Abstract
Background:
In this study we evaluated the feasibility and efficacy of predicting conduction system abnormalities under 3-dimensional (3D) electroanatomic mapping guidance during transcatheter closure of perimembranous ventricular septal defects (pmVSDs) in adults.
Methods and Results:
The distribution of the His-Purkinje system (HPS) close to the margins of pmVSDs in the left ventricle was identified using 3D electroanatomic mapping and near-field HPS was further confirmed by different pacing protocols. Of the 20 patients in the study, 17 (85%) were successfully treated by transcatheter intervention. The minimum distance between the margins of the pmVSD and near-field HPS, as measured by 3D electroanatomic mapping, ranged from 1.3 to 3.9 mm (mean [± SD] 2.5±0.7 mm). Five patients with a minimum distance <2 mm had a higher risk (3/5; 60%) for adverse arrhythmic events, whereas patients with a distance >2 mm were at a much lower risk (1/15; 6.7%) of procedure-related conduction block (P=0.032). No other adverse events were recorded during the follow-up period (median 30 months).
Conclusions:
A minimum distance between the pmVSD and near-field HPS <2 mm was associated with a relatively high risk of closure-related conduction block. 3D electroanatomic mapping may be helpful in guiding decision making for transcatheter closure and reduce the incidence of adverse arrhythmic events.
Perimembranous ventricular septal defects (pmVSDs) account for approximately 80% of all isolated ventricular septal defects (VSDs).1
According to the 2008 American College of Cardiology (ACC)/American Heart Association (AHA) guidelines for adults with congenital heart disease, only muscular VSDs are indicated for device closure.2
Recently, based on safety and efficacy outcomes at mid-term follow-up, transcatheter device closure without cardiopulmonary bypass has emerged as a treatment option for pmVSD.3,4
This method is associated with reduced procedural morbidity compared with the surgical approach, but has its own complications, including complete atrioventricular block (CAVB) and complete left bundle branch block (CLBBB). The proximity of the margins of the pmVSD to the conduction system increases the risk of adverse arrhythmic events related to transcatheter closure.
In a previous study we verified that if low-output pacing at a specific site where a His bundle (HB) potential is recorded from 3-dimensional (3D) electroanatomic mapping and a narrower QRS complex is produced by capturing the HB, then this indicates a near-field His-Purkinje system (HPS), and ablation at these sites results in a higher incidence of conduction block.5
Although mechanical manipulation and occluder compression seem to have less potential for damage to the conduction system than ablation, the incidence of transcatheter closure-related arrhythmia cannot be ignored. On the basis of our previous para-Hisian study,5
we assumed that if 3D electroanatomic mapping can identify the distribution of the HPS, especially for the near-field HPS, and clarify its proximity to the margins of the pmVSD, it may be possible to guide procedural decision making and reduce the risk of arrhythmic events. In this study, we prospectively applied this technique to transcatheter closure in adults with pmVSDs and further explored its feasibility and efficacy.
Methods
Study Subjects
Between January 2016 and August 2018, 20 adults with pmVSD were eligible for transcatheter closure in Guangdong Cardiovascular Institute. The study included 8 male and 12 female patients ranging in age from 18 to 48 years. The study was approved by the Ethics Committee of Guangdong Provincial People’s Hospital and was performed in accordance with the Declaration of Helsinki. Patients provided written informed consent to take part in the study. All patients underwent clinical examination, chest radiography, electrocardiography (ECG), and transthoracic echocardiography (TTE) before the intervention.
The inclusion criteria were as follows: (1) age ≥18 years; (2) pmVSD diagnosed by TTE with a diameter between 2 and 14 mm; (3) a significant left-to-right shunt across the defect; (4) a distance of ≥2 mm from the VSD to the aortic valve; and (5) mean pulmonary artery pressure <70 mmHg, as evaluated by catheterization. Exclusion criteria were: (1) severe aortic or tricuspid regurgitation; (2) severe pulmonary hypertension (≥70 mmHg); (3) a right-to-left shunt across the defect; (4) New York Heart Association (NYHA) Class III and IV; and (5) the presence of other types of congenital heart disease and/or previous surgery.
Procedures
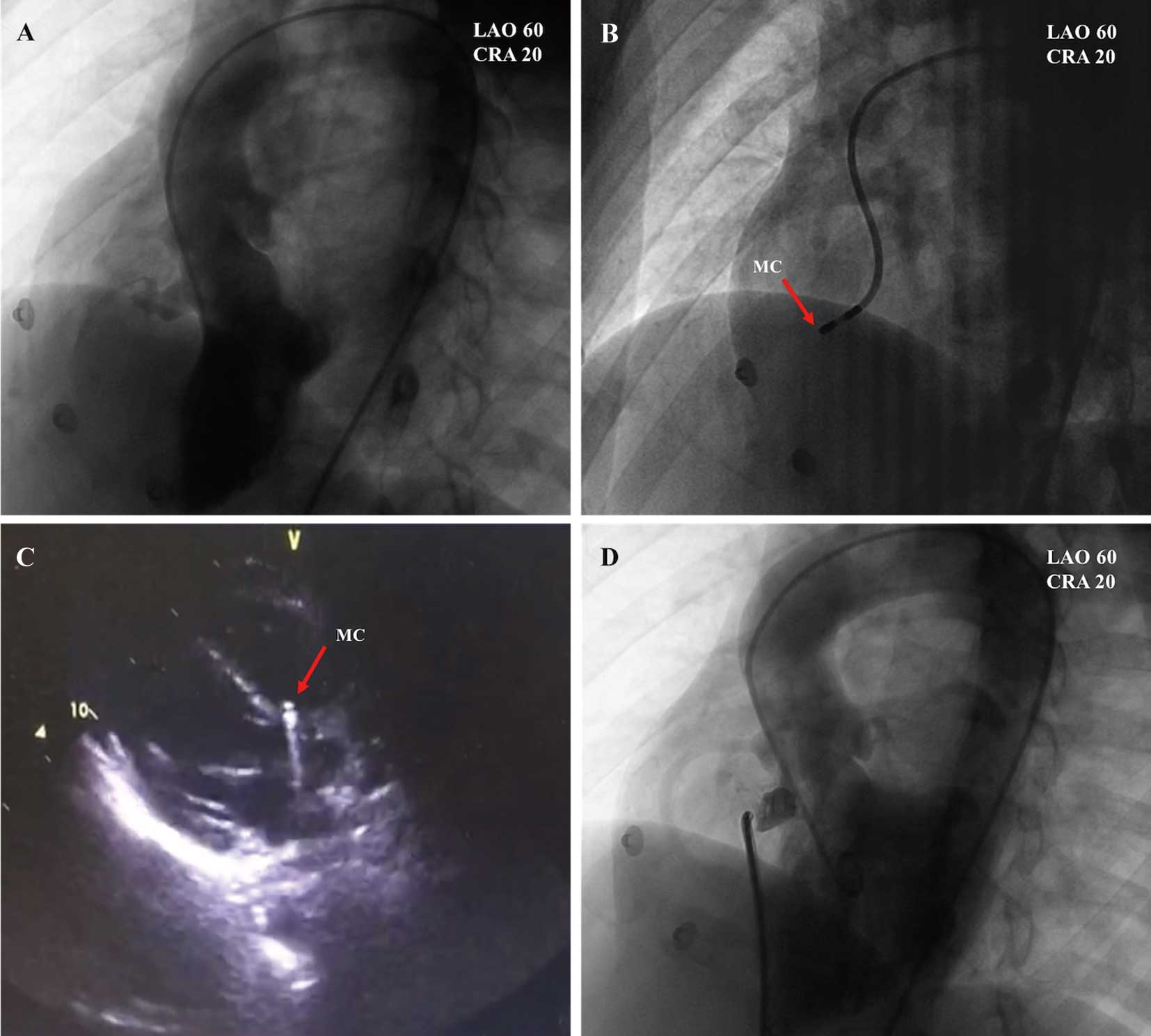
The catheterization procedure was performed in patients under local anesthesia. Heparin (100 IU/kg) was administered intravenously during the procedure. Access was created through the right femoral vein and right femoral artery. Right heart catheterizations were performed to assess the degree of shunting and to evaluate pulmonary vascular resistance. Angiography in the left ventricle (LV) at a 60°/20° left anterior oblique projection/cranial view was performed to detect the location, shape, and size of the pmVSD and its relationship with the aortic valve (Figure 1A). The diameter of the pmVSD was measured during end diastole, and an occluder was selected based on this measurement. A mapping catheter (NaviStar 4 mm; Biosense Webster, Diamond Bar, CA, USA) was used to conduct 3D electroanatomic mapping of the HPS in the LV via a retrograde aortic approach (Figure 1B). This catheter could be used for both mapping and pacing. TTE was also helpful in confirming the location of the mapping catheter (Figure 1C). An arteriovenous circuit was then set up through a femoral vein approach on the same side. A long sheath was advanced to the LV through the arteriovenous circuit and positioned beneath the aortic valve. Through the long sheath, the pmVSD occluder was deployed under fluoroscopic control and echocardiographic guidance. Aortography was also performed before releasing the occluder to ensure that the aortic valve had not been compromised (Figure 1D). After release of the occluder, TTE, aortography, and left ventriculography were performed again to detect tricuspid and/or aortic valve regurgitation and residual shunts.
For patients who presented with arrhythmic events after the procedure, corticosteroid therapy was administered to reduce occluder-related mechanical compression and myocardial edema. Dexamethasone was given at a dose of 10 mg/day for 3–7 days. All patients received oral aspirin (100 mg) daily for 6 months after the procedure.
3D Mapping and Pacing Protocol
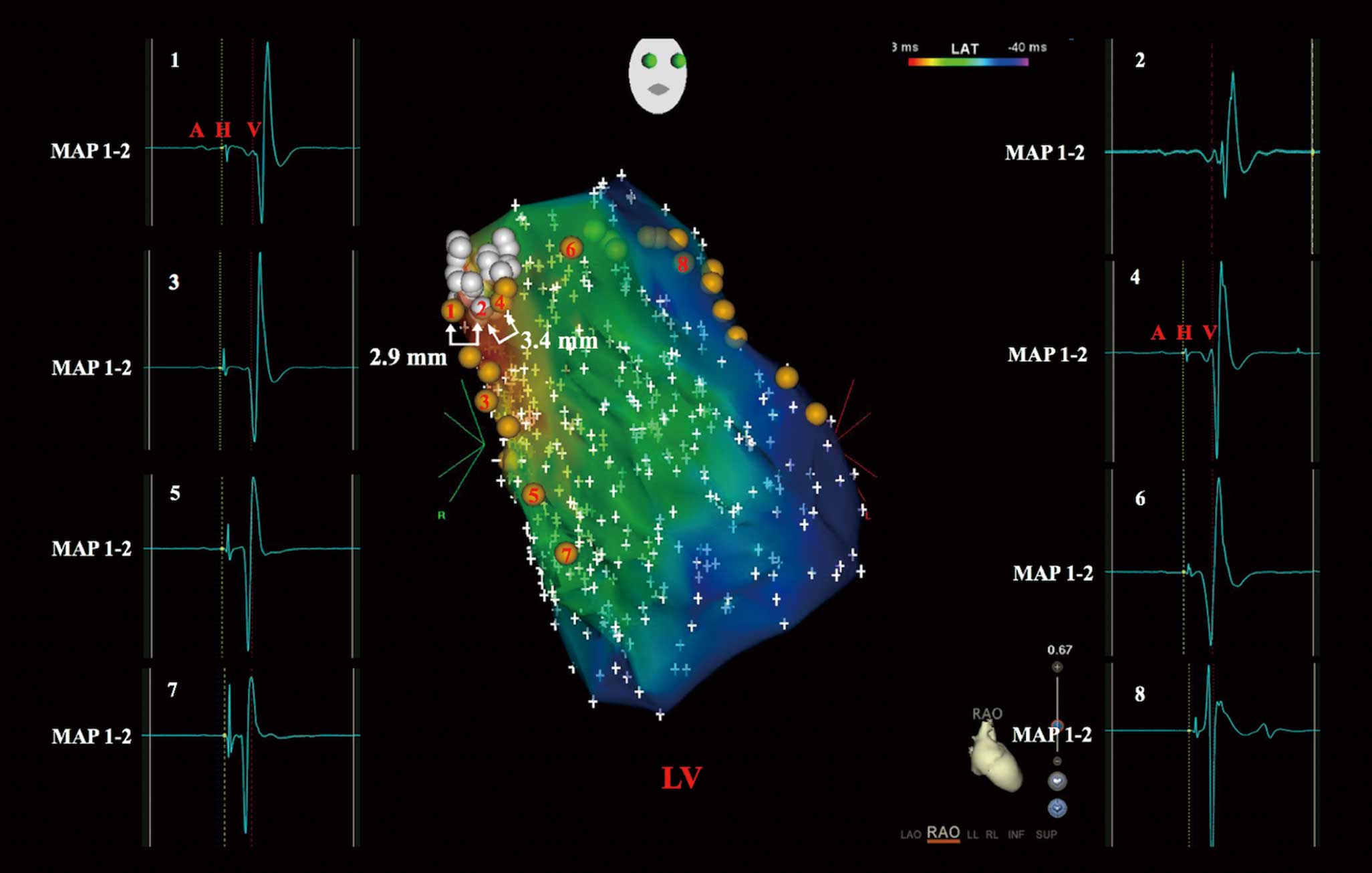
Point-by-point 3D electroanatomic mapping (CARTO; Biosense Webster) was performed to identify a relatively large area of HPS activation adjacent to the pmVSD. During the high-density mapping, the pmVSD, its adjacent HPS activation, and left anterior and posterior fascicular potential sites in the LV were identified and tagged on the 3D map (Figure 2). The sites of near-field HPS potential in the LV were initially defined as the sites with the longest interval from the Purkinje potential to ventricular activation.
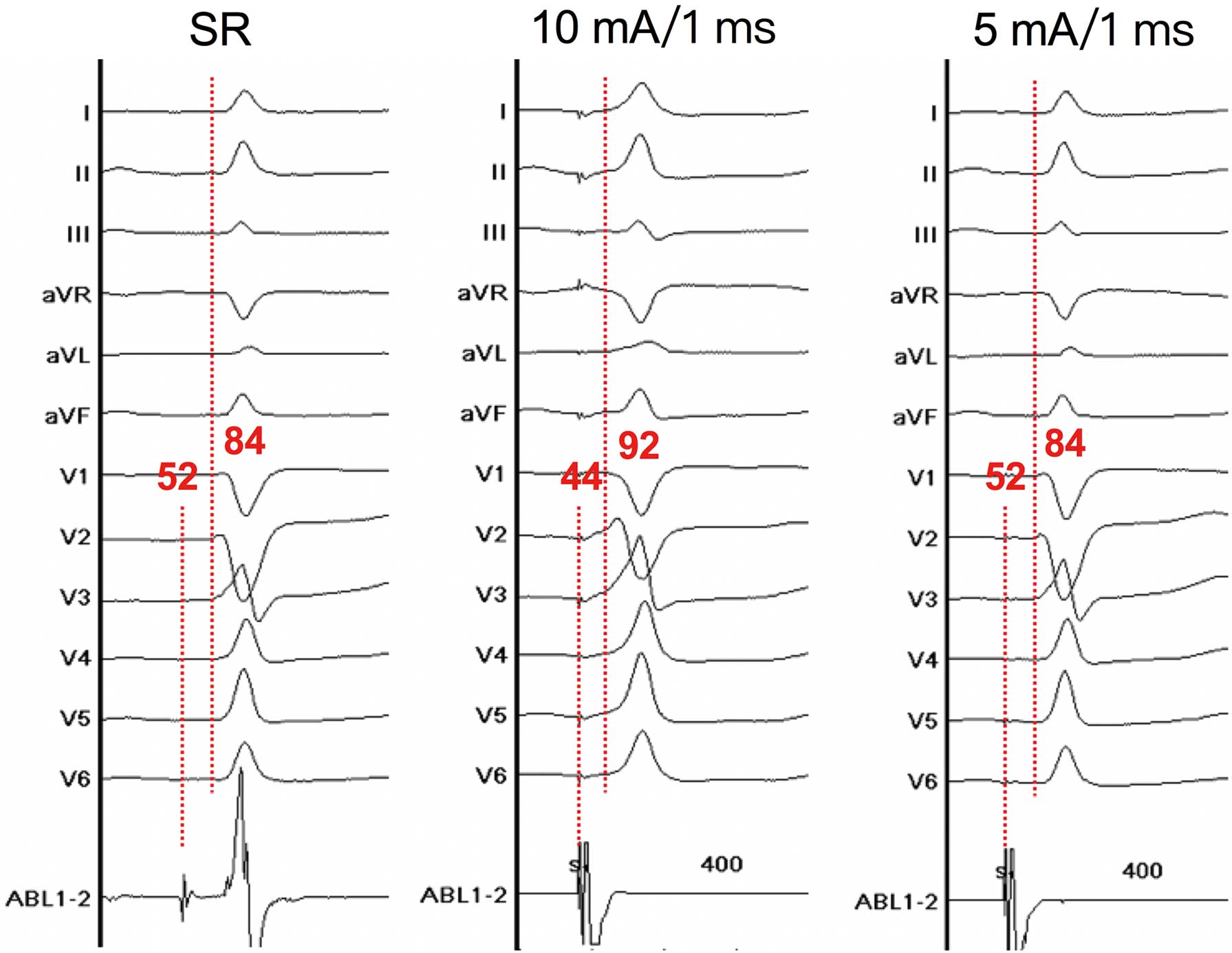
Bipolar pacing from the mapping catheter was performed. The pacing protocol consisted of different pacing current (20, 15, 10, and 5 mA) at a different pulse widths (2.0, 1.5, 1.0, and 0.5 ms). Pacing with a cycle length of 400 ms was performed at the sites recording HPS activation adjacent to the pmVSD. QRS morphology during pacing was interpreted as follows: (1) narrow QRS complexes indicated both myocardial and near-field HPS capture; (2) wide QRS complexes indicated only myocardial capture; and (3) paced QRS morphology identical to that during sinus rhythm and long-stimulus QRS morphology indicated only pure His capture.6
The left-sided near-field HPS was further defined as the presence of His capture with minimal pacing output (<10 mA/2 ms).5
VSD Occluders
Two types of occluder devices were used in this study: a double-disk symmetrical concentric pmVSD occluder (Lifetech Scientific, Shenzhen, China) and a double-disk thin-waist pmVSD occluder (Starway Medical Technology, Beijing, China). These 2 devices are self-expanding double-disk devices composed of 0.127 mm nitinol wire mesh with fabric inside. The waist diameters of the occluders ranged from 5 to 12 mm. The diameters of the right and left disks were uniformly 4 mm larger than the waist of the symmetrical concentric pmVSD occluder. The diameters of the right and left disks were 4 and 8 mm larger, respectively, than the waist of the thin-waist pmVSD occluder. The waist length of the symmetrical concentric occluder and thin-waist occluder were 3 and 5 mm, respectively. The selection of the occluder was based primarily on the location and characteristics of the defect. The device size chosen was usually 1–4 mm larger than the maximum defect size as assessed by either echocardiography or angiography, depending on which was judged most reliable.7
Definition of Adverse Arrhythmic Events and New-Onset Arrhythmic Events
Adverse arrhythmic events were defined as second- and/or third-degree atrioventricular block, complete right bundle branch block (CRBBB), CLBBB, and accelerated junctional rhythm (AJR) during the procedure. Any of the above conduction abnormalities persisting for more than 48 h after the procedure was also regarded as an adverse arrhythmic event. New-onset arrhythmic events were defined as newly developed conduction blocks after the procedure where there had been no conduction block or a different conduction block before the procedure.
Follow-up Protocol
Continuous ECG monitoring was performed during the entire hospitalization period. A standard 12-lead ECG examination was performed within 6 h after the procedure, and subsequently on Days 1, 3, and 7 after the procedure. Clinical examination, TTE, ECG, and/or Holter ECG were performed at 1, 3, 6, and 12 months after the procedure and yearly thereafter. The findings at each visit were recorded in an electronic database by well-trained personnel. All patients were regularly followed-up at the outpatient clinic.
Statistical Analysis
Continuous variables are expressed as the mean±SD and were compared by Student’s t-test or the Mann-Whitney U-test (non-parametric). Categorical variables are reported as counts or percentages and were compared by the Fisher’s exact test. Statistical analyses were performed using SPSS version 24.0 (IBM Corp., Armonk, NY, USA). All tests were 2-sided, and P<0.05 was considered significant.
Results
Clinical and Procedural Patient Data
Of the 20 patients in whom transcatheter closure of the pmVSD was attempted, 17 (85%) were successfully treated by transcatheter intervention. The mean age of the patients was 28±10 years. During the procedure, the mean defect size measured by angiography was 4.9±1.6 mm (range 2.9–8.5 mm), and the mean size of the implanted device was 6.5±1.9 mm (range 5–12 mm). The thin-waist occluder device was selected for 9 patients, and the symmetric occluder device was selected for 8 patients. A septal aneurysm accompanied the defect in 13 patients (65%), as confirmed by TTE (Table 1).
Table 1.
Clinical and Procedural Characteristics of Patients
Case
no. |
Age
(years) |
Sex |
Membranous
aneurysm |
Defect size
(mm) |
Device type |
Device size
(mm) |
Follow-up period
(months) |
| 1 |
25 |
Female |
No |
3.5 |
Thin-waist |
5 |
48 |
| 2 |
48 |
Male |
Yes |
5.6 |
Symmetric |
6 |
38 |
| 3 |
18 |
Male |
No |
2.9 |
Symmetric |
5 |
36 |
| 4 |
32 |
Male |
No |
6 |
Symmetric |
9 |
36 |
| 5 |
19 |
Female |
Yes |
5 |
Thin-waist |
5 |
39 |
| 6 |
22 |
Male |
Yes |
4 |
Thin-waist |
5 |
36 |
| 7 |
20 |
Female |
No |
4 |
Thin-waist |
6 |
36 |
| 8 |
42 |
Male |
Yes |
4 |
Thin-waist |
7 |
37 |
| 9 |
31 |
Female |
No |
6.8 |
– |
– |
36 |
| 10 |
22 |
Female |
Yes |
8 |
Symmetric |
12 |
24 |
| 11 |
18 |
Female |
Yes |
3 |
Thin-waist |
5 |
36 |
| 12 |
30 |
Female |
No |
3.6 |
Thin-waist |
6 |
28 |
| 13 |
47 |
Male |
Yes |
5.3 |
Thin-waist |
6 |
30 |
| 14 |
18 |
Male |
Yes |
5.1 |
Thin-waist |
7 |
24 |
| 15 |
24 |
Female |
Yes |
4.3 |
Symmetric |
6 |
24 |
| 16 |
29 |
Female |
No |
3 |
Symmetric |
6 |
24 |
| 17 |
19 |
Female |
Yes |
6 |
Symmetric |
6 |
24 |
| 18 |
26 |
Male |
Yes |
5 |
– |
– |
20 |
| 19 |
35 |
Female |
Yes |
8.5 |
Symmetric |
9 |
12 |
| 20 |
37 |
Female |
Yes |
4 |
– |
– |
12 |
On average, 718±320 points were taken for 3D mapping of the LV.
Figure 2
shows the 3D electroanatomic mapping of the HPS distribution and the relative location of the pmVSD. As shown in the map, no Purkinje potential was recorded in the pmVSD, with a relatively large area of HPS activation along the defect margins. Pacing with lower outputs (10 mA at 1 ms in 8 patients and 5 mA at 1 ms in 12 patients) in the sites recording HPS potential adjacent to the pmVSD resulted in a narrower QRS morphology by capturing the HB and some myocardium, indicating these sites are near-field HPS that are close to the anatomic HB (Figure 3). The HB-ventricular (HV) and QRS intervals of the sites before pacing were 44±5 ms (range 35–54 ms) and 87±9 ms (range 72–109 ms), respectively. The duration of narrower paced QRS complexes was 90±9 ms (range 76–112 ms). A mean distance of 2.5±0.7 mm (range 1.3–3.9 mm) between the pmVSD and near-field HPS was measured by 3D electroanatomic mapping. A minimum distance <2 mm was detected in 5 patients (25%).
Adverse arrhythmic events were noted in 4 patients (2 with CLBBB, 1 with CRBBB, and 1 with AJR and CLBBB). In 3 cases the adverse arrhythmic events occurred during the procedure, whereas in 1 case the event occurred 4 days after the procedure. A 31-year-old female without conduction abnormality before the procedure developed CLBBB immediately when the occluder was ready to be released (Case 9;
Figure 4A). Similarly, a 26-year-old male with left anterior hemiblock (LAH) before the procedure progressed to AJR and CLBBB when maneuvering the long sheath to release the occluder (Case 18;
Figure 4B). The minimum distance between the pmVSD and near-field HPS in these 2 patients was 1.4 and 1.3 mm, respectively. Sinus rhythm was automatically restored in these 2 patients after removal of the device and delivery system. The first patient (Case 9) refused surgical repair for personal reasons and remained without any symptoms. A conventional surgical repair was conducted in the second patient (Case 18) via the median sternotomy approach under cardiopulmonary bypass. No new-onset arrhythmic events were shown during this patient’s follow-up visits. CRBBB was observed in a 37-year-old female when establishing the arteriovenous circuit during the procedure (Case 20;
Figure 4C), with 3D electroanatomic mapping showing that the minimum distance between the pmVSD and near-field HPS was 2.8 mm. After the delivery sheath was withdrawn, sinus rhythm quickly recovered. Therefore, the procedure was canceled, and subsequent surgical repair was performed in this patient. During the next 12 months of follow-up, neither CRBBB nor other conduction blocks were observed.
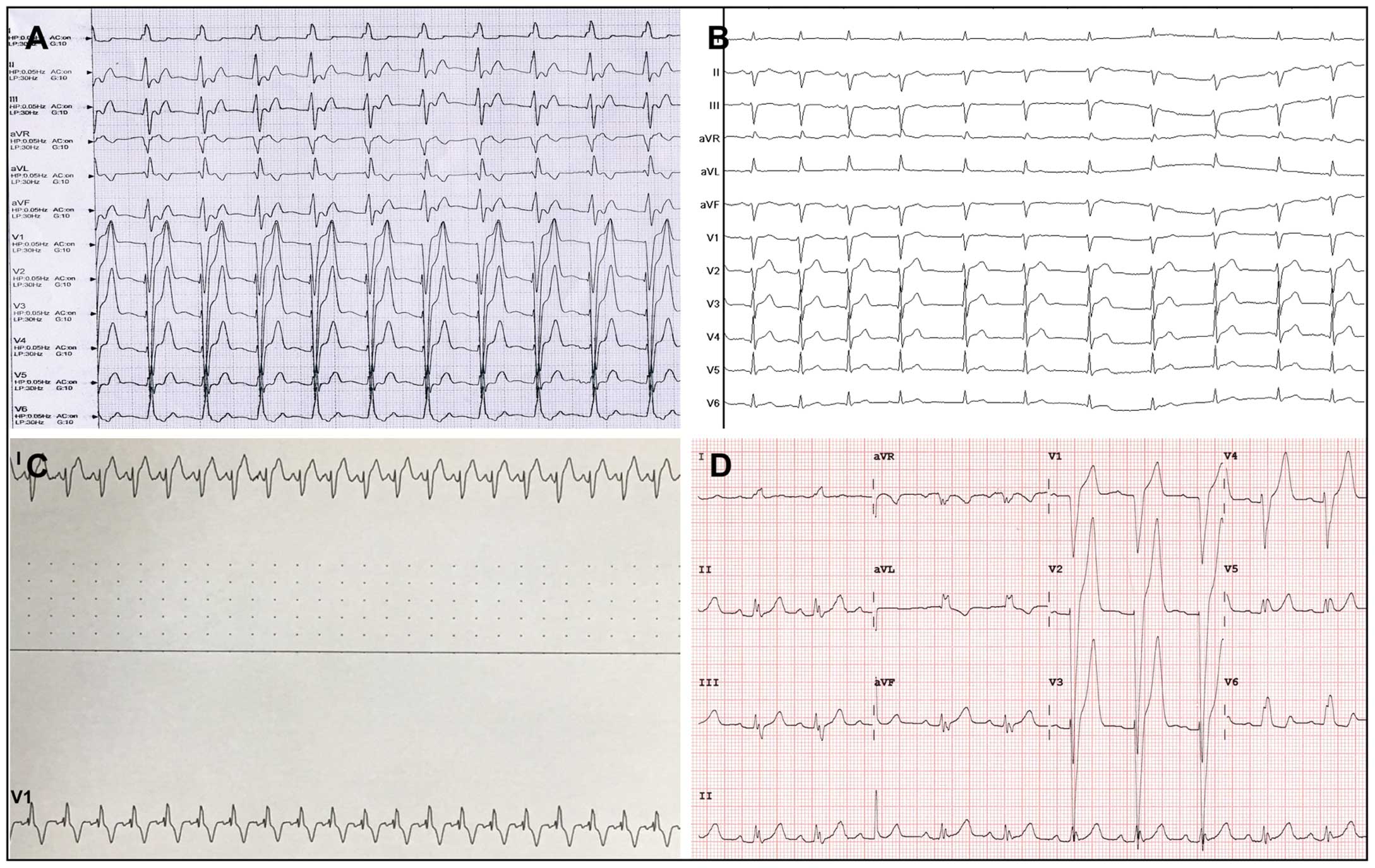
Another CLBBB occurred 4 days after placement of a 6-mm pmVSD occluder in a 30-year-old female (Case 12;
Figure 4D). This patient had a normal ECG before and during the procedure. A minimum distance of 1.5 mm from the defect to the near-field HPS was measured by 3D electroanatomic mapping. After intravenous administration of dexamethasone (10 mg/day) for 7 days, sinus rhythm was restored in this patient and she was discharged.
Risk Factors for Adverse Arrhythmic Events
Univariate analysis was conducted between the groups with and without adverse arrhythmic events (Table 2). Variables, including age, sex, pmVSD diameter, threshold of His capture, and presence of septal aneurysm, were compared between the 2 groups. The results revealed that there were no significant differences in age, sex, defect size, threshold of His capture, and the presence of septal aneurysm between the 2 groups (all P>0.05).
Table 2.
Procedure-Related Risk Factors for Adverse Arrhythmic Events
| |
Adverse arrhythmic events |
P-value* |
| Yes (n=4) |
No (n=16) |
| Male sex |
1 (25.0) |
7 (43.8) |
0.619 |
| Age (years) |
31±5 |
27±10 |
0.513 |
| Diameter of the pmVSD (mm) |
4.9±1.4 |
4.9±1.7 |
0.968 |
| Septal aneurysm |
2 (50.0) |
11 (68.8) |
0.587 |
| Minimum distance between the pmVSD and near-field HPS |
|
|
0.032 |
| <2 mm |
3 (75.0) |
2 (12.5) |
|
| ≥2 mm |
1 (25.0) |
14 (87.5) |
|
| Threshold of His capture |
|
|
0.619 |
| 5 mA at 1 ms |
3 (75.0) |
9 (56.2) |
|
| 10 mA at 1 ms |
1 (25.0) |
7 (43.8) |
|
Values are given as the mean±SD or n (%). *P-values are based on the Student’s t-test, the Mann-Whitney U-test, or Fisher’s exact test, as appropriate. P<0.05 was considered significant. HPS, His-Purkinje system; pmVSD, perimembranous ventricular septal defect.
The minimum distance between the pmVSD and near-field HPS measured by 3D electroanatomic mapping was also compared between the 2 groups using univariate analysis. These results indicated that a distance <2 mm was correlated with procedure-related adverse arrhythmic events (P=0.032).
New-Onset Arrhythmic Events
Four patients had conduction abnormalities before the procedure (3 with LAH and 1 with LAH plus incomplete right bundle branch block [IRBBB]). One patient with LAH developed AJR and CLBBB during the procedure, and the remaining 3 patients did not develop adverse arrhythmic events progressively during the follow-up period (Table 3).
Table 3.
Cardiac Rhythm During the Pre- and Postprocedural Periods
| Case no. |
Preprocedure |
Postprocedure |
| 6 h |
24 h |
72 h |
7 days |
1 month |
3 months |
6 months |
12 months |
24 months |
36 months |
| 1 |
SR |
SR |
SR |
SR |
SR |
SR |
SR |
SR |
SR |
SR |
SR |
| 2 |
PAC |
PAC |
PAC |
PAC |
PAC |
PAC |
PAC |
PAC |
PAC |
PAC |
PAC |
| 3 |
SR |
LAH |
SR |
SR |
SR |
SR |
SR |
SR |
SR |
SR |
SR |
| 4 |
SR |
SR |
SR |
AJR |
SR |
SR |
SR |
SR |
SR |
SR |
SR |
| 5 |
SR |
LAH |
SR |
SR |
SR |
SR |
SR |
SR |
SR |
SR |
SR |
| 6 |
LAH+
IRBBB |
LAH+
IRBBB |
LAH+
IRBBB |
LAH+
IRBBB |
LAH+
IRBBB |
LAH+
IRBBB |
LAH+
IRBBB |
LAH+
IRBBB |
LAH+
IRBBB |
LAH+
IRBBB |
LAH+
IRBBB |
| 7 |
SR |
SR |
SR |
SR |
SR |
SR |
SR |
SR |
SR |
SR |
SR |
| 8 |
LAH |
LAH |
LAH |
LAH |
LAH |
LAH |
LAH |
LAH |
LAH |
LAH |
LAH |
| 9 |
SR |
CLBBB* |
SR |
SR |
SR |
SR |
SR |
SR |
SR |
SR |
SR |
| 10 |
SR |
LAH |
IRBBB |
IRBBB |
IRBBB |
IRBBB |
SR |
SR |
SR |
SR |
– |
| 11 |
SR |
SR |
SR |
SR |
SR |
SR |
SR |
SR |
SR |
SR |
SR |
| 12 |
SR |
SR |
SR |
CLBBB |
CLBBB |
SR |
SR |
SR |
SR |
SR |
– |
| 13 |
SR |
SR |
SR |
SR |
SR |
SR |
SR |
SR |
SR |
SR |
– |
| 14 |
SR |
SR |
SR |
SR |
SR |
SR |
SR |
SR |
SR |
SR |
– |
| 15 |
SR |
LAH |
SR |
SR |
SR |
SR |
SR |
SR |
SR |
SR |
– |
| 16 |
SR |
SR |
SR |
SR |
SR |
SR |
SR |
SR |
SR |
SR |
– |
| 17 |
SR |
LAH |
CRBBB |
AJR |
SR |
SR |
SR |
SR |
SR |
SR |
– |
| 18 |
LAH |
AJR+
CLBBB* |
LAH |
LAH |
LAH |
LAH |
LAH |
LAH |
LAH |
– |
– |
| 19 |
LAH |
LAH |
LAH |
LAH |
LAH |
LAH |
LAH |
LAH |
LAH |
– |
– |
| 20 |
SR |
CRBBB* |
SR |
SR |
SR |
SR |
SR |
SR |
SR |
– |
– |
*Events that occurred during the procedure. AJR, accelerated junctional rhythm; CLBBB, complete left bundle branch block; CRBBB, complete right bundle branch block; IRBBB, incomplete right bundle branch block; LAH, left anterior hemiblock; PAC, premature atrial contraction; SR, sinus rhythm.
New-onset arrhythmic events occurred in 7 patients, with 10 events after the procedure. The time between device placement and the development of new-onset conduction block was usually ≤3 days. Among these cases, LAH was the most common type of arrhythmic event (5/17; 29.4%), followed by right bundle branch block (RBBB; 2/17; 11.7%), AJR (2/17; 11.7%), and left bundle branch block (LBBB; 1/17; 5.9%). Most of these events were transient and asymptomatic phenomena. Of the 7 patients with post-procedural conduction blocks, 6 recovered to normal conduction before discharge, whereas the new-onset conduction block in the remaining patient (IRBBB) did not recover to normal conduction until the 1-month follow-up visit. No CAVB occurred during the entire follow-up period.
Risk Factors for New-Onset Arrhythmic Events
Of the 17 patients who were successfully treated by transcatheter closure, there was no significant difference between patients with and without new-onset arrhythmic events in sex, age, pmVSD diameter, presence of an aneurysm, type of occluder device, or occluder size (all P>0.05). Results of the univariate analysis of potential predisposing factors for new-onset arrhythmic events are summarized in
Table 4. Of note, a shorter distance between the pmVSD and near-field HPS (2.5±0.7 vs. 2.7±0.7 mm) seems to be associated with a higher risk of new-onset arrhythmic events, but this difference was not statistically significant (P=0.568).
Table 4.
Risk Factors for New-Onset Arrhythmic Events After the Procedure
| |
New-onset arrhythmic events |
P-value* |
| Yes (n=7) |
No (n=10) |
| Female sex |
5 (71.4) |
5 (50.0) |
0.622 |
| Age (years) |
23±6 |
30±12 |
0.128 |
| Diameter of the pmVSD (mm) |
5.1±1.7 |
4.6±1.6 |
0.543 |
| Septal aneurysm |
4 (57.1) |
7 (70.0) |
0.644 |
| Thin-waist occluder |
2 (28.6) |
7 (70.0) |
0.153 |
| Occluder size (mm) |
7.0±2.6 |
6.2±1.2 |
0.404 |
| Distance between the pmVSD and near-field HPS (mm) |
2.5±0.7 |
2.7±0.7 |
0.568 |
Values are presented as the mean±SD or as n (%). *P-values are based on the Student’s t-test, the Mann-Whitney U-test, or Fisher’s exact test, as appropriate. P<0.05 was considered significant. HPS, His-Purkinje system; pmVSD, perimembranous ventricular septal defect.
Except for the arrhythmic events mentioned above, there were no deaths, incidents of infective endocarditis, device embolizations or malpositions, thrombus formations, or other morbidities during the follow-up period (median 30 months; range 12–48 months).
Discussion
Main Findings
In the present study we showed that mapping of the distribution of the HPS facilitates visualization of its 3D location in relation to the location of pmVSD. By combining 3D electroanatomic mapping and pacing techniques, the site with denoted His potentials can be further confirmed as near-field HPS. By measuring the distance between the pmVSD and near-field HPS, we reached the conclusion that a minimum distance <2 mm was associated with a higher risk of adverse arrhythmic events related to the procedure.
Using 3D Electroanatomic Mapping and Pacing Techniques to Identify the Near-Field HPS
To the best of our knowledge, this is the first report on the use of 3D electroanatomic mapping to guide transcatheter closure of pmVSD in adults. This novel strategy may help improve our understanding of the anatomy of the pmVSD and its adjacent conduction system, as well as guide the decision making process regarding transcatheter closure. It is known that the HB passes through the central fibrous body before it reaches the lower edge of the membranous septum, and then continues into the penetrating portion of the HB (PHB) that bifurcates into the left and right bundle branches.8
All pmVSDs share the feature that the HB and PHB pass posterior and inferior to them, and the distance between the PHB and the margins of the pmVSD is approximately 2–4 mm.9,10
In a previous study we demonstrated that the site where an HB potential is recorded and the HB can be captured with minimal output represents near-field His activation, indicating close proximity to the HB.5
According to the present study, HPS activation based on 3D mapping can be found in a relatively large area adjacent to the pmVSD. We prospectively applied this technique in left-sided HPS, and further corroborated that pacing at the sites recording HPS activation adjacent to the pmVSD could produce a narrower QRS complex by capturing the HB with a low pacing output (<10 mA/2 ms). Using 3D electroanatomic mapping, we measured a mean distance of 2.5±0.7 mm (range 1.3–3.9 mm) between the pmVSD and near-field HPS, indicating a close relationship with regard to their anatomical characteristics. Anatomically, the near-field HPS that we tagged on the 3D map with a minimum distance from the pmVSD should be located in the PHB, and even in the PHB-left bundle branch region.
Potential Mechanisms of Adverse Arrhythmic Events
Conduction block has been commonly reported in transcatheter closure of pmVSDs due to the proximity of the conduction system to the defect.11
Some previous studies reported that bundle branch block and CAVB occurred in 3.5–8.6% of cases.12–14
In the present study, no CAVB was recorded during the entire follow-up period, whereas other adverse arrhythmic events were noted in 4 patients. Of these, 1 patient with a minimum distance of 2.8 mm from the defect to the near-field HPS was found to have CRBBB when establishing the arteriovenous circuit during the procedure. The patient quickly recovered to normal conduction after the delivery system was withdrawn, suggesting that mechanical stimulation may result in conduction abnormality. Of the other 3 patients with adverse arrhythmic events during closure and after the procedure, there was a minimum distance between the pmVSD and near-field HPS of <2 mm in all cases. These events may be due to the direct compression of the device on the conduction system with a relatively short distance from the pmVSD to the near-field HPS, explaining why the sinus rhythm recovered quickly in patients after removal of the device and delivery system and the initiation of steroid therapy. Notably, CLBBB has been proven to induce left ventricular remodeling and to cause heart failure.15,16
Left ventricular motion is significantly delayed and often dyssynchronous in patients with CLBBB. Meanwhile, the ejection fraction can decrease gradually because of the impaired left ventricular motion, leading to LBBB-induced left ventricular enlargement and cardiomyopathy.17
Therefore, CLBBB should be regarded as an adverse arrhythmic event, underscoring the need for lifelong follow-up.
Potential Mechanisms of New-Onset Arrhythmic Events
New-onset bundle branch block, including LAH and RBBB, was quite common in the present study, probably due to the delicate and fragile features of bundle branch that increase the risk of injury. Most of the new-onset arrhythmic events had a benign course, and resolved spontaneously or were effectively treated with corticosteroid therapy before discharge. The mechanism underlying these events probably involves mechanical compression, progressive device flattening, friction between the device disk and the septal myocardium, and an inflammatory response. In addition, we observed that 50% of new-onset arrhythmic events occurred within 6 h after the procedure, which also confirms that mechanical stimuli may be important causes of arrhythmia. A previous study indicated that asymmetric occluders and large device sizes increased the risk of new-onset arrhythmic events.18
An enlarged left disc of the thin-waist occluder and large device sizes logically increase the risk of compression onto the conduction tissue. However, no difference was found in occluder type and device size between the 2 groups in the present study. Although the mean distance between the pmVSD and near-field HPS was less in patients with than without new-onset arrhythmic events (2.5±0.7 vs. 2.7±0.7 mm), this difference was not significant. Therefore, larger sample sizes and longer follow-up periods are needed to verify the relationship between new-onset arrhythmic events and their related risk factors.
Guide to Decision Making and Treatment Options
In the present study we confirmed that the distance between the pmVSD and near-field HPS measured by 3D mapping is crucial. A minimum distance <2 mm was associated with a higher risk of procedure-related adverse arrhythmic events. Therefore, the choice of treatment should be individualized based on the minimum distance between the pmVSD and the near-field HPS. If the minimum distance between the pmVSD and near-field HPS is ≥2 mm, transcatheter closure is considered to be a relatively safe treatment. If the minimum distance is <2 mm, caution should be exercised when deciding transcatheter closure for the pmVSD. Surgical repair of the pmVSD should be an alternative to transcatheter closure in this situation.
Surgical repair with cardiopulmonary bypass and median sternotomy has been the standard treatment for VSDs. However, this procedure has many complications, such as postoperative pain, thoracostomy scars, infection, a prolonged hospital stay, and psychological trauma. With the recent development of various devices and improvements in interventional techniques, device closure for VSD using a transthoracic or transcatheter approach is more widely performed, and good clinical outcomes have been obtained.19,20
A recent meta-analysis found no significant difference between transcatheter closure and surgical repair of pmVSD with regard to the incidence of adverse arrhythmic events; however, double the number of patients developed adverse arrhythmic events after transcatheter compared with surgical repair.21
Moreover, Fang et al20
reported a similar success rate and comparable rates of adverse arrhythmic events in the different device groups compared with the surgical repair group. Compared with surgical repair, device closure procedures such as transcatheter and periventricular device closure involve real-time monitoring of changes in cardiac rhythm due to the delivery and release of the occluder. On the basis of the findings of the present study, we speculate that the occurrence of adverse arrhythmic events during and after the procedure may be related to the mechanical compression caused by the occluder, because a relatively short distance was found between the pmVSD and near-field HPS in these patients. Therefore, at Guangdong Cardiovascular Institute, surgical repair of pmVSDs with an autologous patch instead of an inserted device is still the preferred method in patients with failed transcatheter closure.
Based on the experience from our previous para-Hisian studies,5,22
we believe that 3D electroanatomic mapping can be of considerable importance in transcatheter closure of pmVSDs. The total mapping time itself was relatively short (20 min) in the present study. High-density 3D electroanatomic mapping can improve our understanding of the local anatomy of the pmVSD and its adjacent conduction system before the procedure, which is critical for all practitioners, including interventional cardiologists, cardiac electrophysiologists, and cardiac surgeons. The time required for mapping is minimal, and 3D electroanatomic mapping should not lengthen the procedure considerably. Additional costs associated with 3D electroanatomic mapping could be an issue, but may be offset by improvements in procedural outcomes and reductions in the incidence of conduction injury.
Study Limitations
Although the present study demonstrated significant benefits of 3D electroanatomic mapping for transcatheter pmVSD closure, it nevertheless has some limitations. First, an ultrasound catheter was not used in the study to improve our understanding of the local anatomy. Second, this study is limited by its small sample size. Larger series with longer follow-up data are needed. Third, the right-sided distance between the pmVSD and near-field HPS could also play an important role in predicting conduction disturbance related to the procedure, but was not evaluated in the present study. Despite these limitations, the methods used in this study can improve the prediction of adverse arrhythmic events during and after transcatheter pmVSD closure.
Conclusions
Transcatheter closure of pmVSDs in adults guided by 3D electroanatomic mapping and pacing techniques is feasible and useful for delineation of the cardiac conduction system in close proximity. It allows for intracardiac identification of the near-field HPS adjacent to the pmVSD, thus guiding occlusion procedures and possibly reducing the incidence of procedure-related adverse arrhythmic events.
Acknowledgement
The authors thank LetPub (www.letpub.com) for linguistic assistance during the preparation of this manuscript.
Sources of Funding
Y.X. is supported by a research grant from the National Natural Science Foundation of China (NSFC-81870254). S.W. is supported by a research grant from the Science and Technology Program of Guangzhou City, China (No. 201508020261).
Conflict of Interest
The authors do not have any conflicts of interest to declare.
References
- 1.
Baumgartner H, Bonhoeffer P, De Groot NM, de Haan F, Deanfield JE, Galie N, et al. ESC guidelines for the management of grown-up congenital heart disease (new version 2010). Eur Heart J 2010; 31: 2915–2957.
- 2.
Warnes CA, Williams RG, Bashore TM, Child JS, Connolly HM, Dearani JA, et al. ACC/AHA 2008 guidelines for the management of adults with congenital heart disease: A report of the American College of Cardiology/American Heart Association Task Force on Practice Guidelines (writing committee to develop guidelines on the management of adults with congenital heart disease). Circulation 2008; 118: e714–e833.
- 3.
Wang J, Zuo J, Yu S, Yi D, Yang X, Zhu X, et al. Effectiveness and safety of transcatheter closure of perimembranous ventricular septal defects in adults. Am J Cardiol 2016; 117: 980–987.
- 4.
Stout KK, Daniels CJ, Aboulhosn JA, Bozkurt B, Broberg CS, Colman JM, et al. 2018 AHA/ACC guideline for the management of adults with congenital heart disease: A report of the American College of Cardiology/American Heart Association Task Force on Clinical Practice Guidelines. J Am Coll Cardiol 2019; 73: e81–e192.
- 5.
Xue Y, Zhan X, Wu S, Wang H, Liu Y, Liao Z, et al. Experimental, pathologic, and clinical fingdings of radiofrequency catheter ablation of para-hisian region from the right ventricle in dogs and humans. Circ Arrhythm Electrophysiol 2017; 10: e005207.
- 6.
Takatsuki S, Mitamura H, Tanimoto K, Fukuda Y, Ieda M, Miyoshi S, et al. Clinical implications of “pure” Hisian pacing in addition to para-Hisian pacing for the diagnosis of supraventricular tachycardia. Heart Rhythm 2006; 3: 1412–1418.
- 7.
Wang L, Cao S, Li J, Yang L, Liu Y, Ren J, et al. Transcatheter closure of congenital perimembranous ventricular septal defect in children using symmetric occluders: An 8-year multiinstitutional experience. Ann Thorac Surg 2012; 94: 592–598.
- 8.
Mutharasan RK, Nagaraj A, Hamilton AJ, McPherson DD, Bharati S. Computer three-dimensional reconstruction of the atrioventricular conduction system. Pacing Clin Electrophysiol 2004; 27: 740–748.
- 9.
Moore JP, Aboulhosn JA. Introduction to the congenital heart defects: Anatomy of the conduction system. Card Electrophysiol Clin 2017; 9: 167–175.
- 10.
Milo S, Ho SY, Wilkinson JL, Anderson RH. Surgical anatomy and atrioventricular conduction tissues of hearts with isolated ventricular septal defects. J Thorac Cardiovasc Surg 1980; 79: 244–255.
- 11.
Bai Y, Xu XD, Li CY, Zhu JQ, Wu H, Chen SP, et al. Complete atrioventricular block after percutaneous device closure of perimembranous ventricular septal defect: A single-center experience on 1046 cases. Heart Rhythm 2015; 12: 2132–2140.
- 12.
Knauth AL, Lock JE, Perry SB, McElhinney DB, Gauvreau K, Landzberg MJ, et al. Transcatheter device closure of congenital and postoperative residual ventricular septal defects. Circulation 2004; 110: 501–507.
- 13.
Sullivan ID. Transcatheter closure of perimembranous ventricular septal defect: Is the risk of heart block too high a price? Heart 2007; 93: 284–286.
- 14.
Carminati M, Butera G, Chessa M, De Giovanni J, Fisher G, Gewillig M, et al. Transcatheter closure of congenital ventricular septal defects: Result of the European Registry. Eur Heart J 2007; 28: 2361–2368.
- 15.
Zannad F, Huvelle E, Dickstein K, van Veldhuisen DJ, Stellbrink C, Køber L, et al. Left bundle branch block as a risk factor for progression to heart failure. Eur J Heart Fail 2007; 9: 7–14.
- 16.
Vernooy K, Verbeek XA, Peschar M, Crijins HJ, Arts T, Cornelussen RN, et al. Left bundle branch block induces ventricular remodelling and functional septal hypoperfusion. Eur Heart J 2005; 26: 91–98.
- 17.
Blanc JJ, Fatemi M, Bertault V, Baraket F, Etienne Y. Evaluation of left bundle branch block as a reversible cause of non-ischaemic dilated cardiomyopathy with severe heart failure. A new concept of left ventricular dyssynchrony-induced cardiomyopathy. Europace 2005; 7: 604–610.
- 18.
Zhao LJ, Han B, Zhang JJ, Yi YC, Jiang DD, Lyu JL. Postprocedural outcomes and risk factors for arrhythmias following transcatheter closure of congenital perimembranous ventricular septal defect: A single-center retrospective study. Chin Med J (Engl) 2017; 130: 516–521.
- 19.
Chen Q, Wu WX, Huang JS, Chen LW, Fang GH. Transthoracic device closure, transcatheter device closure, and surgical repair via right submammary thoracotomy for restrictive ventricular septal defect, a respective comparative study. J Invest Surg 2019; 1: 1–6.
- 20.
Fang GH, Chen Q, Hong ZN, Lin ZW, Zhang GC, Cao H, et al. The comparison of perventricular device closure with transcatheter device closure and the surgical repair via median sternotomy for perimembranous ventricular septal defect. Ann Thorac Cardiovasc Surg 2018; 24: 308–314.
- 21.
El-Kadeem S, El Nemr S, El Amrousy D, Zoair A. Comparison of transcatheter versus surgical closure of perimembranous ventricular septal defect in pediatric patients: A systematic review and meta-analysis. J Saudi Heart Assoc 2019; 31: 188–197.
- 22.
Luo S, Zhan X, Ouyang F, Xue Y, Fang X, Liao H, et al. Catheter ablation of right-sided para-Hisian ventricular arrhythmias using a simple pacing strategy. Heart Rhythm 2019; 16: 380–387.





