vs. 10.0%, P=0.02). The unadjusted incidence of major bleeding was significantly lower in the prasugrel group than in the clopidogrel group (3.8% vs. 7.8% for BARC type 3 or 5 bleeding, P<0.001; 1.6% vs. 3.3% for TIMI major bleeding, P=0.01). All-cause death was less frequent in the prasugrel group (6.0% vs. 11.7%, P<0.001). No significant difference between groups was seen in the incidence of MI, stroke or stent thrombosis.
| Overall (n=3,069) |
Prasugrel (n=2,607) |
Clopidogrel (n=462) |
P value | |
|---|---|---|---|---|
| Cardiovascular death, non-fatal MI or non-fatal stroke (primary endpoint) | 7.3 | 6.8 | 10.0 | 0.02 |
| BARC type 3 or 5 bleeding | 4.4 | 3.8 | 7.8 | <0.001 |
| TIMI major bleeding | 1.9 | 1.6 | 3.3 | 0.02 |
| Composite of primary endpoint and TIMI major bleeding | 8.4 | 7.9 | 11.3 | 0.02 |
| All-cause death | 6.9 | 6.0 | 11.7 | <0.001 |
| Cardiovascualr death | 3.8 | 3.5 | 5.6 | 0.03 |
| MI | 1.0 | 1.0 | 1.3 | 0.50 |
| Stroke | 0.5 | 0.5 | 0.7 | 0.59 |
| Stent thrombosis | 0.4 | 0.5 | 0 | 0.15 |
| BARC type 2, 3, or 5 bleeding | 6.1 | 5.4 | 9.7 | <0.001 |
| TIMI major or minor bleeding | 2.7 | 2.2 | 5.4 | <0.001 |
| Fatal bleeding | 0.5 | 0.4 | 1.1 | 0.04 |
| Intracranial bleeding | 0.6 | 0.5 | 0.9 | 0.32 |
| Blood transfusion due to bleeding event | 2.0 | 1.4 | 5.0 | <0.001 |
Data are given as percent. BARC, Bleeding Academic Research Consortium; MI, myocardial infarction; TIMI, Thrombolysis in Myocardial Infarction.
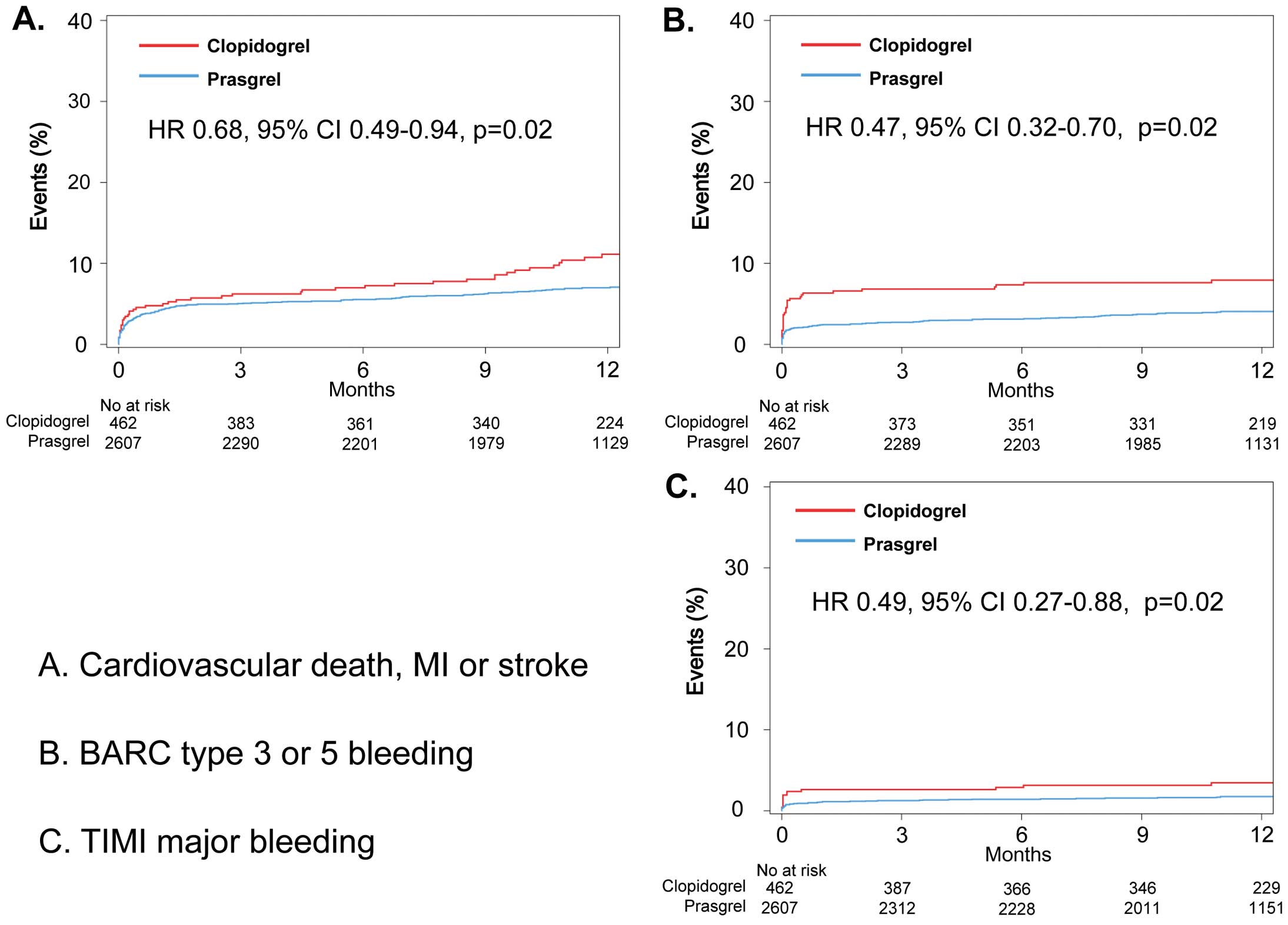
Comparison of unadjusted Kaplan-Meier curves for clinical outcomes between the clopidogrel (red line) and prasugrel (blue line) groupd. (A) Cardiovascular death, non-fatal MI or non-fatal stroke. (B) Bleeding Academic Research Consortium (BARC) type 3 or 5 bleeding. (C) Thrombolysis for Myocardial Infarction (TIMI) major bleeding. CI, confidence interval; HR, hazard ratio; MI, myocardial infarction.
Table 4 summarizes the cumulative hazards of the clinical outcomes in patients treated with prasugrel vs. those treated with clopidogrel. In the weighted Cox proportional hazards regression models using the IPTW method, no significant difference in the primary endpoint was seen between the prasugrel and clopidogrel groups (adjusted HR 1.07, 95% CI 0.67–1.72, P=0.78, Figure 3A). With regard to safety outcomes, risk of BARC type 3 or 5 bleeding was significantly lower in the prasugrel group than in the clopidogrel group (adjusted HR 0.62, 95% CI 0.39–0.99, P=0.042, Figure 3B). No significant difference in risk of TIMI major bleeding was seen between groups (adjusted HR 0.75, 95% CI 0.35–1.62, P=0.46, Figure 3C). When the IPTW adjustment was further augmented by multiple Cox regression models (IPTW+multivariable), HRs of the primary outcome (adjusted HR 1.04, 95% CI 0.63–1.71, P=0.88) and major bleeding (adjusted HR 0.61, 95% CI 0.39–0.96, P=0.03 for BARC type 3 or 5 bleeding, adjusted HR 0.69, 95% CI 0.31–1.50, P=0.34 for TIMI major bleeding) were similar to those in the weighted Cox regression model using IPTW.
| Unadjusted | Multivariable† | IPTW | IPTW+multivariable* | |||||
|---|---|---|---|---|---|---|---|---|
| HR (95% CI) |
P value | HR (95% CI) |
P value | HR (95% CI) |
P value | HR (95% CI) |
P value | |
| Cardiovascular death, non-fatal MI or non-fatal stroke (primary endpoint) |
0.68 (0.49–0.94) |
0.02 | 1.21 (0.80–1.84) |
0.37 | 1.07 (0.67–1.72) |
0.78 | 1.04 (0.63–1.71) |
0.88 |
| BARC type 3 or 5 bleeding | 0.47 (0.32–0.70) |
0.001 | 0.58 (0.37–0.92) |
0.02 | 0.62 (0.39–0.99) |
0.046 | 0.61 (0.39–0.96) |
0.03 |
| TIMI major bleeding | 0.49 (0.27–0.88) |
0.02 | 0.67 (0.33–1.38) |
0.28 | 0.75 (0.35–1.62) |
0.46 | 0.69 (0.31–1.50) |
0.34 |
Hazard ratios (HR) were calculated for the prasugrel treatment compared with clopidogrel. *Adjustment was performed through the use of IPTW and covariates used in the multivariable Cox hazard model. †Multivariable Cox hazard modeling using clinically relevant covariates including age, sex, body weight, presence of STEMI, use of anticoagulants, history of cerebrovascular disease, transradial approach procedure, Killip class ≥2, eGFR, history of previous MI or PCI, history of malignancy. CI, confidence interval; IPTW, inverse probability of treatment weighting. Other abbreviations as in Tables 1,3.

Comparison of adjusted Kaplan-Meier curves for clinical outcomes between clopidogrel (red line) and prasugrel (blue line) groups. (A) Cardiovascular death, non-fatal MI or non-fatal stroke. (B) BARC type 3 or 5 bleeding. (C) TIMI major bleeding. Survival curves are adjusted using inverse probability of treatment weighting (IPTW). Abbreviations as in Figure 2.
As concomitant use of oral anticoagulant could affect clinical outcomes, we analyzed ischemic and bleeding events between patients treated with prasugrel and those with clopidogrel, excluding patients who received oral anticoagulant during hospitalization (n=372) (Supplementary Table 1). In the remaining 2,697 patients, the incidence of the primary outcome was comparable between the prasugrel and clopidogrel group (adjusted HR 1.02, 95% CI 0.61–1.71, P=0.93). The incidence of BARC type 3 or 5 bleeding was significantly lower in the prasugrel group than in the clopidogrel group (adjusted HR 0.57, 95% CI 0.35–0.93, P=0.03). No difference was observed in the incidence of TIMI major bleeding between groups (adjusted HR 0.69, 95% CI 0.31–1.53, P=0.36) (Supplementary Table 2).
Subgroup AnalysisTo determine whether the outcomes for prasugrel and clopidogrel observed in the overall population were consistent across complex patient subgroups, adjusted HRs for BARC type 3 or 5 bleeding were calculated for subgroups stratified by age, sex, clinical presentation (STEMI or non-STEMI), body weight (≤50 kg), transradial approach, eGFR <30 mL/min/1.73 m2, or history of cerebrovascular disease. On subgroup analysis, a lower risk of BARC type 3 or 5 bleeding associated with prasugrel was consistent across subgroups (Figure 4).

Adjusted HRs for the BARC type 3 or 5 bleeding in various subgroups. eGFR, estimated glomerular filtration rate; STEMI, ST-elevation myocardial infarction; TIA, transient ischemic attack. Other abbreviations as in Figure 2.
In the present study, 125 patients underwent switching between prasugrel and clopidogrel during the hospitalization (Figure 1). Switching patient characteristics, angiographic and interventional characteristics, and clinical outcomes are shown in Supplementary Tables 3–5; 92% of the switching group was initially treated with prasugrel, then switched to clopidogrel. The remaining 8% underwent switching from clopidogrel to prasugrel. Median time of switching was 6.5 days following AMI onset. Similar to the prasugrel group, the switching group was younger, less likely to be female, and more likely to have STEMI compared with the clopidogrel group. However, the switching group showed more comorbidities such as history of cerebrovascular disease and atrial fibrillation. The switching group was more likely to have heart failure (Killip class ≥2), more likely to undergo PCI using the transfemoral approach, and more likely to undergo intra-aortic balloon pumping and venous-arterial extracorporeal membrane oxygenation than the prasugrel group. Peak CK level was significantly higher in the switching group than in either the clopidogrel or prasugrel group. The incidence of the primary endpoint in the switching group was comparable with that in the prasugrel group, but significantly lower than that in the clopidogrel group (switching 4.8%, prasugrel 6.8%, clopidogrel 10.0%). Incidence of major bleeding in the switching group was significantly higher than in either the prasugrel or clopidogrel group (BARC type 3 or 5 bleeding; switching 16.0%, prasugrel 3.8%, clopidogrel 7.8%).
To examine the risk of ischemic and bleeding events in 3,194 patients, including those who underwent switching of P2Y12 inhibitors during hospitalization, we re-allocated patients into prasugrel (n=2,722) and clopidogrel groups (n=472) according to the P2Y12 inhibitor initially prescribed during hospitalization. Consistent with the main analysis, prasugrel showed a comparable risk of the primary endpoint with the clopidogrel group, while the risk of BARC type 3 or 5 bleeding was lower with prasugrel than with clopidogrel (Supplementary Table 6).
The present analysis of this nationwide prospective registry in Japan found that a large proportion of patients with AMI were treated with prasugrel in real-world practice. Prasugrel was used predominantly in younger, male STEMI patients with fewer comorbidities than clopidogrel-treated patients. Prasugrel was associated with comparable ischemic risk to clopidogrel, whereas the risk of major bleeding as assessed by BARC type 3 or 5 bleeding was significantly lower in prasugrel-treated patients. The relative benefit of prasugrel for the lower risk of major bleeding was consistent across subgroups including the elderly, patients with low body weight and those with a history of cerebrovascular disease.
To the best of our knowledge, the JAMIR is the first examination of current antiplatelet therapy and clinical outcomes of Japanese patients with AMI since the launch of prasugrel in 2014. In the present study, prasugrel was used 5-fold more frequently than clopidogrel (2,607 patients treated with prasugrel vs. 462 patients treated with clopidogrel), which differs from the previous AMI registry in Japan. In the J-AMI study, a total of 2,161 patients with STEMI were enrolled between May 2011 and October 2011 from 213 hospitals. DAPT was given to 84.8% and most patients (76.3%) was treated with clopidogrel, followed by ticlopidine (5.6%).18 Our findings were consistent with a 2016 report based on a Japanese registry of patients undergoing PCI (J-PCI), which showed that use of prasugrel was 66% in patients with AMI, while use of clopidogrel was only 19.5%.19 The widespread use of prasugrel in contemporary clinical practice reinforces the need for evaluation of its efficacy and safety in Japanese AMI patients.
In the PRASFIT ACS study, an adjusted dose of prasugrel was associated with a numerically lower incidence of the primary composite endpoint of cardiovascular death, non-fatal MI and non-fatal stroke compared with clopidogrel at 24 weeks (prasugrel 9.4% vs. clopidogrel 11.8%) and at 48 weeks (prasugrel 11.1% vs. clopidogrel 12.7%) without any increase in the incidence of TIMI major bleeding (prasugrel 1.9% vs. clopidogrel 2.2%). In the present study, the unadjusted incidences of the primary endpoint and major bleeding events were significantly lower in the prasugrel group than in the clopidogrel group. The current study population treated with prasugrel in the real-world setting of the JAMIR differs from that of the prasugrel arm in the PRASFIT ACS study in terms of the higher proportion of STEMI, inclusion of patients with comorbidities such as severe heart failure and those with a history of cerebrovascular disease. Nevertheless, the incidence of the primary outcomes in prasugrel-treated patients seems lower than that of the prasugrel arm in the PRASFIT ACS study (JAMIR 6.8% vs. PRASFIT ACS 11.1%), while the incidence of major bleeding as assessed by TIMI criteria was comparable with the prasugrel arm in the PLASFIT ACS (JAMIR 1.6% vs. PRASFIT ACS 1.9%). Importantly, unlike the PRASFIT ACS study, a number of differences in patients’ characteristics existed between the prasugrel and clopidogrel groups of the JAMIR. Use of prasugrel was associated with STEMI, and higher peak CK levels, suggesting that prasugrel-treated patients showed a higher risk of ischemic events. On the other hand, prasugrel-treated patients in the JAMIR were less likely to have characteristics indicating an increased risk of bleeding, such as older age, lower body weight and history of cerebrovascular disease.7,20 Differences in the patients’ characteristics between the prasugrel and clopidogrel groups appeared to reflect physician decisions to balance the benefits of potent antiplatelet therapy and risk of bleeding and may have affected the present results. However, the lower risk of bleeding in the prasugrel group remained significant even after adjusting for potential confounders by IPTW. Our results are consistent with the results of the PRASFIT ACS study, which found that the adjusted dose of prasugrel did not apparently increase major bleeding events compared with clopidogrel in Japanese ACS patients. Furthermore, subgroup analyses showed no excess risk of bleeding associated with prasugrel across subgroups including patients ≥75 years old, those with low body weight, and those with a history of cerebrovascular disease, which were identified as the high-risk subgroups for bleeding in the TRITON-TIMI 38 trial.7 The present study thus provides further support for the safety of the adjusted-dose of prasugrel in Japanese patients with AMI in real-world settings. On the other hand, no clear difference was noted in the primary endpoint between patients treated with prasugrel and those treated with clopidogrel after adjustment by IPTW. One potential explanation of this result is that the present study was underpowered to detect differences in the primary outcome because of the small number of ischemic events.
Recent randomized trials of the new potent P2Y12 inhibitors, prasugrel and ticagrelor, have shown a substantial reduction in ischemic events. In the TRITON-TIMI 38 trial, a 60-mg loading dose and 10-mg maintenance dose of prasugrel reduced the incidence of ischemic events compared with clopidogrel (9.9% vs. 12.1%; HR 0.81, 95% CI 0.73–0.90) at the expense of an increased rate of bleeding events (2.4% vs. 1.8%; HR 1.32, 95% CI 1.03–1.68).7 In the Platelet Inhibiton and Patient Outcomes (PLATO) trial, ticagrelor reduced the incidence of the primary endpoint of cardiovascular death, MI or stroke compared with clopidogrel in patients with ACS (9.8% vs. 11.7%; HR 0.84, 95% CI 0.72–9.92, P<0.001). The rate of major bleeding was similar between groups (11.6% vs. 11.2%, respectively, P=0.43). As a result of these studies, current guidelines recommend using ticagrelor or prasugrel over clopidogrel in patients with AMI undergoing PCI.21,22 Recently, national registries of ACS in the USA and European countries have demonstrated an increasing use of the potent P2Y12 inhibitors and their efficacy in clinical practice.23–26 However, the benefits of potent P2Y12 inhibitors in the Asian population have not been clarified. The multicenter, double-blind, randomized PHILO trial compared the efficacy and safety of ticagrelor vs. clopidogrel in 801 patients with ACS in East Asian countries including Japan.27 In contrast to the PLATO study, the PHILO trial demonstrated that ticagrelor was associated with a numerically higher incidence of the primary composite outcome of cardiovascular death, MI and stroke (9.0% vs. 6.3%) and major bleeding (10.3% vs. 6.8%) compared with clopidogrel. Furthermore, the Korea Acute Myocardial Infarction Registry (KAMIR) showed that use of the potent P2Y12 inhibitors, prasugrel and ticagrelor did not reduce the incidence of ischemic events, but significantly increased the risk of bleeding compared with clopidogrel.28,29 These inconsistent outcomes under potent P2Y12 inhibitor use between East Asian and Caucasian populations could be related to different responses to the antiplatelet drugs. Several pharmacological studies have shown that the degree of platelet inhibition following administration of potent P2Y12 inhibitor is significantly higher among East Asians compared with Caucasians.30,31 The present results support the notion that lower doses of potent P2Y12 inhibitors may be better to balance the benefits and risks of antiplatelet therapy in Asian population.8 Further studies are required to examine the optimal dose of potent P2Y12 inhibitors in Asian patients with AMI, who present with a unique genetic background including a high prevalence of CYP2C19 loss-of-function alleles.
It should be noted that there were 3.6% of patients (n=125) who underwent switching P2Y12 inhibitors during hospitalization in real-word practice. The availability of different oral P2Y12 inhibitors (clopidogrel, prasugrel, ticagrelor) has enabled physicians to contemplate switching among therapies because of specific clinical scenarios.32 In the present study, the majority of the switching group was initially treated with prasugrel, and was characterized by a higher prevalence of comorbidities and more invasive procedures, such as PCI by transfemoral approach, use of intra-aortic balloon pumping, and venous-arterial extracorporeal membrane oxygenation, compared with the prasugrel group. Indeed, the incidence of bleeding was higher in the switching group than in the prasugrel or clopidogrel group. These results suggested that switching might have occurred as a result of increased risk of bleeding or unfavorable events such as bleeding or stent thrombosis. Practice guidelines have not fully elaborated on how to switch therapies, leaving clinicians with limited guidance on when and how best to switch therapies. Differences in the pharmacology of P2Y12-receptor inhibitors, such as binding sites (competitive or noncompetitive), half-life, and speed of onset and offset of action, are important factors that might lead to drug interactions when switching between agents.
The major limitation of this study relates to the observational nature of the study, and residual or unmeasured confounding is likely to persist. For example, there were a number of differences between patients treated with prasugrel and those treated with clopidogrel, and the differences in ischemic and bleeding events could potentially be related to selective prescribing. Although we performed IPTW analysis to adjust potential confounders, no statistical method of adjustment can completely abolish this limitation. Given that prasugrel shows a more consistent, rapid and pronounced inhibition of platelet activity than clopidogrel, careful patient evaluation for selection of P2Y12 inhibitor is likely to be important. Second, bleeding might be the subject of underreporting in registries, but this is true for both clopidogrel and prasugrel and severe bleeding events are considered less likely to be missed. Third, our analysis might have been underpowered to detect significant differences in TIMI major bleeding or each component of ischemic events such as MI, stroke and stent thrombosis. In addition, subgroup analyses of BARC type 3 or 5 bleeding events reduced the sample size, and most of them might be underpowered. Fourth, although there was a difference in the incidence of switching of P2Y12 inhibitor after discharge between the prasugrel and clopidogrel groups, the reasons for switching were not available in our study. Fifth, data regarding prehospital medication such as other antiplatelet drugs, thrombotic drugs, or nonsteroidal anti-inflammatory drugs and heparin were not available in the present study. Finally, although participation was nationwide, the present study was conducted in 50 institutions in Japan, so the generalizability of our findings to non-participating centers remains to be established.
The real-world database of the JAMIR demonstrated frequent use of prasugrel in Japanese patients with AMI. The prasugrel group showed comparable rates of 1-year ischemic events to clopidogrel, although the risk of bleeding was lower with prasugrel than with clopidogrel. Given the observational nature of the present study, further studies are required to confirm the efficacy and safety of adjusted-dose prasugrel in Japanese AMI patients.
We thank all the investigators, clinical research coordinators, and data managers involved in the JAMIR study for their contributions. Institutes and members of the JAMIR are listed in the Supplementary File.
This work was planned by the Japan Cardiovascular Research Foundation and is financially supported by Daiichi Sankyo Co., Ltd.
Dr. Yasuda reports remuneration for lectures from Takeda, Daiichi Sankyo, and Bristol-Myers Squibb, and trust research/joint research funds from Takeda and Daiichi Sankyo; Dr. Takayama reports lecture fees from Daiichi Sankyo; Dr. Ogawa reports lecture fees and research grants from AstraZeneca, Bayer, Boehringer Ingelheim, Daiichi Sankyo, Pfizer, and Sanofi. No of the other authors have any conflicts of interest to declare.
Please find supplementary file(s);
http://dx.doi.org/10.1253/circj.CJ-19-0145