Abbreviations
| AC |
arm circumference |
| ACCF |
American College of Cardiology Foundation |
| ADL |
activities of daily living |
| AHA |
American Heart Association |
| AI |
adequate intake |
| AMA |
arm muscle area |
| AMC |
arm muscle circumference |
| ASPEN |
American Society for Parenteral and Enteral Nutrition |
| BIA |
bioelectrical impedance analysis |
| BMI |
body mass index |
| CC |
calf circumference |
| CNAQ |
Council on Nutrition Appetite Questionnaire |
| CONUT |
controlling nutritional status |
| CRP |
C-reactive protein |
| DASH |
Dietary Approaches to Stop Hypertension |
| DG |
tentative dietary goal for preventing life-style related diseases |
| DHA |
docosahexaenoic acid |
| DXA |
dual energy X-ray absorptiometry |
| EAR |
estimated average requirement |
| ECW |
extracellular water |
| EER |
estimated energy requirement |
| EPA |
eicosapentaenoic acid |
| ESC |
European Society of Cardiology |
| ESPEN |
European Society for Clinical Nutrition and Metabolism |
| FAACT |
Functional Assessment of Anorexia /Cachexia Therapy |
| FT |
food test |
| GNRI |
geriatric nutritional risk index |
| GRV |
gastric residual volume |
| HDL |
high density lipoprotein |
| IABP |
intra-aortic balloon pump |
| ICU |
intensive care unit |
| LDL |
low density lipoprotein |
| LPL |
lipoprotein lipase |
| MNA® |
Mini Nutritional Assessment |
| MNA-SF® |
Mini Nutritional Assessment-Short Form |
| MWST |
modified water swallow test |
| NPPV |
noninvasive positive pressure ventilation |
| NRS |
nutritional risk screening |
| NST |
nutrition support team |
| ONS |
oral nutrition supplementation |
| PNI |
prognostic nutritional index |
| PPN |
peripheral parenteral nutrition |
| QOL |
quality of life |
| RBP |
retinol binding protein |
| RDA |
recommended dietary allowance |
| REE |
resting energy expenditure |
| RSST |
repetitive saliva swallowing test |
| SCCM |
Society of Critical Care Medicine |
| SENPE |
Spanish Society of Parenteral and Enteral Nutrition |
| SGA |
subjective global assessment |
| SNAQ |
Simplified Nutritional Appetite Questionnaire |
| TBW |
total body water |
| Tf |
transferrin |
| TNF |
tumor necrotic factor |
| TPN |
total parenteral nutrition |
| TSF |
triceps skinfolds |
| UL |
tolerable upper intake level |
| V̇O2 |
oxygen uptake |
| V̇CO2 |
carbon dioxide output |
| VE |
videoendoscopic examination of swallowing |
| VF |
videofluoroscopic examination of swallowing |
| WHO |
World Health Organization |
Introduction
In Japan, heart failure patients continue to be increasing rapidly, and the state is called a “heart failure pandemic”. Heart failure is basically impairment of cardiac function, but its pathology is greatly influenced by non-cardiac factors including complications, and this constitutes a difference compared with cancer. In such circumstances, attention is directed to nutritional disorders, a non-cardiac factor, as a new target of therapeutic approaches to heart failure patients. Nutritional disorders have been shown by a number of epidemiological studies to be a risk factor for heart failure and events subsequent to heart failure. After heart failure, a negative spiral is considered to be set off with not only progression of heart failure exacerbating nutritional disorder but also exacerbation of nutritional disorder accelerating progression of heart failure. Nutritional disorders are also a factor of frailty, which is a problem in recently increasing aged heart failure patients.
Presently, however, the accumulation of scientific knowledge remains insufficient for the preparation of guidelines for nutritional management of heart failure patients. Therefore, the Japanese Heart Failure Society prepared this statement as a summary of expert consensus at the current level based on the knowledge available at present with participation by experts in various fields of many disciplines. It is our wish that this statement urges medical service workers to reflect deeply about nutritional support of heart failure patients, consequently promotes the evolution of new knowledge, and eventually contributes to the development of this field, which is presently an unexplored territory, into a rich and productive discipline.
Chapter I. What Is Nutrition?
Section 1. Nutrition and Nutrients
In humans, nutrition is defined as supporting life by maintaining vital phenomena such as respiration, digestion and absorption, excretion, exercise, growth, and reproduction, taking in materials necessary for healthy daily living from the environment and using them, and excreting wastes. Therefore, nutrition is a phenomenon rather than materials.1,2
In contrast, nutrients are materials which must be taken in from the environment to sustain vital phenomena, examples of which are protein and calcium.1
Thus, nutrition and nutrients differ clearly but are occasionally confused or misunderstood.
Section 2. Intake of Nutrients and Their Physiological Activities
Nutrients essential for living are classified into five groups, namely, proteins, lipids, carbohydrates, vitamins, and minerals. Each nutrient bears physiological activities and roles necessary to sustain life. Of these nutrients, proteins, lipids, and carbohydrates are called energy-yielding nutrients, because they produce energy in the body. Carbohydrates and proteins produce about 4 kcal/g of energy, and lipids produce about 9 kcal/g of energy. Therefore, the amount of energy contained in a particular food item or dish or nutritional preparation is determined by the amounts of various energy-yielding nutrients. Vitamins and minerals do not produce energy in the body, but they are indispensable for structuring of biological tissues and regulation of vital functions. Also, since water is closely involved in digestion, absorption, excretion, and structuring of biological tissues as well as control of body temperature, it is regarded as a material comparable to a nutrient.
Deficiency or excess (imbalance) of nutritional intake is widely known to not only invite disorders but also strongly affect the development and progression (exacerbation) of diseases. Therefore, appropriate nutritional intake is indispensable for sustaining or promoting health and preventing and treating diseases. However, we usually ingest nutrients as meals rather than plain nutrients. Therefore, the contents, amount, and balance of diet are important (Figure I-1).
Section 3. Nutritional Disorders, Deficiencies, and Excesses
Lack of nutrients necessary for an individual and consequent inability to maintain normal physiological functions is called nutritional deficiency. Also, the inability to maintain normal physiological functions due to excessive nutritional intake is called nutritional excess. In addition, physical disorders caused by nutritional deficiency or excess are called nutritional disorders.
Nutritional disorders are caused by not only excessive or deficient intake of essential nutrients for some reason but also malabsorption, impairment of utilization, increases in consumption or demand, and disorder of excretion alone or in combination. Nutritional disorders may also be caused by the effects of drugs on absorption, utilization, or metabolism of nutrients.
A. Nutritional Disorders Due to Protein or Energy Deficiency
A nutritional disorder caused by the lack of protein is called kwashiorkor. It results from qualitative and quantitative inadequacy of protein and has been reported to be observed in infants in regions with a low standard of living, but is recently considered also to be caused by protein deficiency combined with factors such as infection and trauma. Nutritional disorder caused by simultaneous deficiency of protein and energy is called marasmus. It is observed in adults and older people as well as infants in regions with a low standard of living and is considered to be caused primarily by low energy intake associated with an absolute lack of food intake.3
B. Vitamin Deficiency and Excess
Vitamins consist of four lipid-soluble vitamins (vitamins A, D, E, and K) and nine water-soluble vitamins (vitamins B1, B2, B6, B12, niacin, pantothenic acid, folic acid, biotin, and vitamin C).
Lipid-soluble vitamin deficiency is caused by decreases in intake of lipid-soluble vitamins and lipid absorption. Also, if lipid-soluble vitamins are ingested or administered in excess, they are accumulated in the liver and fat and may cause hypervitaminosis.
Since water-soluble vitamins are readily excreted, their storage in the body is low. Therefore, they may be deficient, and disorders due to their excess are rare.3,4
C. Mineral Deficiency and Excess
Minerals include sodium, potassium, calcium, phosphorus, and magnesium, which are classified as macro-minerals, and iron, zinc, copper, manganese, chromium, iodine, molybdenum, and selenium, which are classified as trace elements (called micro-minerals in the Dietary Reference Intakes for Japanese (2020)). The time until deficiency or excess becomes apparent varies widely among minerals.3,5
D. Interrelations Among Nutrients
In iron deficiency anemia, which is frequently observed in Japan, for example, appropriate intake of vitamin C is necessary to promote absorption of dietary non-heme iron along with increased intake of iron for symptomatic relief. Thus, approaches with sufficient consideration of interrelations among nutrients are required for the prevention and treatment of nutritional deficiency or excess.
Section 4. Dietary Reference Intakes for Japanese
The amount a nutrient necessary for a person (minimum amount necessary to avoid deficiency) is unknown in many cases, because its accurate determination is extremely complex and practically impossible. Even if the sex, age, height, and activity level are assumed to be the same, necessary amounts of nutrients vary, and consideration of individual differences is necessary. Therefore, the current nutrition science predicts and organizes deficits and surpluses of intakes and recommendable intakes of nutrients based on scientific evidence including the results of nutrient consumption and expenditure studies and nutritional surveys and by introducing approaches of probability theory. In Japan, this is implemented in the Dietary Reference Intakes for Japanese compiled by the Ministry of Health, Labour and Welfare.
In the Dietary Reference Intakes for Japanese (2020),6
indices are adopted from three viewpoints, namely 1) avoidance of insufficient intake, 2) avoidance of health damage due to excessive intake, and 3) prevention of lifestyle-related diseases.
There are three indices adopted for the avoidance of insufficient intake. The estimated average requirement (EAR) is considered to be the amount that meets the requirement in half the population (the probabilities of fulfillment and lack are both 50%). The recommended dietary allowance (RDA) is adopted as an index that complements EAR. RDA is considered the amount that meets the requirement in most people (probability of fulfillment: 97.5%). In addition, the adequate intake (AI) is used if EAR or RDA cannot be determined due to the lack of scientific evidence. AI is considered the amount sufficient to maintain a nutritional level at or above which the risk of deficiency is nearly zero.
As the index aiming at avoidance of health damage due to excessive intake, the tolerable upper intake level (UL) is adopted.
In addition, as the intake that current Japanese should provisionally aim at for the prevention of lifestyle-related diseases, the tentative dietary goal for preventing life-style related diseases (DG) is adopted.
A conceptual diagram that helps with the understanding of these indices is shown in
Figure I-2.6

Concerning energy, the body mass index (BMI) is adopted as an index to check the balance between intake and consumption of energy (energy balance).6
However, BMI is difficult to apply to school children, because both the height and weight change with growth. Therefore, it is necessary to understand and confirm the condition including changes with time using objective indices such as the obesity assessment and growth curve used in school health statistical survey by the Ministry of Education, Culture, Sports, Science and Technology.6,7
In the Dietary Reference Intakes for Japanese (2020), a provisional range of BMI that should be targeted is determined based on comprehensive judgments in consideration of the relationships of the overall mortality and incidences of various diseases with BMI and the relationships between causes of death and BMI reported by observational epidemiological studies as well as the reality of BMI in Japanese (Table I-1).6
Particularly, in those aged 65 years and above, there was a gap between BMI at which the overall mortality was lowest and the reality, and the provisional target range of BMI is set at 21.5–24.9 kg/m2
partly because of the necessity to consider the prevention of both frailty and lifestyle-related diseases.
Table I-1.
Target BMI Range (18 Years and Older)*
1,*
2
| Age (years) |
Target BMI (kg/m2) |
| 18–49 |
18.5–24.9 |
| 50–64 |
20.0–24.9 |
| 65–74*3 |
21.5–24.9 |
| 75+*3 |
21.5–24.9 |
*1For both males and females. These values shall be used merely as a reference.
*2The target ranges were set comprehensively based on BMI at which the overall mortality reported in observational epidemiological studies was lowest in consideration of the relationship between the incidence of each disease with BMI, relationship between each cause of death and BMI, effects of smoking and complications on BMI and mortality risk, and actual BMI in Japanese.
*3In older people, a provisional target range of BMI was set as 21.5–24.9 kg/m2 in view of the necessity of consideration of the prevention of both frailty and lifestyle-related disease.
Adapted from Ministry of Health, Labour and Welfare. “Dietary Reference Intakes for Japanese (2015)” Preparation Committee Report (March 2014).6
Incidentally, check of energy balance using BMI does not lead to practical measures without a specific guidelines or recommendation about energy intake. Therefore, the Dietary Reference Intakes for Japanese (2020) presents a formula (method) for the calculation of the estimated energy requirement (EER) as a product of basal metabolic rate and physical activity level based on monitoring of the condition of each person.6
Section 5. Evaluation of Nutrient Intakes
The amounts of nutrients that we ingest as diet constantly fluctuate. Therefore, it is not appropriate to evaluate nutrient intakes by checking the dietary contents on a particular day. The Dietary Reference Intakes for Japanese recommends the evaluation by determining the mean intakes of nutrients over a period of about 1 month as habitual intakes except those that vary widely and comparing them with the reference intakes. However, it is fairly bothersome to weigh dietary contents and record them for 1 month. Thus, in practice, methods in which the dietary contents are weighed and recorded on a few separate days, and the habitual intakes are estimated from the values obtained, or in which the amounts of food groups ingested over a given period and the frequency of their intakes are asked, and the nutrient intakes during the period are estimated (food intake frequency survey method) are often employed. However, since there are advantages and disadvantages in any nutritional/dietary survey method, one or several survey methods and evaluation procedures that are appropriate must be selected depending on what are evaluated and for what purpose they are evaluated. Also, comprehensive assessment by measuring, if necessary, the serum albumin level and amounts of urinary excretion of urea nitrogen and sodium is often performed.
Section 6. Recommendable Nutrient Intakes
Based on the results of the National Health and Nutrition Survey conducted annually by the ministry of Health, Labour and Welfare and many other nutritional/dietary surveys, nutrients that may be underingested or overingested have been generally identified from the dietary contents of average Japanese people. Therefore, from this perspective, measures and management to encourage strict adherence to EAR, RDA, or AI are necessary. However, actual dietary nutrient intakes are not so simple.
Many foods contain several nutrients, and meals are prepared as dishes consisting of one or more foods. Thus, if excessive attention is paid to nutrients that may be under- or overeaten, meals themselves become less appetizing, and dietary intakes as a whole may decrease, or meals may even cease to be meals. For optimal nutrient intakes, habitual intakes should be adjusted to remain in a tolerable range rather than confining the intake of energy or each nutrient to a set value. Specifically, the intakes of nutrients that may be underingested should be maintained at or above EAR, and the intakes of nutrients ingested above UL by the use of oral nutrition supplementation (ONS), should be controlled by stopping or reducing the intake of ONS, aiming both to bring them closer to RDA or AI (Figure I-3).7

Incidentally, the sodium (salt) intake of Japanese is higher than the global average. According to the Dietary Reference Intakes for Japanese (2020), EAR of sodium in adults is 600 mg/day (equivalent to 1.5 g/day of salt). The sodium intake of Japanese is not usually considered to fall below this level without marked perspiration if they can eat normally. Instead, it is more important to reduce the sodium intake, and DG as salt is set at <7.5 g/day for males and <6.5 g/day for females.7
Section 7. Changes in Energy and Nutrient Requirements With Growth and Aging
It is necessary that humans ingest amounts of energy and nutrients appropriate for changes in physical conditions from birth to death.
According to the Dietary Reference Intakes for Japanese (2020), the basal metabolism reference value (kcal/kg body weight/day) that serves as the basis for estimation of basal metabolism, which is the minimum necessary amount of energy to sustain life, is 61.0 and 59.7 in males and females, respectively, for 1–2-year-olds, 37.4 and 34.8 for 10–11-year-olds, 23.7 and 22.1 for 18–29-year-olds, and 21.5 and 20.7 for those aged ≥75 years, showing decreases with aging.6
However, the height, which is another factor, also changes with growth and aging. Therefore, the basal metabolic expenditure varies with changes in these two factors. Individual EER can be calculated by multiplying the basal metabolic expenditure by the physical activity level.
To calculate EER of a growing child, it is necessary to add the energy needed for the increased tissue to the energy required for physical activities and tissue synthesis. In adults, also, for pregnant women, it is necessary to add the sum of the change in total energy consumption due to pregnancy and the accumulated amount of energy. For breast-feeding women, the energy for breast-feeding must be added to EER before pregnancy.6
Special attention is needed for nutrition in many women, because additional nutrient intakes may be necessary in consideration of menstruation, pregnancy, and breast-feeding.
Section 8. Characteristics of Nutritional Therapy for Treatment
The Dietary Reference Intakes for Japanese (2020) is prepared in consideration of the prevention of undernutrition and frailty in older people as well as the prevention of lifestyle-related diseases and their progression. It is also targeted to healthy individuals and a population consisting primarily of healthy individuals including people with risk factors for lifestyle-related diseases, older people with risk factors for frailty capable of generally independent living, and a population consisting primarily of such people.6
For these reasons, it practically concerns only nutrients and energy contained in orally ingested foods.
On the other hand, for the management of energy and nutrient intakes for therapeutic purposes in individuals and groups with diseases or at a high risk for diseases, it is necessary to use guidelines for nutritional management, such as treatment guidelines for particular diseases with the understanding of basic principles of the Dietary Reference Intakes toward energy and nutrient intakes, and, eventually, the utmost priority is given to the judgments and instructions of the physician in charge. Naturally, there are situations in which nutritional methods including enteral and intravenous nutrition are employed alone or in combination in addition to situations manageable by ordinary oral nutrition alone. In nutritional therapy for treatment, the prescribed (provided) amounts of energy and nutrients vary with not only the physical characteristics but also the pathologic condition of the patient. In addition, the tolerable ranges of the amounts of energy and nutrients are usually narrower than those in healthy individuals. Moreover, in those with critical conditions or complications, the ranges are further narrowed, and more strict management is necessary.
Chapter II. Nutritional Disorders in Heart Failure
Section 1. Paradigm Shift From Restrictive Nutritional Guidance
In the general population, obesity has been shown to be an independent risk factor for heart failure as well as future events of cardiovascular disease.8
However, Anker et al. reported that, in heart failure patients, loss of body weight is a poor prognostic factor and that stability of body weight is associated with a good prognosis.9
Thereafter, similar evaluation was made by large-scale multicenter studies. As a low body mass index (BMI) was consistently associated with a poor outcome, heart failure guidelines in Western countries began to warn about a low body weight10,11
Although there are physical differences between Western and Japanese people, similar phenomena have also been reported in Japanese12–14
(Figure II-1). Under these circumstances, the necessity of change in the direction of nutritional guidance from conventional restriction of energy intake to maintenance of BMI has begun to be recognized.
Section 2. Concepts of Sarcopenia, Frailty, and Cachexia
Sarcopenia, frailty, and cachexia are pathological conditions with low body weight and undernutrition.
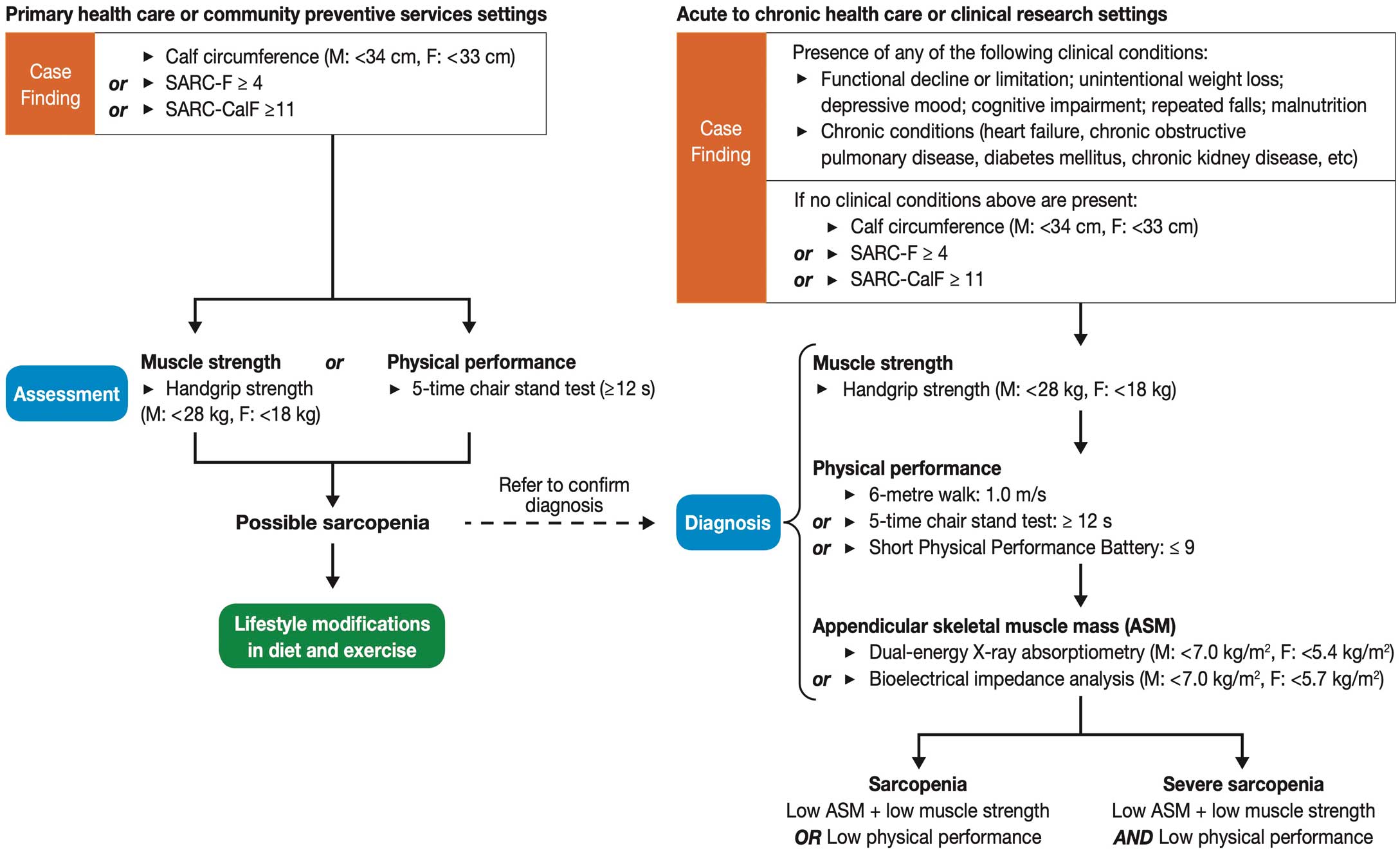
Sarcopenia means declines in muscle strength and physical functions due to loss of muscle mass. The diagnostic criteria for Asians by the Asian Working Group for Sarcopenia are shown in
Figure II-2.15
Frailty means a state of increased vulnerability to health damage based on age-associated declines in various functions, and sarcopenia is an important element of frailty. Frailty contains the reversibility, i.e., the possibility of recovery of health. Along with the conventional diagnostic criteria for frailty shown by Fried16
(Table II-1), a basic check list incorporating the assessment of psychological and social aspects has also been proposed by the Ministry of Health, Labour and Welfare.17
Since low body weight and undernutrition in heart failure were considered historically to be caused primarily by cachexia, or alteration of humoral factors, the following discussion of the pathology centers around cachexia.
Table II-1.
Phenotypic Definition of Frailty
| 1. Weight loss |
A critical mass of characteristics, defined as three or more, had to
be present for an individual to be considered frail. Those with one
or two characteristics were hypothesized to be in an intermediate,
possibly prefrail, stage clinically. Fried et al. developed a phenotypic
definition of frailty based on readily identifiable physical aspects. |
| 2. Slow gait speed |
| 3. Weakness (grip strength) |
| 4. Exhaustion |
| 5. Low physical activity level |
Source: Prepared based on Fried LP, et al.
J Gerontol A Biol Sci Med Sci
2001.16
At the Cachexia Consensus Conference held in 2008 in Washington, DC, USA, the concept and definition of cachexia observed in heart failure were proposed (Table II-2).18
According to the report, cachexia is a concept that integrates many factors including protein catabolism, lipid degradation, and bone mineral loss based on sympathetic nerve hyperactivity, increased inflammatory cytokines, and increased insulin resistance.
Table II-2.
Diagnostic Criteria for Cachexia in Adults
Weight loss of at least 5%*1 in 12 months or less in the presence of underlying illness,*2 PLUS THREE of the following criteria:
• Decreased muscle strength (lowest tertile)
• Fatigue*3
• Anorexia*4
• Low fat-free mass index#
• Abnormal biochemistry
a) increased inflammatory markers CRP (>5.0 mg/L), IL-6 >4.0 pg/mL
b) Anemia (<12 g/dL)
c) Low serum albumin (<3.2 g/dL) |
The literature on cachexia is growing but still somewhat limited. This is particularly true of specific diagnosis criteria. The criteria, below, represents the clinical experiences of the clinicians on the consensus panel and the limited data on patients with cachexia. The following needs to be excluded: starvation, malabsorption, primary depression, hyperthyroidism and age-related loss of muscle mass.
*1Edema-free.
*2In cases where wright loss cannot be documents a BMI <20.0 mg/m2 is sufficient.
*3Fatigue is defined as physical and/or mental weariness resulting from exertion; an inability to continue exercise at the same intensity with a resultant deterioration in performance.
*4Limited food intake (i.e. total caloric intake less than 20 kcal/kg body weight/d; <70% of usual food intake) or poor appetite.
#Lean tissue depletion (i.e. mid upper arm muscle circumference <10th percent for age and gender; appendicle skeletal muscle index by DEXA (kg/m2) by DXA <5.45 in female and <7.25 in males.)
Adapted from Evans WJ, et al. Clin Nutr 2008.18
©2008 Elsevier Ltd and European Society for Clinical Nutrition and Metabolism, with permission from Elsevier.
In healthy individuals, 250–350 g/day of muscle protein is degraded into amino acids. They are partly reused for protein synthesis and partly stored in the blood. Part of the amino acids in blood is converted in the liver by glyconeogenesis and used as energy.
In heart failure, the blood levels of cortisol, catecholamines, and inflammatory cytokines increase, and insulin resistance and a decrease in testosterone are observed, suggesting imbalance between protein catabolism and anabolism (Figure II-3).19
Section 4. Decrease in Fat Mass
The decrease in adipose tissue observed in cachexia is considered to be caused by promotion of lipid degradation. The activity of lipoprotein lipase (LPL) in adipose tissue is enhanced by the actions of catecholamines associated with activation of the sympathetic nervous system and tumor necrosis factor (TNF)-α associated with promotion of inflammation, and fat in fat cells is degraded. Also, insulin inhibits lipid degradation, but insulin resistance is observed in heart failure, suggesting predominance of lipid degradation.20
Moreover, natriuretic peptides have been reported to have a lipid degrading activity.21
Section 5. Modification of Disease Condition in Acute Heart Failure
In acute heart failure, the nutritional condition is considered to be more likely to deteriorate than in chronic heart failure (Table II-3). Albumin has a long half-life and exhibits mirror-image fluctuations of the fluctuations of C-reactive protein (CRP), which is an index of inflammation. Although it does not necessarily reflect the nutritional condition alone, progressive hypoalbuminemia observed in patients with acute heart failure after admission is related to exacerbation of the outcome.22
Table II-3.
Factors That Exacerbate the Nutritional State in Acute Heart Failure (Compared With Chronic Heart Failure)
1. Increased protein catabolism and lipid degradation due to further activation of inflammatory cytokines, catecholamines,
and natriuretic peptides |
| 2. Increased work of respiratory muscles due to labored respiration |
| 3. Reduced albumin generation due to congestion of the liver |
| 4. Reduced nutrient absorption due to intestinal edema |
| 5. Reduced dietary intake |
Chapter III. Nutritional Assessment
Section 1. What Is Nutritional Evaluation?
Nutritional evaluation is can be divided into nutritional screening and nutritional assessment.23
Nutritional screening is performed to identify patients with malnutrition and those possibly at risk for nutrition-related disorders. Nutritional assessment, on the other hand, is a process in which clinical data, food intake data, body composition data, and biochemical data are collected, patients with malnutrition are identified, and appropriate nutritional therapy is designed. The JSPEN Guidelines for Parental and Enteral Nutrition- Guidelines for the Proper Use of Parenteral and Enteral Nutrition24
define nutritional assessment as “a method for comprehensive evaluation of the nutritional status using medical history, nutritional history, physical findings, body measurement data, clinical laboratory data, etc.” (Table III-1).
Table III-1.
Items of Nutritional Assessment
| Medical history |
History of present disease: Fever and cough, gastrointestinal symptoms, etc. When
they occurred? |
| Past history: Metabolic diseases (diabetes, chronic kidney disease, etc.) |
| History of surgery, oral medications, socioeconomic condition, etc. |
Nutritional history: Appetite, dietary contents, changes in food intake, body weight
changes, gastrointestinal symptoms, food preferences, food allergy, etc. |
| Physical examinations |
Edema, ascites, findings related to deficiency of particular nutrients, etc. |
| Body measurements |
Height, body weight, BMI |
| Muscle mass (AC, TSF, AMC, AMA, CC, etc.) |
| Body composition (DXA, BIA) |
| Biochemical tests |
Transferrin (Tf), transthyretin, albumin, retinol-binding protein (RBP), total cholesterol,
liver function tests, kidney function tests, etc. |
| Body function assessment |
Respiratory function, swallowing function, ADL, etc. |
Adapted from Japanese Society for Parenteral & Enteral Nutrition, JSPEN Guidelines for Parenteral and Enteral Nutrition (3rd edition), 201324 with modifications.
The more information is available for nutritional assessment, the more appropriate the assessment can be. The results of body measurements, biochemical tests, and physical function tests are usually used for nutritional assessment, but more detailed nutritional assessment can be made by collecting a wide variety of information including the family structure, living environment, economic status, oral environment, and state of medication as well as the age and sex of the patient.
Section 2. Nutritional Screening
Nutritional screening is performed to select patients at nutritional risk. As it is important to screen all patients, a method that can be performed readily in a short time even by non-experts with minimum interrater variation in the results is necessary.
Although the methods for nutritional screening unavoidably vary among institutions, it is important to extract patients at risk for nutritional disorders and undernourished patients without omission. If the criterion of the risk of nutritional disorder is too high, patients who should be extracted may be overlooked, but if it is low, too many patients are extracted. Concerning the items examined for nutritional screening, those that can be readily obtained at each institution should be selected.
Section 3. Nutritional Assessment
A. Items of Nutritional Assessment
Medical History
Concerning the history of the present disease, whether there is a chronic lack of food intake or decline in activities of daily living (ADL) can be evaluated by carefully checking for the time of onset of fever, cough, and gastrointestinal symptoms. In taking a medical history, metabolic diseases are important, and diabetes mellitus and chronic kidney disease must be taken into consideration in calculating the nutrient requirements.
In heart failure patients, the number, frequency, and time of past hospitalization due to heart failure must also be checked. Nutritional therapy appropriate for the stage of heart failure is necessary.
Body Measurement Data
1) Body mass index (BMI)
Measurements of the body are important in nutritional assessment. The Guidelines for the Management of Obesity Disease, the Japan Society for the Study of Obesity 201625
defines a body mass index (BMI) of ≥25 kg/m2
as obesity, which is classified by WHO as “pre-obese”, and obesity accompanied by health damage as obesity disease and proposes therapeutic intervention to be necessary (Table III-2).
Table III-2.
Comparison of JASSO Obesity Classification and WHO Criteria
| BMI (kg/m2) |
JASSO classification |
WHO criteria |
| <18.5 |
Low body weight |
Underweight |
| 18.5≤ ∼ <25 |
Normal body weight |
Normal range |
| 25≤ ∼ <30 |
Obesity (grade 1) |
Pre-obese |
| 30≤ ∼ <35 |
Obesity (grade 2) |
Obese class I |
| 35≤ ∼ <40 |
Obesity (grade 3) |
Obese class II |
| 40≤ |
Obesity (grade 4) |
Obese class III |
JASSO, Japan Society for the Study of Obesity; WHO, World Health Organization.
Note 1) Obesity (BMI ≥25 kg/m2) is not necessarily a condition that medically requires weight control. The standard body weight (ideal body weight; kg) is the value calculated as height (m2) × 22, using a BMI of 22 kg/m2, at which morbidity is lowest, as a reference.
Note 2) Severe obesity is defined as a BMI ≥35 kg/m2.
Adapted from Japan Society for the Study of Obesity, Guidelines for the Management of Obesity Disease, 2016.25
Among studies in healthy individuals, there is a report that the incidence of heart failure increases with BMI.4
However, studies in the United States and Japan reported that the prognosis was more favorable in high BMI patients.26,27
This means that obesity is a risk factor for heart failure but that heart failure patients have a better prognosis as they tend to be obese. A meta-analysis also showed that the mortality rate and rehospitalization rate were higher in low-BMI heart failure patients.28
The mortality rate was reported to be higher in obese heart failure patients, if they lost 5% or more weight, than in non-obese patients.29
In heart failure patients, body weight changes are also important for the assessment of the disease state. A short-term body weight gain is useful as an index of fluid retention, and a weight gain of 2 kg or more within a few days strongly suggests acute exacerbation of heart failure.30
Also, if there is a weight loss over a long period, decreases in fat and muscle mass are suspected, and progression of undernutrition is suggested.
2) Arm circumference, triceps skinfold, arm muscle circumference, and calf circumference
From the arm circumference (AC) and triceps skinfold (TSF), the arm muscle circumference (AMC) can be determined, and, then, the arm muscle area (AMA) can be calculated (Figure III-1). These indices are useful for long-term follow-up of the nutritional state. The degree of depletion of muscle protein can be evaluated from changes in AC, and changes in the energy accumulation rate can be evaluated from changes in TSF. In addition, skeletal muscle mass can be evaluated from AMC and AMA.31
A study in heart failure patients has suggested that the addition of AC to BMI as an evaluation item improves the prognostic accuracy.32
More appropriate nutritional assessment is made possible by combining multiple items rather than depending on a single evaluation item.

The calf circumference (CC) is effective for the evaluation of calf skeletal muscles and is reflected in ADL. It is important to measure CC along with the assessment of ADL, such as whether the patients can walk or sit, by interviews. However, heart failure patients often show calf edema, and caution is needed, because the assessment of muscle mass according to CC is inaccurate in patients with edema.
3) Body composition
The assessment of not only body weight but also body composition is needed. Methods for body composition assessment include dual energy X-ray absorptiometry (DXA) and bioelectrical impedance analysis (BIA).
DXA is considered to be the most reliable method for body composition assessment. BIA is a simple and minimally invasive assessment method.33
It is possible to know the tendency of body fluid retention by serially evaluating the same patient.34
There have been reports that the ratio between extracellular water (ECW) and total body water (TBW) determined by BIA (ECW/TBW) is useful for the assessment of body fluid distribution and prognosis of patients with heart failure or end-stage renal disease and critically ill patients.34–37
It is considered possible to evaluate increases or decreases in the amount of body fat in a particular patient and estimate changes in body fluid retention and nutritional status using the ECW/TBW ratio as a reference.
Biochemical Tests
1) Serum albumin and transthyretin (prealbumin)
Serum albumin is widely used as an index of the nutritional status, particularly secondary to inflammation. Concerning the relationship between the serum albumin level and prognosis, the annual mortality rate was reported to be significantly higher when the serum albumin level was <3.4 g/dL in a study of 428 acute heart failure patients.38
Transthyretin has a shorter half-life than albumin and is used as an index for nutritional assessment in a shorter period. In a study of 514 heart failure patients, the mortality rate after 6 months was shown to be high when the transthyretin level was ≤15 mg/dL.39
Also, when used in combination with mini nutritional assessment (MNA)®, which is a nutritional assessment tool, transthyretin serves as a prognostic factor in patients with acute heart failure.40
2) Total cholesterol
The total cholesterol level is considered to reflect long-term nutritional disorders. In a study of 114 patients with chronic heart failure, the 3-year mortality rate was highest in the group with the lowest total cholesterol level (100.0–173.4 mg/dL) compared with other groups.41
However, when the total cholesterol level is used for nutritional assessment, it is also necessary to know whether drugs for treating dyslipidemia are used.
Assessment of Swallowing Function
Swallowing function is evaluated by the repetitive saliva swallowing test (RSST), modified water swallow test (MWST), and food test (FT)42
(Table III-3).
Table III-3.
Tests of Swallowing Function
| Repetitive saliva swallowing test (RSST) |
| Abnormal |
≤2 times for 30 seconds |
| Normal |
≥3 times for 30 seconds |
| Modified water swallowing test (MWST) |
| 1. |
No swallowing, choking, and/or respiratory distress |
| 2. |
Swallowing observed, respiratory distress (silent aspiration is suspected) |
| 3. |
Swallowing observed, adequate respiration, choking, and/or wet hoarseness |
| 4. |
Swallowing observed, adequate respiration, no choking |
| 5. |
In addition to 4, 2 additional swallowing movements (sham swallowing) possible within 30 seconds |
| Food test (FT) |
| 1. |
No swallowing, accompanied by choking or respiratory change |
| 2. |
Swallowing observed, accompanied by respiratory change |
| 3. |
Swallowing observed, accompanied by choking, wet hoarseness, or food retention, but no respiratory
change |
| 4. |
Swallowing observed, no respiratory change, choking, or wet hoarseness, and retention of food in
the mouth is resolved by additional swallowing |
| 5. |
In addition to 4, 2 additional swallowing movements (sham swallowing) possible within 30 seconds |
Source: Prepared based on the Japanese Society of Dysphagia Rehabilitation, JSDR Dysphagia Diet Committee. Dysphagia Assessment (brief version) 2015.42
RSST is a simple test of counting the number of saliva swallowing that the subject can make during a 30-second period.
MWST is a test to evaluate respiratory changes and whether the subject chokes in addition to whether swallowing occurs after infusing 3 mL of cold water into the mouth.
In FT, the subject is asked to swallow a teaspoonful (3–4 g) of food, such as pudding, and the condition of swallowing is evaluated.
In videofluoroscopic examination of swallowing (VF), the processes from the entry of food into the mouth to swallowing are observed by videofluoroscopy by having the subject swallow a contrast medium.
In videoendoscopic evaluation of swallowing (VE), the condition of swallowing is observed directly with a transnasally inserted nasopharyngoscope.
B. Tools for Nutritional Evaluation
It is recommended to combine multiple nutritional assessment items rather than using a single item, because more accurate evaluation of the nutritional status is possible by combining multiple evaluation items.
MNA® (Mini Nutritional Assessment)
MNA®
was developed primarily for the early detection and early treatment of undernutrition syndrome in older patients.43
Presently, MNA®-short form (MNA®-SF) is widely used (Table III-4).
Table III-4.
Mini Nutritional Assessment-Short Form
In a study of 50 heart failure patients evaluated using MNA®
and MNA®-SF, similar results were obtained, and the mortality rate and rehospitalization rate were higher in patients classified according to MNA®-SF in the undernutrition group.44
CC is an evaluation item in MNA®. Its cut-off value is originally 31 cm, but it is considered desirable to set the cut-off level at 28 cm for Asians.45
GNRI (Geriatric Nutritional Risk index)
The GNRI is an evaluation method reported by Bouillanne et al. in 2005, and it has been reported to be an accurate predictive index of the mortality rate in older people.46
The nutritional status is predicted according to the value obtained using a calculation formula consisting of the serum albumin level and body weight alone (Table III-5). In a study of 152 heart failure patients with a mean age of 77 years, the mortality rate significantly increased when GNRI was <92.47
Table III-5.
GNRI (Geriatric Nutrition Risk Index)
| The GNRI formula is as follows: |
Nutrition-related risk from GNRI values
<82: Major
82 to <92: Moderate
92 to <98: Low
≥98: Absent |
| GNRI = [14.89 × Serum albumin (g/dL)] + 41.7 × (Weight / Ideal weight)] |
| or |
| 14.89 × Serum albumin(g/dL) + 41.7 × (BMI / 22) |
Source: Prepared based on Bouillanne O, et al.
Am J Clin Nutr
2005.46
CONUT (Controlling Nutritional Status)
The CONUT was developed as a tool for the assessment of the nutritional status from 3 biological indices, i.e., protein metabolism, immunological competence, and lipid metabolism. The nutritional status is evaluated comprehensively and multifacetedly from the 3 biological indices (Table III-6).
Table III-6.
CONUT (Controlling Nutritional Status)
| Serum Albumin (g/dL) |
≥3.50 |
3.49–3.00 |
2.99–2.50 |
2.50> |
| Score |
0 |
2 |
4 |
6 |
| Total Lymphocytes (/μL) |
≥1,600 |
1,599–1,200 |
1,199–800 |
800> |
| Score |
0 |
1 |
2 |
3 |
| Total Cholesterol (mg/dL) |
≥180 |
179–140 |
139–100 |
100> |
| Score |
0 |
1 |
2 |
3 |
| Assessment |
Normal |
Light |
Moderate |
Severe |
| Screening Total Score |
0–1 |
2–4 |
5–8 |
9–12 |
Source: Prepared based on Ignacio de Ulíbarri J, et al.
Nutr Hosp
2005.48
http://scielo.isciii.es/scielo.php?script=sci_arttext&pid=S0212–16112005000100006&lng=en&nrm=iso
CONUT has been reported to be an index useful in early screening of heart failure patients for undernutrition,48
but it must be carefully interpreted if the patient is taking a drug for treating dyslipidemia for such diseases including coronary artery disease.
PNI (Prognostic Nutritional Index)
PNI is a tool for nutritional evaluation using the serum albumin level and total lymphocyte count.49
In patients with acute heart failure, the PNI score is useful as a prognostic index, and its evaluation in combination with the CONUT score is recommended.50
SGA (Subjective Global Assessment)
SGA is a tool for nutritional evaluation reported by Detsky et al. in 1987.51
It can be performed readily by physical examinations alone without special equipment and is widely used. It is applicable to inpatients in the acute phase, patients admitted to care institutions, and home-cared patients and is also used for outpatient nutritional evaluation.
NRS (Nutritional Risk Screening) 2002
NRS 2002 is a tool for nutritional evaluation prepared by the European Society for Clinical Nutrition and Metabolism (ESPEN) and is characterized by evaluation based on scoring of the severity of nutritional disorder and disease.52
The severity of nutritional disorder is evaluated by scoring each of the body weight loss during a 3-month period, BMI, and weekly food intake 0–3 points.
Chapter IV. Methods for Nutritional Support
It is widely known that the patient’s nutritional status is a very important determinant of the onset and prognosis of heart failure. For nutritional management, oral nutrition, which is the most physiologic to the body, is recommended, and oral intake of food yields many benefits. However, patients difficult to orally ingest nutrients have increased with the increase in older patients, and poor dietary intake due to malaise and sense of dyspnea is often observed, particularly, in heart failure patients. Conventionally, such patients were managed by peripheral parenteral nutrition (PPN), but the problems such as insufficient energy intake were caused by the possibility of angiitis and angialgia associated with an increase in osmotic pressure if the energy supply was increased. Therefore, since the 1960 s, total parenteral nutrition (TPN) has become prevalent, resulting in improvements in the therapeutic results of many diseases. From the viewpoint of “When the gut works, use it!”, elemental diet has been developed for enteral nutrition, and the administration of readily absorbable high-energy, high-protein preparations became possible.
Presently, methods for nutritional support are classified into three major groups, namely, 1) oral nutrition, 2) enteral nutrition, and 3) parenteral nutrition. Each of them has advantages and disadvantages and must be selected appropriately alone or in combination for individual patients (Table IV-1).
Table IV-1.
Advantages and Disadvantages of Oral, Enteral, and Parenteral Nutrition
| |
Advantages |
Disadvantages |
| Oral nutrition |
• The structure of the digestive tract can be
physiologically maintained.
• Blood oxygen supplied to the brain is increased by
increased energy required by the stomach and intestine
for digestion.
• There are physical and psychological/social aspects.
• One psychological/social aspect is activation of
neurotransmitters in the brain.
• Communication through eating develops.
• The cerebrum is stimulated via receptors of vision,
taste, smell, hearing, touch, etc.
• Maintenance of oral hygiene is promoted. |
• It is impossible to provide all necessary nutrients
because of preferences.
• The amount of intake is affected by appetite. |
| Enteral nutrition |
• The structure of the digestive tract can be
physiologically maintained.
• Medical professionals can provide nutrients in planned
amounts.
• Necessary amounts of nutrients for different disease
conditions can be provided. |
• There is concern over the development of
gastrointestinal symptoms.
• There is concern over self-extubation (accidental
extubation).
• There is a sense of discomfort about the tube. |
| Parenteral nutrition |
• Necessary water, electrolytes, and nutrients can be
administered regardless of appetite.
• Can be performed for patients with difficulty in using
their digestive tract. |
• Long-term parenteral feeding may make it
physiologically impossible to maintain the structure of
the digestive tract.
• Serious complications, such as hyperglycemia, phlebitis,
and sepsis, may be induced. |
A. Significance of Oral Nutrition
The fundamental principle of nutritional management is avoiding a meaningless fasting period by using the gastrointestinal tract as much as possible. Needless to say, oral intake is the best route of nutritional supply, and it is the ultimate goal of nutritional support.
Eating from the mouth has physiological significance not confined to simple supply of nutrients.53
B. Significance of Enteral Nutrition
Unlike TPN via the intravenous route, enteral nutrition is a physiological method for nutritional management, by which the dynamics of gastrointestinal hormones can also be maintained more normally. Enteral nutrition requires no special technique if nutritional preparations are administered by inserting a gastric tube orally or transnasally without creating a stoma to the stomach or intestine. Long-standing TPN is associated with the possibility of atrophy of the intestinal mucosal epithelium and consequent bacterial translocation, a phenomenon in which bacteria or endotoxin produced pass through the intestinal mucosa.54
C. Significance of Parenteral Nutrition
Although oral or enteral nutrition should be selected preferentially, parenteral nutrition is the first choice for patients with severe swallowing disorder, gastrointestinal dysfunction, or circulatory instability. The recommendations of the guidelines for nutrition support therapy for critically ill patients by the Japanese Society of Intensive Care Medicine55
are: “The preference of enteral nutrition is strongly recommended (recommendation level: 1A)” to the clinical question, “Which route of nutritional support should be preferred between enteral and parenteral routes?”, but “Delaying the initiation of enteral nutrition is weakly recommended to patients with hemodynamic instability such as those administered a high dose of vasopressor or requiring massive fluid infusion or blood transfusion until resuscitation and stabilization of the hemodynamics (recommendation level: 2C)” to the clinical question, “Is enteral nutrition possible in hemodynamic instability?”.
Section 2. Oral Nutrition in Heart Failure
A. Taste Abnormality and Oral Nutritional Supplements
Taste is sensed by taste buds on the tongue surface or oral mucosa, but the total number of taste buds decreases with aging to about 1/2 to 1/3 in older people compared with neonates, and taste disturbance or disorder is a physiological phenomenon associated with aging. Older people are less sensitive to tastes of foods56
(Figure IV-1).
In heart failure patients difficult to eat orally due to malaise or respiratory distress, the lack of energy intake is usually complemented by oral nutrition supplementation (ONS).
B. Types of Oral Nutrition
Low-Salt Diet
Generally, low-salt diet is diet in which the salt content is restricted to prevent edema and reduce the renal burden. It is classified into 1) salt-free diet, 2) moderately low-salt diet (1–3 g/day), and 3) mildly low-salt diet (5–8 g/day). They are used for the treatment of conditions such as hypertension, congestive heart failure, and renal edema. The Guidelines for Diagnosis and Treatment of Acute and Chronic Heart Failure (2017 edition) recommends a salt intake of “<6 g/day”.57
Minced Diet
Minced diet is food cut into a masticable size for patients with declined mastication ability or muscle strength. The perception that minced food is inappropriate for patients with swallowing disorder is beginning to be established.
Blender Diet
Blender diet is prepared by processing ordinary or soft diet in a blender. It is prepared by adjusting the viscosity to about potage so it can be eaten without mastication. For patients with impaired swallowing ability, it is smoothed using a thickener (polysaccharide thickener), but caution is necessary as pharyngeal residue increases if the food is excessively thickened.
Dysphagia Diet
With progressive aging also of heart failure patients, many of them develop swallowing disorder. Dysphagia diet is prepared by adjusting the shape, thickness, and adherability of bolus according to the level of swallowing ability for patients with declined swallowing or masticatory function.
Section 3. Enteral Nutrition for Heart Failure
A. Types of Enteral Nutrition Products
In selecting enteral nutrition products, it is important to assess the abilities of digestion and absorption and consider the ratio of the 3 major nutrients, i.e., protein, lipids, and carbohydrates, and the type of each nutrient. Commercially available enteral nutrition products can be classified into 1) elemental diets, 2) oligomeric formulas, 3) polymeric formulas, and 4) natural thickened liquids58,59
(Table IV-2).
Table IV-2.
Classification of Enteral Nutrient Preparations by the Nitrogen Source and Their Characteristics
| |
Elemental nutrients |
Digested nutrient
preparations |
Half-digested nutrient
preparations |
Natural thick liquid diet |
| Nitrogen source |
Amino acids |
Amino acids, dipeptides,
and tripeptides |
Polypeptides, protein |
Proteins |
| Lipids |
Long-chain and medium-
chain fatty acids |
Long-chain and medium-
chain fatty acids |
Long-chain and medium-
chain fatty acids |
Long-chain fatty acids |
| Carbohydrates |
Dextrin |
Dextrin |
Dextrin, etc. |
Maltodextrin |
| Fiber components |
None |
None |
Water-soluble and insoluble
dietary fiber added in many
products |
Water-soluble dietary fiber
added |
| Characteristics |
Very low lipid content |
Nitrogen source is primarily
peptides |
Rich in variety |
Primarily natural foods are
compounded |
| Digestion |
Unnecessary |
Partially necessary |
Necessary to an extent |
Necessary |
| Absorption |
Necessary |
Necessary |
Necessary |
Necessary |
| Residue |
Very little |
Very little |
Little |
Large amount |
Recommended
feeding tube size |
Φ1–1.5 mm (5 Fr) |
Φ2–3 mm (8 Fr) |
≥Φ2–3 mm (8 Fr) |
≥Φ4 mm (12 Fr) |
| Drug/food |
Drugs only |
Drugs/food |
Drugs/food |
Food |
Adapted from Ohama O. Enteral Nutrition: Classification and Characteristics of Enteral Nutritional Preparations. Shimada, S. et al. (ed.). Elsevier Japan: 200359
with modifications.
Elemental Diets
Elemental diets have chemically definitive composition and need little digestion. Their major characteristic is that proteins are composed of non-antigenic amino acids. In long-term administration, attention to deficiency of vitamins and trace elements is necessary, and there is the possibility of the occurrence of selenium deficiency, in particular.60
Because of the very low lipid contents, the risk of essential fatty acid deficiency must also be considered.61
Oligomeric Formulas
Since dipeptides consisting of 2 bound amino acid molecules and tripeptides consisting of 3 bound amino acid molecules are absorbed via peptide-specific peptide carriers, oligomeric formulas containing low-molecular-weight peptides as the nitrogen source require little digestion and show a short intestinal retention time.
Polymeric Formulas
Polymeric formulas range from general enteral nutrition products developed by adjusting the compositional ratio of the 3 major nutrients to the nutrient requirements or dietary reference intakes of Japanese to products prepared by adjusting the type and compositional ratio of each nutrient for various disease states. The preparations are enriched with vitamins and trace elements in addition to the 3 major nutrients, and many contain dietary fiber. A disadvantage is the possible occurrence of diarrhea if there is maldigestion or malabsorption. Also, the fat energy ratio is high in many products, and caution is needed if there is impairment of lipid absorption, but some products contain a high percentage of middle-chain fatty acids, which can be absorbed even in such cases. They are formulas appropriate for long-term management by total enteral nutrition, and new products that cater to individual disease states or compensate for defects of conventional formulas are developed at a rapid pace.
Natural Thick Liquids
Before natural thick liquids began to be commercially distributed, liquid meals prepared by stirring ordinary meals with a blender were used. Since natural thick liquids are made from natural foods, they have a low osmotic pressure and are less likely to cause diarrhea even if a large amount is administered in a short time. However, they are not indicated for patients with reduced digestion/absorption ability. Also, they are difficult to pass through a thin tube because of poor fluidity.
For these reasons, oligomeric and thick polymeric diets are recommended for enteric nutrition of heart failure patients. Many heart failure patients also require fluid restriction. Usual nutritional preparations are adjusted to 1.0 kcal/mL with a water content of about 80%. The energy content of thickened nutritional products is adjusted to 1.5–2.0 kcal/mL, and 10% of water intake can be reduced with a product at 2.0 kcal/mL, in which the water content is 70%, compared with usual nutritional products when the same amount of energy is administered. In addition, the daily energy requirement is high in heart failure patients,62
and thickened nutrition products are also advantageous from this viewpoint.
B. Measures to Prevent Complications of Enteral Nutrition
The greatest complication of enteral nutrition is gastrointestinal symptoms. To avoid them, it is recommended to initiate the administration at a low dose and to carefully increase the dose while concomitantly using parenteral nutrition to supplement the lack.
Section 4. Parenteral Nutrition for Heart Failure
Although evidence concerning nutritional management for patients with severe heart failure is insufficient, a few studies have been performed concerning critically ill patients who require intensive care such as those with sepsis.
There is also a report that the target dose of energy administration was reached by enteral nutrition in 40% of the patients using catecholamines,63
and intestinal absorption of nutrients is considered possible even in critically ill patients.64
While enteral nutrition is also considered possible in patients using catecholamines, stabilization of the disease state such as stabilization of the blood pressure, completion of massive fluid administration or transfusion, and no need to increase the dose of catecholamines is considered necessary for the initiation of enteral nutrition. Usually, enteral nutrition induces increases in the oxygen consumption of the digestive tract and intestinal blood flow, but, in patients with low cardiac output or circulatory disorders, it is difficult to increase the intestinal blood flow, and enteral nutrition may trigger a decrease in blood pressure or even intestinal ischemia and necrosis. The prognosis of intestinal ischemia is poor, and the mortality rate has been reported to be 58% in an observational study.65
The Society of Critical Care Medicine (SCCM)/American Society for Parenteral and Enteral Nutrition (ASPEN) Guidelines recommend avoidance of the initiation of enteral nutrition during a large amount of catecholamines administration and massive fluid infusion until stabilization of the hemodynamics,66
with a mean blood pressure of 60 mmHg as a provisional criterion.
Chapter V. Outcome Indices and Evidence of Nutritional Therapy
Nutritional therapy is expected to benefit physical functions, quality of life (QOL), and outcome of chronic heart failure patients. In this chapter, the framework of outcome indices and present status of evidence in the efficacy evaluation of nutritional therapy are described.
A conceptual chart of outcomes used in phase III clinical trials in chronic and acute heart failure patients is shown in
Figure V-1.67
In addition to these outcomes, outcomes related to the assessment of nutritional state, physical functions, frailty, sarcopenia, and cachexia are used in intervention with nutritional therapy. An outcome model of nutritional therapy for heart failure based on the outcome models by the Clinical Nutrition Guideline Group, European Society for Clinical Nutrition and Metabolism (ESPEN)68
is shown in
Figure V-2. In these models, outcomes in nutritional therapy are classified into medical outcomes, patient-reported outcomes, medical economics outcomes, outcomes concerning medical guidelines, and complex outcomes. In many studies on nutritional therapy, medical outcomes were used as the primary indices, but comprehensive assessment with the concomitant use of other outcomes is recommended.2

Interventional studies concerning nutritional therapy for stage C/D symptomatic heart failure patients are very few.10,57,69,70
More research results are awaited.
There have been a number of systematic reviews and meta-analyses of nutritional therapy for older and malnourished as well as heart failure patients, and they are summarized in
Table V-1.71–82
According to the results of meta-analyses, there have been many reports of improvements in indices that reflect the nutritional state including energy intake, protein intake, body weight, body mass index (BMI), arm circumference (AC), and arm muscle circumference (AMC) as a result of some nutritional intervention such as ONS. However, the results of studies using muscle strength, activities of daily living (ADL), QOL, re-admission, or death as outcomes have not been consistent.
Table V-1.
Evidence of Nutritional Therapy Primarily for Older People and Patients
| Outcome |
Outline |
Authors |
Study design |
Journals |
Year |
Reference
No. |
Nutritional
state |
Improvements are observed in
indices of the nutritional state, such
as energy intake, protein intake,
body weight, BMI, AC, and AMC,
due to nutritional support with oral
nutritional supplements, etc. |
Milne AC |
Meta-analysis |
Ann Intern Med |
2006 |
71 |
| Milne AC |
Meta-analysis |
Cochrane Database
Syst Rev |
2009 |
72 |
| Cawood AL |
Meta-analysis |
Ageing Res Rev |
2012 |
73 |
| Beck AM |
Meta-analysis |
Clin Rehabil |
2013 |
74 |
| Bally MR |
Meta-analysis |
JAMA Intern Med |
2016 |
75 |
Physical
function
(primarily
muscle
strength) |
There are reports of some effects,
but the increase in muscle strength
due to nutritional supplements, such
as protein, is not observed or not
significant. |
Milne AC |
Meta-analysis |
Ann Intern Med |
2006 |
71 |
| Milne AC |
Meta-analysis |
Cochrane Database
Syst Rev |
2009 |
72 |
| Cawood AL |
Meta-analysis |
Ageing Res Rev |
2012 |
73 |
| Cermak NM |
Meta-analysis |
Am J Cin Nutr |
2012 |
76 |
| Finger D |
Meta-analysis |
Sports Med |
2015 |
77 |
| Bally MR |
Meta-analysis |
JAMA Intern Med |
2016 |
75 |
| Thomas DK |
Meta-analysis |
J Am Med Dir Assoc |
2016 |
78 |
| Beaudart C |
Systematic review |
Osteoporos Int |
2017 |
79 |
| Yoshimura Y |
Meta-analysis |
J Am Med Dir Assoc |
2017 |
80 |
| QOL |
No consistent results have been
obtained. |
Milne AC |
Meta-analysis |
Ann Intern Med |
2006 |
71 |
| Cawood AL |
Meta-analysis |
Ageing Res Rev |
2012 |
73 |
| Re-admission |
No consistent results have been
obtained. |
Cawood AL |
Meta-analysis |
Ageing Res Rev |
2012 |
73 |
| Beck AM |
Meta-analysis |
Clin Rehabil |
2013 |
74 |
| Stratton RJ |
Meta-analysis |
Ageing Res Rev |
2013 |
81 |
| Bally MR |
Meta-analysis |
JAMA Intern Med |
2016 |
75 |
| Deutz NE |
Double-blind RCT
including 157 heart
failure patients |
Clin Nutr |
2016 |
82 |
| Death |
No consistent results have been
obtained. |
Milne AC |
Meta-analysis |
Ann Intern Med |
2006 |
71 |
| Milne AC |
Meta-analysis |
Cochrane Database
Syst Rev |
2009 |
72 |
| Cawood AL |
Meta-analysis |
Ageing Res Rev |
2012 |
73 |
| Beck AM |
Meta-analysis |
Clin Rehabil |
2013 |
74 |
| Bally MR |
Meta-analysis |
JAMA Intern Med |
2016 |
75 |
| Deutz NE |
Double-blind RCT
including 157 heart
failure patients |
Clin Nutr |
2016 |
82 |
Concerning studies carried out for the prevention or treatment of sarcopenia, there have been some reports of synergy by the addition of nutritional supplementation to exercise, but the evidence remains insufficient.83,84
More research results are anticipated.
Chapter VI. Nutritional Therapy According to the Disease Stage
Section 1. Nutritional Therapy for Stage A/B Heart Failure
A. What Is Appropriate Salt Intake?
Excessive intake of salt increases the blood pressure and exacerbates the risk of cardiovascular disease. The World Health Organization (WHO) proposes a target salt intake of <5 g (2 g of Na) /day, and the American Heart Association (AHA) recommends a salt intake of <3.8 g (1.5 g of Na)/day for hypertensive patients.85
Japan ranks high in salt intake86
with a mean daily salt intake of about 10 g.87
A recent observational study reported that a low salt intake is also related to cardiovascular events.88
Since excessive salt restriction may also be harmful, the setting of the salt control goal remains controversial. According to Japanese guidelines, the target of salt reduction is set at <6 g/day for stage A/B heart failure patients such as those with hypertension and myocardial infarction in consideration of the Japanese dietary habit.89,90
B. What Is the Optimal Body Weight for the Prevention of Cardiovascular Events?
The WHO defines a body mass index (BMI) of ≥25 kg/m2
as pre-obese and ≥30 kg/m2
as obese.91
Since complications such as glucose intolerance, dyslipidemia, and hypertension increase when BMI exceeds 25 kg/m2, a BMI of 18.5–24.9 kg/m2
is defined as normal weight and ≥25 kg/m2
as obesity25
(see
Chapter III, Table III-2). In Japanese, the incidence of coronary artery disease increases two-fold in males and 1.58-fold in females with a BMI of ≥27 kg/m2
compared with those with a BMI of 23.0–24.9 kg/m2.92
Low body weight is also a problem, and the risk of cerebral infarction and cerebral hemorrhage increases in those with a BMI of <18.5 kg/m2.92
Body weight gains after reaching adulthood is also important, and the risk of coronary artery disease doubles in those who had a BMI of <21.7 kg/m2
in their 20 s and gained 10 kg or more thereafter.93
C. Evidence About Various Foods for the Prevention of Cardiovascular Events
Red Meat
The harm of a meat diet has been emphasized as a risk factor of cardiovascular disease, but the relationship between the intake of red meat and the occurrence of cardiovascular disease is considered weak in recent reports.94,95
A study in Japanese showed that moderate meat intake (<100 g) does not lead to an increase in deaths due to cardiovascular disease.96
In contrast, intake of processed meat such as ham and sausage at ≥75 g/day increases the risk of heart failure 1.28-fold and the risk of death due to heart failure 2.43-fold compared with their intake at <25 g/day.97
Fish
Fatty blue-skinned fish contain large amounts of ω3 polyunsaturated fatty acids such as eicosapentaenoic acid (EPA) and docosahexaenoic acid (DHA) and may the prevent coronary artery disease. The AHA recommends eating fish at least 2 times a week.85
Vegetables/Fruits
Vegetables and fruits are rich in vitamins, minerals, and fiber1
and may reduce deaths due to cardiovascular disease.98–100
Whole-Grain Cereals
Whole-grain cereals are rich in fiber, vitamin B, and minerals and have been reported to be effective for preventing coronary artery disease and reducing the risk of deaths due to cardiovascular disease.101
Nuts
Nuts are rich in unsaturated fatty acids, fiber, vitamins, and minerals and have been reported to reduce the risk of coronary artery disease.102
Alcohol
Mild intake of alcohol reduces the risk of heart failure.103
Regular drinkers are recommended to restrict drinking under 30 g/day in terms of pure alcohol.89
D. Evidence Concerning the Dietary Pattern for the Prevention of Cardiovascular Events
A regular diet consists of combinations of various food items, and evaluation based on the dietary pattern is important. Mediterranean diet104
and Dietary Approaches to Stop Hypertension (DASH) diet (Table VI-1) have been reported as useful dietary patterns to prevent cardiovascular events.105,106
Japanese food is attracting global attention as a healthy diet, and a relationship between the Japan Diet (characterized by low meat and fat intakes and high intakes of soybeans, fish, vegetables, seaweeds, mushrooms, and fruits107) and reduced risk of death due to cardiovascular disease has been reported.108,109
The frequency of cardiovascular events is reported to be low in Japanese with a dietary pattern in compliance with the Japanese Food Guide Spinning Top (Figure VI-1)110
prepared by the Ministry of Agriculture, Forestry and Fisheries based on the Dietary Reference Intakes for Japanese.111
Table VI-1.
The DASH Eating Plan
| Food |
Daily/Weekly servings |
Serving indication |
| Grains |
6 to 8 SVs per day |
1 slice bread
1 oz* dry cereal
1/2 cup** cooked rice, pasta, or cereal |
| Vegetables |
4 to 5 SVs per day |
1 cup raw leafy vegetable
1/2 cup cooked vegetable
1/2 cup vegetable juice |
| Fruits |
4 to 5 SVs per day |
1 medium fruit
1/4 cup dried fruit
1/2 cup fresh, frozen, or canned fruit
1/2 cup fruit juice |
Fat-free or low-fat milk and milk
products |
2 to 3 SVs per day |
1 cup milk
1 cup yogurt
11/2 oz cheese |
| Lean meats, poultry, and fish |
<6 oz per day |
1 oz cooked lean meats, poultry, fish
1 egg |
| Nuts, seeds, and legumes |
4 to 5 SVs per week |
1/3 cup (11/2 oz) nuts
2 tbsp peanut butter
1/2 cup cooked beans or peas |
| Fats and oils |
2 to 3 SVs per day |
1 tsp soft margarine
1 tsp vegetable oil
1 tbsp mayonnaise
2 tbsp salad dressing |
| Sweets and sugars |
5 or fewer SVs per week |
1 tbsp sugar
1 tbsp jelly or jam |
*1 oz=28 g, **1 cup=236 mL. Assuming the daily total energy intake as 2,000 kcal, the necessary intake of each food item is shown in the dietary unit of serving (SV). Source: Prepared based on National Institutes of Health. Description of the DASH Eating Plan.105

E. Soft Drinks (Sweetened Beverages)
A high intake of soft drinks or sweetened beverages increases the risk of obesity and diabetes mellitus.85,112
In addition, so-called zero-calorie soft drinks containing artificial sweeteners may also be involved in the pathogenesis of diabetes.113
F. Oral Nutritional Supplementation
The effectiveness of oral nutrition supplementation (ONS) has not been established, and the AHA is not recommending the use of ONS.85
G. Evidence Concerning Fatty Acids
Fatty acids consist of atoms of 3 elements, i.e., carbon (C), hydrogen (H), and oxygen (O),114
and are divided into saturated fatty acids with no carbon double bonds and unsaturated fatty acids with carbon double bonds (Figure VI-2). Unsaturated fatty acids are divided into monounsaturated fatty acids with one carbon double bond and polyunsaturated fatty acids with multiple carbon double bonds, and polyunsaturated fatty acids are divided into ω3 and ω6 fatty acids depending on the position of the first double bond from the terminal methyl group (CH3).6
Unsaturated fatty acids with hydrogen atoms on the same side of the double bond are called cis, and those with hydrogen atoms on different sides of the double bond are called trans-fatty acids.
Saturated Fatty Acids
Saturated fatty acids are contained in large amounts in animal fat in meat, dairy products, and vegetable oils such as palm oil used in processed foods.114
Excessive intake of saturated fatty acids is considered to increase the blood level of low density lipoprotein (LDL) cholesterol and promote atherosclerosis. Although recent meta-analyses have suggested that saturated fatty acids have no marked effect on atherosclerosis,115,116
cardiovascular events have been reported to be reduced by replacing saturated fatty acids with polyunsaturated fatty acids derived from vegetable oils.117
Unsaturated Fatty Acids
1) Monounsaturated fatty acids
Oleic acid, a typical monounsaturated fatty acid, is contained in large amounts in animal fat and cooking oils such as olive oil.114
Compared with saturated fatty acids, monounsaturated fatty acids do not markedly increase LDL cholesterol and are considered to prevent cardiovascular disease. However, it has been reported that monounsaturated fatty acids derived from olive oil reduce deaths due to cardiovascular disease but that those derived from animals or vegetables have no such effect.94,118
2) Polyunsaturated fatty acids
① ω6 Fatty Acids
Linoleic acid is a typical polyunsaturated fatty acid, and it is ingested primarily as vegetable oils such as soybean oil and corn oil.114
Since they may prevent cardiovascular diseases, they are recommended as substitutes for saturated fatty acids.94
However, as ω6 fatty acids are converted in the body to prostaglandins and leukotrienes, which cause inflammation, there is concern over the safety of their excessive intake,6
and the possibility of an increase in coronary artery disease due to their excessive intake has also been suggested.119,120
② ω3 Fatty Acids
ω3 fatty acids include α-linolenic acid derived from cooking oil and EPA and DHA derived from fish.114
The effectiveness of EPA preparations for the secondary prevention of coronary artery disease has been reported.121
No consensus has been reached concerning their effectiveness for the primary prevention.122
3) Trans-Fatty Acids
Trans-fatty acids are generated in the production of semisolid or solid oils and fats from liquid vegetable or fish oils at room temperature by the processing technique called hydrogenation. Typical trans-fatty acids are margarine, fat spread, and shortening, and they are contained in bread, cake, doughnut, etc., as ingredients.114
If trans-fatty acids are ingested in large amounts, LDL cholesterol increases, high density lipoprotein (HDL) cholesterol decreases, and the risk of coronary artery disease increases.117
Table VI-2
shows typical fatty acids, their relationships with cardiovascular diseases, and the foods that contain them in large amounts.
Table VI-2.
Effects of Fatty Acids on Cardiovascular Diseases, Foods High in Fatty Acids, and Sufficient Intake
| |
Relationships with
cardiovascular diseases |
Adequate intake*6,114 |
Representative fatty acids
and high-content foods** |
| Saturated fatty acids |
There are reports that affirm and
those that deny the relationship
with the risk of coronary artery
disease.115,116,123
The risk of coronary artery disease
is reduced by replacement with
polyunsaturated fatty acids.117 |
<7% of total energy intake |
Acetic acid (sour taste of vinegar)
Lactic acid (butter and cheese)
Palmitic acid (palm oil)
Stearic acid (cocoa butter) |
Unsaturated
fatty
acids |
Monounsaturated
fatty acids |
There are reports that support
preventive effects against coronary
artery disease and those that
suggest an increased risk94,118 |
DG not set
(Excessive intake may increase
the risk of coronary artery disease/
obesity, and caution is necessary.) |
Oleic acid (olive oil) |
Polyunsaturated
fatty
acids |
ω6 fatty acids |
The risk of coronary artery disease
is reduced by replacement of
saturated fatty acids with ω6 fatty
acids.94
There are reports that the risk of
coronary artery disease is
increased by excessive intake.119,120 |
DG not set
(Excessive intake may induce
inflammation, and caution is
necessary.) |
Linoleic acid (vegetable oils such
as soybean oil, corn oil, and
safflower oil)
γ-Linolenic acid (evening primrose
oil, etc.)
Arachidonic acid (meat, egg, fish,
liver oil, etc.) |
| ω3 fatty acids |
The administration of EPA
preparations is useful for secondary
prevention of coronary artery
disease.121
The effectiveness of EPA
preparations was not demonstrated
by recent studies.94,124 |
DG not set
(Harmful substances, such as
mercury, pose problems depending
on the fish type. Caution against
excess intake is necessary,
particularly, in pregnant women.) |
α-Linolenic acid (shiso oil, perilla oil,
canola oil, soybean oil, etc.)
EPA (fish oil)
DHA (fish oil) |
| Trans fatty acids |
Related to the risk of coronary
artery disease.118 |
<1% of total energy intake |
Too
numerous
to list114 |
Margarine, shortening,
etc., and Western
confectioneries and
fried food in which
they are ingredients |
*Sufficient intakes are based on the Dietary Reference Intakes for Japanese (2015 edition), Ministry of Health, Labour and Welfare. **Representative fatty acids and high-content foods are based on the Dietary Reference Intakes for Japanese (2010 edition), Ministry of Health, Labour and Welfare.
H. Frequency and Timing of Eating
In modern society, the daily schedule of activities is diversified, and mealtime is irregular. In those who skip breakfast, the risk of obesity,125
diabetes,126
and cardiovascular events127
increases. Also, eating late dinners in addition to skipping breakfast increases the risk of metabolic syndrome.128
Section 2. Nutritional Therapies for Stage C/D Acute Heart Failure
A. Selection and Objectives of Parenteral and Enteral Nutrition
Some guidelines recommend enteral rather than parenteral nutrition as the route of nutritional support for critically ill patients.55
Enteral nutrition is considered to control infection,129
shorten the duration of hospitalization,130
and reduce the medical expenditure131
compared with parenteral nutrition although it does not improve the mortality rate.132
There has not been a randomized controlled study that compared parenteral and enteral nutrition in acute heart failure patients, and which of parenteral and enteral nutrition is more beneficial for acute heart failure patients is presently unknown (Table VI-3).
Table VI-3.
Nutritional Therapy for Acute Heart Failure in Stages C and D
| |
Selection of parenteral or enteric
nutrition, time of initiation |
DGs of energy, protein, and
salt administration |
Severely ill
patients
requiring
intensive
care |
As control of infections, shortening of hospitalization, and reduction of
medical cost are expected by enteral nutrition compared with parenteral
nutrition, enteral nutrition should be selected if enteral intubation is
possible. Enteral nutrition should be initiated within 24 hours after the
beginning of treatment and within 48 hours at the latest. However, if
the hemodynamics are unstable, it is recommended to avoid initiating
enteral nutrition. There is no evidence regarding the time of initiation
of parenteral nutrition. |
The DG of energy is approximately 25–30 kcal/kg of
body weight/day, and it is recommended to administer
less energy than the calculated DG for 1 week in the
acute period. The DG of protein is recommended to be
at least ≥1.2 g/kg/day. The DG of salt is unknown. |
Heart failure
patients |
In the stage of acute heart failure, whether parenteral or enteral
nutrition is more beneficial is unclear, but it is recommended to
promptly initiate enteral nutrition after stabilization of the hemodynamics.
There is no evidence regarding the time of initiation of parenteral
nutrition. |
In acute heart failure, there is little evidence for setting
clear targets of energy and protein administration.
Concerning salt, the usefulness of restricted intake in
the acute period has not been established, and the
optimal dose of salt is unknown. |
B. Determination of the Time of Initiation
In this section, the time of initiation of nutritional therapy for critically ill patients requiring intensive care is discussed. Whether these results can be applied to acute heart failure patients is unknown.
Time of Initiation of Enteral Nutrition
The Japanese Guidelines for Nutrition Support Therapy in the Adult and Pediatric Critically Ill Patients55
recommend the initiation of enteral nutrition within 24 hours as much as possible and within 48 hours at the latest after the beginning of treatment of critically ill patients. However, if the hemodynamics is unstable, the guidelines also consider it necessary to withhold the initiation of enteral nutrition. In severe heart failure, the hemodynamics often affects the use of the intestine, and enteral nutrition may invite intestinal ischemia or further exacerbation of hemodynamic instability. It is recommended that enteral nutrition should be withheld when the mean blood pressure is ≤60 mmHg due to cardiogenic shock133
and that its initiation should be evaluated after stabilization of the blood pressure and end of high-dose fluid supplementation or transfusion.
There is a report that the intestine could be used without complications such as intestinal ischemia when oral and enteral nutrition was initiated during treatment using hypertensive drugs, dialysis, and the intra-aortic balloon pump (IABP) in 70 patients after heart surgery using the heart-lung machine.63
Time of Initiation of Parenteral Nutrition
Presently, no consensus has been reached about the time of initiation of parenteral nutrition. In the EPaNIC Trial, a group in which nutritional support was initiated within 48 hours by parenteral nutrition (early group) and a group in which parenteral nutritional support was not performed (late group) were compared and showed increases in the incidence of infection, and the durations of support with a respirator and stay in the intensive care unit (ICU) in the early group.134
In the Early PN Trial, on the other hand, the durations of support with a respirator and thrombocytopenia were shortened by initiating parenteral nutrition early after admission to the ICU.135
It is difficult to directly apply the results of the EPaNIC Trial to Japanese patients, because intensive insulin therapy, which is not recommended at present, was used in the EPaNIC Trial, and because BMI of the patients in both studies was high around 28 kg/m2.
C. Target Nutrient Intakes
Presently, research results that provide the basis for target nutritional intakes for acute heart failure patients are not available. In this section, target nutritional intakes for severely ill patients requiring intensive care are discussed.
Energy
First, to determine the target amount of energy to be administered, it is necessary to estimate energy expenditure. In heart failure patients, the resting energy expenditure (REE) is reported to be about 18% higher compared with healthy individuals.136
It is recommended to set the target amount of energy to be administered based on the measurement of the energy expenditure by indirect calorimetry137
or its calculation using an estimation equation.
For the calculation of the target amount of energy to be administered using an estimation equation, a simplified formula assuming the energy expenditure per kg of body weight as 25–30 kcal/day and Harris-Benedict equation are widely used today. Indirect calorimetry can be substituted for estimation of the energy expenditure using an estimation equation.138
In critically ill patients, it is recommended to administer energy at a dose smaller than the calculated target amount during the first week of the acute phase. In a group in which tolerable restriction of nutritional intake was imposed, the hospital death rate was significantly lower, durations of the stay in the ICU and the period of support using a respirator were shorter,139
and the incidence of hyperglycemia was lower,140
compared with a group in which energy was administered to the target dose. In a study of the relationship between the fulfillment rate of the energy requirement with the mortality rate in ICU patients, a U-shaped curve was obtained, indicating that the risk of death was high when the energy intake was excessive or deficient.138
Protein
In an observational study of patients admitted to the ICU, the mortality rate was shown to decrease inversely with protein intake.141
The administration of at least 1.2 g/kg/day of protein is necessary in patients in the acute phase of trauma to improve nitrogen balance.142
However, a systematic review indicated that the grounds concerning the optimal protein dose are insufficient.143
Since the subjects of these studies were not acute heart failure patients, the optimal protein intake for acute heart failure patients is unclear.
Salt
One of the exacerbating factors of chronic heart failure is excessive intake of salt, but there has been no study that showed the optimal salt intake in acute heart failure patients.
In patients capable of oral feeding, excessive salt restriction may invite anorexia, leading to energy deficiency due to insufficient dietary intake. According to an interventional study, there was no advantage in the combination of strict salt restriction and fluid restriction.144
D. Types and Selection of Enteral Nutrient Preparations
In heart failure patients, the risk of diarrhea associated with enteral nutrition increases because of intestinal edema and reduced digestion/absorption ability.
There is no significant difference between oligomeric nutrient preparations (peptide type nutrient preparations) and polymeric nutrient preparations in the duration of hospitalization, incidence of infection, or mortality rate.91
Most of the nutrient preparations usable in Japan contain 1 kcal of energy per mL, but the use of concentrated products with energy content of 1.5 or 2 kcal/mL should be considered for acute heart failure patients with fluid restriction.
E. Management of Patients During Enteral Nutrition Therapy
Points of attention in the management of enteral nutrition in critically ill patients are described, although they are not specific to heart failure patients.
Body Position During Enteral Nutrition Therapy
Sufficient caution against aspiration is necessary during enteral nutrition therapy. In enteral feeding of tracheally intubated patients, the incidence of pneumonia can be reduced by placing them in the semi-Fowler’s position, in which the cranial end of the bed is elevated 30–45°.145
It is recommended to maintain semi-Fowler’s position as much as possible in consideration of the risk of aspiration.
Nutritional Management During Noninvasive Positive Pressure Ventilation
During noninvasive positive pressure ventilation (NPPV), there is the possibility of aspiration if the gastric contents reach the oral cavity due to vomiting or gastroesophageal reflux, but the NPPV guidelines recommend enteral nutrition for patients incapable of oral feeding.146
Even in patients requiring NPPV, avoidance of unnecessary fasting and early initiation of oral or enteral feeding are also recommended if the use of intestine is possible.
Estimation of Gastric Residual Volume
The amount of nutrient preparation and digestive juice retained in the stomach during the management of enteral feeding is called the gastric residual volume (GRV). Generally, GRV is measured every 4–6 hours.
GRV is used as an index for the assessment of gastric motility. There is a report that GRV is unrelated to the incidence of aspiration,147
but there was also a report that the frequency of aspiration was high when GRV was large.148
The joint guidelines by the Society of Critical Care Medicine (SCCM) and American Society for Parenteral and Enteral Nutrition (ASPEN)149
and Canadian guidelines150
recommend continuation of enteral feeding without interruption if GRV is ≤500 mL, however, careful management is required in Japanese patients even if GRV is ≤500 mL.
Intermittent and Continuous Administration
Comparison between intermittent and continuous administration showed that the target amount of energy administration could be reached more quickly by intermittent administration.151
However, it is necessary to consider that the subjects were American patients admitted to the trauma ICU and that they were young with a mean age in the 40 s.
Although there has been no study in which intermittent and continuous administration was compared in heart failure patients, the frequency of diarrhea was reported to be lower by continuous administration in burn patients152
and older people.153
From these observations, continuous administration is recommended, if possible, for enteral nutritional management in the acute phase.
F. Composition of Parenteral Nutrition
In parenteral nutrition, the administration of at least the 3 primary nutrients, vitamins, and trace elements should be considered rather than glucose alone. In the Early PN Trial,135
the outcome was not exacerbated even when parenteral nutrition, which contained the 3 primary nutrients, was initiated early. The recommended doses of vitamins and trace elements for not only heart failure patients but also critically ill patients are presently unclear.154
Section 3. Nutritional Therapy for Stage C/D Chronic Heart Failure
A. Therapeutic Goals of Chronic Heart Failure and the Position and Basic Principles of Its Nutritional Therapy
Chronic heart failure is a progressive syndrome in which gradual progression of declines in cardiac function, physical functions, and nutritional state is observed with repeated alternation of exacerbation and remission. Exacerbation of the nutritional state, decreases in muscle mass and muscle strength, and declines in physical functions are considered to occur as results of phenomena due to circulatory insufficiency such as dysfunction of viscera and organs of the whole body, activation of the renin-angiotensin system and sympathetic nervous system, and activation of inflammatory cytokines.
While the stage of heart failure gradually progresses, physical functions and nutritional state change in parallel, and the nutritional state is exacerbated as the terminal phase approaches. The serum albumin and cholesterol levels have been reported to be prognostic factors independent of age or the severity of heart failure.38,41
The goal of nutritional therapy for chronic heart failure is to prevent exacerbation of heart failure and improve the outcome while maintaining the nutritional state and aiming to maintain or improve the ability of physical activities. To achieve this goal, it is important to reduce salt intake, which may increase fluid retention, while maintaining appropriate energy intake. Generally, since the nutritional state is often intact if chronic heart failure is stabilized in stage C, nutritional therapy often centers around modification of salt intake, i.e., nutritional guidance primarily consisting of salt restriction. However, if intake of a necessary amount of energy is difficult to secure due to salt restriction, priority should be placed on securing energy intake. In addition, there is a report that Mediterranean diet reduced the re-admission rate of heart failure patients while it did not improve the prognosis, and the combination of foods (diet pattern) is also considered important.155
If the nutritional state is exacerbated due to progression of the stage of heart failure, even higher priority is given to appropriate energy intake. The course of chronic heart failure, nutritional state, contents of nutritional therapy, and objectives of concomitant exercise therapy are summarized in
Figure VI-3.156

B. Sarcopenia, Frailty, and Cachexia Associated With Chronic Heart Failure and the Prognosis
Chronic heart failure is complicated by sarcopenia in 25–50% of the patients.157,158
Moreover, a condition with increased vulnerability to health damage due to declines in various functions accompanied by impairment of cognitive function, psychological/psychiatric problems such as depression, and social problems such as solitary living and economic difficulties in addition to physical problems due to sarcopenia is defined as frailty,16
and the degree of frailty is correlated with the development of new heart failure159
and the outcome of heart failure patients.160
In the course of progression of heart failure, 5–15% of the patients lapse into a systemic wasting condition called cachexia (see
Chapter II, Table II-2).18,161,162
Cardiac cachexia is an independent poor prognostic factor for heart failure.9
Dietary therapy and exercise therapy with reevaluation and optimization of drug therapy for heart failure are important for improving cardiac cachexia.163
C. Chronic Heart Failure and Obesity, Optimal BMI
Presently, no evidence concerning the optimal BMI for chronic heart failure patients has been established. In heart failure patients, the prognosis has been reported to be poorer as BMI is lower and, inversely, to be better as BMI is higher, a phenomenon known as “obesity paradox”.9,14
However, no evidence concerning the relationship between intentional body weight gain or loss and the prognosis has been established in heart failure patients.
D. Reality of Nutritional Therapy for Chronic Heart Failure
Energy Requirement
In nutritional therapy for chronic heart failure patients, consideration of energy intake, salt/water intake, and intakes of protein and vitamins/minerals is necessary. As the basic dietary pattern for chronic heart failure, the Mediterranean diet consisting primarily of fish, vegetables, fruits, wine, and whole-grain cereals is recommended.
In heart failure patients, basal metabolism is increased,136
and the energy requirement is greater than usual.
For the estimation of the energy requirement, the REE is considered to be calculated most accurately by indirect calorimetry:
REE (kcal/day) =
{(3.941 × V̇O2 (mL/min)) + (1.106 × V̇CO2 (mL/min))} × 1.44–2.17 × urinary urea nitrogen (mg/day)
V̇O2: oxygen uptake, V̇CO2: carbon dioxide output
or using a simplified formula:
REE (kcal/day) =
{(3.94 × V̇O2 (mL/min)) + (1.11 × V̇CO2 (mL/min))} × 1.44 164
REE calculated using this formula multiplied by the activity index (Table VI-4) is the estimated daily energy requirement. However, as expired gas analysis, which is often difficult to perform, is necessary for indirect calorimetry, a method based on the Harris-Benedict equation165
is widely used as a substitute for the calculation of REE166
(Table VI-4). However, there is also no mention about the stress index of chronic heart failure patients in this equation. In addition, in Japanese, particularly, older women, the energy requirement calculated using this equation tends to be higher than the value obtained by indirect calorimetry.
Table VI-4.
Estimation of Necessary Amount of Energy Using the Harris-Benedict Equation
Basal metabolic
expenditure |
Male: 66.5 + (13.8 × body weight in kg) + (5.0 × height in cm) − (6.8 × age)
Female: 655.1 + (9.6 × body weight in kg) + (1.8 × height in cm) − (4.7 × age) |
| Activity index |
Recumbent position: 1.0, wheelchair (resting on the bed): 1.2, walking (out-of-bed activities):
1.3–1.4, mild exertion: 1.5, moderate exertion: 1.7, heavy exertion: 1.9 |
| Stress index |
After surgery (no complications): 1.0, fever: 1.2–1.5, fracture: 1.15–1.3, bedsore: 1.2–1.6,
cancer, COPD, pneumonia, sepsis: 1.1–1.3, use of steroid: 1.6–1.7, severe infection, multiple
traumas: 1.2–1.4, multiple organ failure, burn injury: 1.2–2.0 |
Necessary amount
of energy |
Basal metabolic expenditure × activity index × stress index |
Source: Prepared based on Japanese Society for Parenteral & Enteral Nutrition. Guidelines for Parenteral/Enteral Nutrition for Comedicals. Nankodo; 2000.166
As more convenient methods, a simplified formula for the calculation of basal metabolic rate in Japanese166
[14.1 × current body weight (kg) + 620 for males, 10.8 × current body weight (kg) + 620 for females] and addition or subtraction of 25–30 kcal/kg depending on the degree of stress are also used frequently. Whichever estimation equation may be used for the calculation of energy requirement, assessment of the patient’s nutritional state must be repeated during the course of treatment.
If the actual body weight is ≥125% of the standard body weight (22 × height (m)2), it is recommended to perform the calculation using the corrected body weight calculated as:
Corrected body weight (kg) =
{(actual body weight) − (standard body weight)} × 0.25 + standard body weight167
Salt Restriction and Fluid Restriction
As salt increases fluid retention and the report that half-hearted salt/fluid restriction is the most frequent factor of re-admission due to exacerbation of heart failure, salt restriction is important in nutritional management of heart failure patients. However, no specific recommended intake of salt has been established. According to Japanese guidelines, a salt intake of <6 g/day is recommended, and more strict salt restriction should be evaluated for severe heart failure,57
but caution is necessary, particularly, in older patients, because excessively strict salt restriction reduces appetite and may lead to exacerbation of the nutritional state. It is recommended to reduce salt intake within a tolerable range by individually assessing the patient’s nutritional state and dietary intake and considering the actual salt intake without sticking to salt-restricted diet.
Guidance about the necessity of fluid restriction in heart failure is often similar to that about salt restriction, but intake of a certain amount of fluid is necessary to maintain fluid homeostasis. Generally, the daily fluid requirement is considered to be 30–40 mL/kg/day including dietary water content, but it varies with age, and the minimum requirement in those aged ≥75 years is considered to be 25 mL/kg/day.168
There is no clear evidence concerning fluid restriction in heart failure patients. In the European Society of Cardiology (ESC) guidelines, also, avoidance of excessive intake of fluid is recommended, but they only state that restriction of fluid intake to 1.5–2.0 L/day may be evaluated to alleviate symptoms and congestion in patients with severe heart failure and recommend adjustments such as increasing the fluid intake depending on the environment and physical condition.69
Furthermore, in older patients, appropriate support for fluid intake is necessary in consideration of the age-associated decline in thirst center function.57
However, fluid restriction is necessary if the patient with severe heart failure shows dilutional hyponatremia. The amount of water contained in food is often assumed to be about 400 mL per 1,000 kcal or about 600 mL per 1 kg of usual diet.
Protein Intake
In the absence of moderate or severe renal dysfunction, protein intake of patients with chronic diseases including chronic heart failure is recommended to be increased to 1.2–1.5 g/kg/day.169,170
If the estimated glomerular filtration rate (eGFR) is <60 mL/min/1.73 m2, and if there is moderate or severe renal dysfunction with grade 3b chronic renal disease, protein restriction to 0.6–0.8 g/kg/day is considered. In such an event, also, it is recommended to ingest a sufficient amount of energy, or ≥30 kcal/kg, with attention to sarcopenia and frail.169,171
E. Possible Effectiveness of Oral Supplements in Heart Failure
Of the essential amino acids, branched-chain amino acids such as leucine, isoleucine, and valine (particularly leucine) are known to be substrates in muscle protein synthesis and, at the same time, promote protein synthesis. Many studies that evaluated the effects of nutritional supplementation therapy using oral amino acids to promote muscle protein synthesis in chronic heart failure reported increases in muscle protein synthesis and improvements in skeletal muscle mass and exercise tolerance.172,173
However, since each of these studies was performed in a small number of patients and did not show the evidence of improvement in prognosis, further evaluation is still necessary.
EPA and DHA, which are ω3 polyunsaturated fatty acids and essential fatty acids contained in large amounts in fish oil, are known to have anti-arteriosclerotic, anti-inflammatory, and anti-inflammatory cytokine actions and are expected to have suppressive effects on heart failure. In both the American College of Cardiology Foundation (ACCF)/AHA and ESC guidelines, intake of ω3 polyunsaturated fatty acids is recommended as nutritional supplementary therapy despite a difference in recommendation grade.69,174
In chronic heart failure, deficiency of vitamins and trace elements is also likely to occur.175
Regarding ONS, the effects of agents such as coenzyme Q10,176
carnitine,177
taurine,178
antioxidants179
or hormone therapy using growth hormones180
and thyroid hormones181
have been reported. However, no evidence has been established.
F. Possibility of Synergy Between Exercise and Nutrition Therapies
The effectiveness of exercise therapy in chronic heart failure is well established, and various effects including improvements in the outcome, neurohumoral factors, skeletal muscle mass and quality, and vascular endothelial function in addition to exercise tolerance have been reported.69,174,182
Exercises recommended for heart failure patients are low-intensity resistance training and aerobic exercise such as walking, bicycle ergometer, and light aerobic exercise.182
If muscle weakness is severe, combining resistance training (low/moderate-intensity repetitious muscle-developing exercise) with systemic aerobic exercise is reportedly safe and effective for improving exercise tolerance and QOL.182
Generally, the importance of resistance training relative to aerobic exercise is greater as skeletal muscle mass and muscle strength are more reduced, and as exercise tolerance is more impaired. The exercise pattern that recovers loss of skeletal muscle mass is resistance training, and its combination with nutritional therapy is important.183
It has been found that synergistic effects were obtained in muscle mass and motor function by combining exercise therapy primarily consisting of low-intensity resistance training with amino acid supplementation therapy in older Japanese women with sarcopenia.184
Section 4. Diet in Palliative Care of Heart Failure Patients
In the terminal phase of heart failure, symptoms including anorexia, nausea/vomiting, respiratory distress, and a sense of abdominal fullness associated with the disease state are observed, and food intake decreases. This also causes a decrease in salt intake. There are also reports that suggest that excessive restriction of salt intake is related to a poor prognosis.185,186
The objective of palliative care is to support patients to maintain QOL so that they can spend their remaining time as they wish. It is necessary to evaluate diet individually, and uniform excessive salt reduction should be avoided.
Although, eventually, patients show natural progressive loss of appetite, forced nutritional support should be avoided in this period. Also, uniform fluid infusion to compensate for reduced dietary intake is not recommended in the terminal phase, because it may exacerbate dyspnea and symptoms of congestion such as edema.187
Chapter VII. Team Care for Heart Failure in Nutritional Therapy
Section 1. Significance of Nutritional Therapy for Heart Failure by Multidisciplinary Collaboration
In conducting nutritional therapy for heart failure, it is necessary to consider social problems such as the family structure, presence or absence of caregivers, and economic state and problems other than the disease including mental/psychological condition in addition to the assessment of the nutritional state and understanding of the complex pathology. Multidisciplinary teamwork is important, because it is difficult to cope with nutritional disorders in heart failure by a single profession alone, which may result in inadequacy of medical services and operations and increased burden on medical workers.
The Guidelines for Diagnosis and Treatment of Acute and Chronic Heart Failure of Japan also recommend a team approach for the management of heart failure patients (class I),57
and multidisciplinary inclusive nutritional intervention has been reported to improve the nutritional state in older people with a risk for malnutrition including heart failure patients.188
Team care means, “Care appropriate for the patient’s condition provided by a team of various medical professionals based on a high level of expertise of each member by sharing the objective and information, dividing duties, but collaborating and complementing each other”.189
This is consistent with the concept of “skill-mix”, which is also called “multidisciplinary collaboration”. Skill-mix is not simply division of roles among professionals but involves mutual complementation of roles, transfer of authority and responsibility, creation of new professionals, and functional differentiation (Figure VII-1).190
Section 2. Stage-Specific Setting of Team Goals
A. Acute Phase (Acute Phase/Recovery Phase)
In the acute phase of heart failure, the team of nutritional therapy aims to quickly and accurately evaluate the nutritional state and perform nutritional support by an appropriate interventional method (enteral, parenteral, oral feeding). Once the intervention method has been determined, attention to fluid management is necessary simultaneously with the assessment of the nutritional state. After stabilization of heart failure, nutritional guidance is given early, and arrangements for nutritional management after discharge are made.
B. Chronic Phase (Maintenance Phase/Living Phase)
The mean duration of hospitalization of heart failure patients is 33.9 days,191
and it is difficult to improve the nutritional state during hospitalization or to form an appropriate habit of nutrient intake. Therefore, the team goals after discharge are: 1) to have the patients themselves fully understand the significance of nutritional therapy, 2) to provide information about necessary nutrient intake behavior, and 3) to continuously monitor the state of implementation of the therapy and nutritional state.
Section 3. Roles of Various Professions (Figure VII-2)
A. Roles of Physicians
Role of Physicians in Multidisciplinary Collaboration
Physicians play a coordinating role of communicating with members of the multidisciplinary team, making adjustments among professions by drawing out their opinions, issuing necessary instructions and advice to other professions according to the results of assessment of the disease and circumstances of the diagnosis of treatment, and transmitting the opinions shared within the team to the physician in charge.
Role of Physicians in Nutritional Assessment
In heart failure, the values of nutritional indices may change under the effects of systemic congestion and neurohumoral factors.40
For this reason, physicians must implement nutritional evaluation in consideration of the effects of the disease state of heart failure based on the results of nutritional assessment carried out by various professions.
Role of Physicians in Intervention With Nutritional Therapy
Risk management is important in nutritional management of heart failure, which exhibit complicated pathological conditions. Particularly, in hemodynamically unstable situations such as the acute phase and exacerbating phase of heart failure, physicians must evaluate risks in nutritional supplementation that require caution in the management of heart failure such as bowel rest and restriction of fluid intake and give other professions instructions about measures to take.
B. Role of Registered Dietitians
Registered dietitians play an important role in clinical practice as experts of nutritional management/guidance and nutritional evaluation/judgment.192
Registered dietitians evaluate the nutritional state by collecting a wide range of information concerning nutrition and draw up nutritional management plans. If the dietary intake is insufficient, registered dieticians propose the use of oral nutrition supplementation (ONS) and change of the route of administration by sharing information concerning eating function, disease state, treatment plan, and treatment environment, within the team and specifically showing expected effects and points of attention in the management. To cope with changing disease states, registered dietitians select the evaluation items and re-evaluate/re-design the contents of nutritional management in multidisciplinary approach.
In the acute phase, registered dieticians predict excess or deficiency of energy and nutrients according to the administration route in collaboration with pharmacists and design mutually complementary nutritional therapies. In the chronic phase, self-management of disease by the patients is important.193
If the patient’s self-management is inadequate, measures to obtain cooperation from family or caregivers must be taken. Depending on the treatment environment, home delivery of meals and home care service are proposed to the team as options. If adherence to dietary therapy is difficult to obtain, treatment should be conducted under conditions close to the actual dietary environment by proposing management according to the characteristics and preferences of the patient.
In the terminal phase, the approach to dietary therapy is evaluated together with the patient and family by taking their wishes in consideration and providing sufficient information including the risks of readmission and prolongation of hospitalization.
C. Role of Nurses
Collection of Information Concerning the Life Circumstances, Assessment of Problems With Nutritional Therapy, and Adjustment With Family and Other Professions
In introducing nutritional therapy, nurses play a central role in the assessment for the judgment of its technical and economic feasibility to the patient and family. Occasionally, nutritional therapy necessary for the patient cannot be implemented because of the lack of understanding or cooperation by the family living with the patient even if the patient himself/herself understands the necessity of nutritional therapy. In such situations, consideration of the family is also necessary.
Role of Deepening the Understanding of Patients
Since diet is closely related to emotions (see
Chapter IV, Section 1-A), medical professionals must understand the values of the patient/family about diet, perception of the patient/family about nutritional therapy, and patient’s dietary practice. For this purpose, patients should be listened to. By talking, patients are also expected to reflect on the present status, sort out problems, and modify their behavior.194
Evaluation of the Body Weight, Dietary Intake, and Salt Intake
It is necessary for nurses to monitor signs and symptoms of heart failure with serial evaluation of the body weight, body composition, daily dietary intake, and salt intake even after discharge of the patients. The absolute salt intake can also be estimated by examination of casual urine samples.195
Assessment of Anorexia
Concerning decreases in dietary intake, the reality may be difficult to capture by medical interviews if there is cognitive impairment. The cause of anorexia may be postprandial hypotension or orthostatic hypotension, and observation by nurses, who have long contact with patients, is important.
D. Roles of Physiotherapists, Occupational Therapists, and Speech Therapists
Physiotherapists and Occupational Therapists
The nutritional state is closely related to physical functions and activities of daily living (ADL). Physiotherapists and occupational therapists assess physical functions and constitution, posture, cognitive function, and ADL and evaluate how they are related to the nutritional state. If impairment of physical function or ADL is observed, they formulate ADL supporting programs with exercise loads appropriate for the disease condition, nutritional state, and the state of physical functions. Older patients have low reserve of physical functions and exhibit complications and complex impairment of physical functions. Paying attention to the endurance of, and fatigue in, feeding activities, they propose appropriate posture and actions of eating, eating pace, and support equipment. They also assess the respiratory function including the ability to cough and respiratory reserve and provide guidance in coughing and respiration training, if necessary, for the risk management against aspiration.
Speech Therapists
Speech therapists evaluate eating and swallowing disorders and devise programs for basic training in eating function and improving the functions of oropharyngeal and laryngeal organs. Eating/swallowing training can be classified into direct training in food ingestion and indirect training to improve the basic swallowing function. Speech therapists evaluate the indications of these training methods according to the level of consciousness, general condition, appetite, and cognitive function. In addition, they propose diets adjusted to the oral and swallowing functions.
E. Roles of Pharmacists, Dental Hygienists, and Medical Social Workers
Pharmacists
Pharmacists support nutritional therapy by helping with the selection of the feeding route and pharmaceutical formulation from pharmacological viewpoints. They propose nutritional preparations and proper methods for their use based on the knowledge of drug/food interactions. They also provide explanation and guidance about the prevention of complications and avoidance of risks associated with the use of nutritional preparations.
Dental Hygienists
Dental hygienists examine and evaluate the state of oral hygiene and give expert oral care using medical devices and drugs. They also evaluate oral contamination, which may cause taste disorders, and the state of mastication environment or dentures, which affect bolus formation.
Medical Social Workers
Medical social workers play the role of coordinators for the use of social services. They help patients continue to have appropriate nutritional management through consultation, adjustment, and support concerning psychological, social, and economic problems that the patients and families encounter.
Section 4. Actual Nutritional Therapy by Multidisciplinary Collaboration
A. Multidisciplinary Collaboration in Nutritional Therapy for Severe Heart Failure
In severe heart failure, nutritional therapy must be conducted based on the understanding of each profession about the high hemodynamic variability and consequent destabilization of the general condition as well as the possibility of the occurrence of complications associated with nutritional therapy.
Nutritional Evaluation
Nurses and registered dieticians must hear from the patient or family about the nutritional state and ADL before admission and judge whether the patient was at risk or was in a state of malnutrition. It is also indispensable to collect information such as the dietary intake before admission and drinking history for the risk assessment of refeeding syndrome.
The results of body measurements are the basis of nutritional evaluation, and they are usually evaluated by registered dieticians, physiotherapists, and nurses. In severe heart failure, however, as heart failure itself affects the nutritional assessment items (see
Chapter III, Section 3-A), nutritional evaluation in consideration of the physician’s evaluation of the disease state is necessary.
Selection of the Administration Method of Nutritional Preparations and Points of Attention
Monitoring of changes in hemodynamic indices (vital signs, urine volume) and checking of gastrointestinal symptoms after the beginning of enteral nutrition are made by nurses, who spends much time at the bedside, according to the physicians’ evaluation of the cardiac function and hemodynamics. If nutritional requirements cannot be fulfilled due to the difficulty in initiating or increasing enteral nutrition, registered dieticians or pharmacists must make proposals about indications of total parenteral nutrition (TPN) and peripheral parenteral nutrition (PPN).
Pharmacists must send reminders about the effects of drugs used on nutritional management while placing priority to the use of drugs for the management of heart failure and make relevant proposals if additions, reductions, or adjustment of the dose are necessary for nutritional management.
Nutritional Management in the Transition From the Acute Phase to the Recovery or Maintenance Phase
In conducting oral nutrition, speech therapists and nurses perform risk management against aspiration including the assessment of swallowing function. If the risk of aspiration, low output syndrome, or exacerbation of the hemodynamics is high, physicians must consider changing the feeding method, if necessary, by consulting with registered dieticians.
If the condition is confirmed to tolerate the transition to oral feeding, the settings of the amount of feeding and salt intake are re-checked. It is necessary to share the state of the patient’s activities in the ward and contents of rehabilitation among professions on the basis of evaluation of the general condition.
If anorexia is observed, its cause must be identified among the professions. Methods for the evaluation of anorexia include the Functional Assessment of Anorexia /Cachexia Therapy (FAACT) Appetite Scale196
and, as simpler methods, the Council on Nutrition Appetite Questionnaire (CNAQ) and Simplified Nutritional Appetite Questionnaire (SNAQ).197
Physicians must evaluate whether anorexia is caused by heart failure or not. Pharmacists must check the presence or absence of drugs with zinc chelator actions and evaluate the possibility of zinc deficiency, which may cause taste disorders,198
nurses and registered dieticians evaluate changes in the patient’s preferences, dietary contents, and eating environment (place, tableware) and, in cooperation with speech therapists and dental hygienists, evaluate the oral environment including oral dryness and unfit dentures. When the cause of anorexia is identified, measures are devised multidisciplinarily, and if the dietary intake does not fulfill the nutritional requirements, the addition of supplements, concomitant use of enteral/parenteral feeding should be evaluated, as necessary, under the initiative of registered dieticians. If the possibility of an involvement of depression or anxiety cannot be excluded, arrangements are made for intervention by psychiatrists or clinical psychologists from the viewpoint of mental health.
In severe heart failure, to sustain ADL and quality of life (QOL), it is very important to preserve or improve skeletal muscle mass and muscle strength (see
Chapter VI, Section 3-F). The state of energy and protein intakes are evaluated under the initiative of registered dieticians, and physiotherapists conduct exercise therapy based the pathological assessment primarily by physicians. In the nutritional management in the transition from the recovery to maintenance phase, the state of body weight management and salt/fluid intake are checked primarily by nurses and registered dieticians, and guidance is given to the patients and families. In patients with malnutrition or at risk of malnutrition, attention to decreases in dietary intake continues to be necessary. Since the patient’s general condition, personality and mental condition, living and economic status, and the presence or absence of caregivers greatly affect nutritional therapy for severe heart failure patients, it is recommended to formulate measures by consultation among all professions involved.
B. Collaboration With the Nutrition Support Team in the Treatment of Heart Failure
If there are difficulties in nutritional management, intervention by a team for safe and effective nutritional management in collaboration with many experts and professions trained in nutritional management such as the nutrition support team (NST)24
should be considered. Since heart failure is accompanied by rapid changes in the disease state and body fluid volume, close collaboration is necessary. Intervention by an expert team is expected to have an educational effect on the staff of the heart failure team concerning nutritional management.
C. Home Nutritional Therapy for Frail Patients
Heart failure patients are likely to progress into frailty (see
Chapter II, Section 2, Table II-1).16
If a patient is judged to be frail, priority should be placed on securing the quality and quantity of nutrition and exercise rather than restriction of salt intake. For this, early detection of frailty is important. Nurses, who have long contact with patients, are expected to bear the role of frailty screening.
Reliable interventional studies on frailty with nutritional therapy alone are still few.199,200
According to studies in average older people as references, measures such as increasing the intakes of energy, protein, and branched-chain amino acids including leucine and trace nutritional element supplementation are possible optaions.201–206
Home exercise therapy is also necessary to increase muscle mass and strength. Such interventions should be performed under the appropriate supervision of nutritionists and therapists.
For home management of frail heart failure patients, collaboration between the medical staff specializing in heart failure and home caregivers is indispensable.
Conflicts of interest of the parties involved in developing the statement
The Japanese Heart Failure Society Expert Consensus Writing Committee for “A Scientific Statement on Nutritional Assessment and Management in Heart Failure Patients” obtained declarations of the conflict of interest status over the past one year from each committee member and author based on the following criteria regarding the financial relationship between the drafting committee members and companies involved in the relevant fields.
1. Compensation received for serving as an executive officer or consultant of companies or organizations (should be stated if the compensation from a single company / organization exceeds ¥1 million annually)
2. Holding shares of stock and earning profits from the stock (should be stated if profits earned from the stocks of a single company exceed ¥1 million annually, or holding ownership of 5% or more of the company’s entire share)
3. Patent royalties paid from companies / organizations (should be stated if a single royalty exceeds ¥1 million annually)
4. Daily allowance from companies / organizations for time and effort spent for attendance (making a presentation) at a conference (such as lecture fees)(should be stated if the annual total amount of daily allowance from a single company / organization [such as lecture fees] exceeds ¥500,000)
5. A manuscript fee from companies / organizations (should be stated if the annual total manuscript fee from a single company / organization exceeds ¥500,000)
6. A research grant or subsidy from companies / organizations (should be stated if the annual total amount of the grants paid by a single company / organization for medical research [such as commissioned research funds and collaborative research funds] exceeds ¥1 million)
7. Scholarship or donation from companies / organizations (should be stated if the annual total amount of scholarship or donation paid from a single company / organization to a declaring person, a laboratory representative, or a department [course or class] to which a declaring person belongs exceeds ¥1 million)
8. Affiliating to a donated fund laboratory provided by a company / organization
9. Acceptance of travel expenses or gifts unrelated to research (should be stated if the total annual amount received from a single company / organization exceeds ¥50,000)
Notes
1: None
2: None
3: None
4: Bayer Yakuhin Ltd., Daiichi Sankyo Company Limited, Mitsubishi Tanabe Pharma Corporation, MSD K.K., Nippon Boehringer Ingelheim Co., Ltd., Novartis Pharma K.K., Ono Pharmaceutical Co. Ltd., Otsuka Pharmaceutical Co., Ltd., Pfizer Japan Inc., Takeda Pharmaceutical Company Limited, Teijin Pharma Limited
5: None
6: Actelion Pharmaceuticals Japan Ltd., EP-CRSU Co. Ltd., HuBit genomix, Japan Tobacco Inc, Nippon Boehringer Ingelheim Co., Ltd.
7: Actelion Pharmaceuticals Japan Ltd., Astellas Pharma Inc., Bayer Yakuhin Ltd., BIOTRONIK Japan, Inc., Boston Scientific Corporation, Daiichi Sankyo Company Limited, FUKUDA DENSHI CO., LTD., Fukuda Lifetech Chugoku Co., Ltd., Japan Lifeline Co., Ltd., Johnson & Johnson K.K., Kowa Pharmaceutical Co. Ltd., Mitsubishi Tanabe Pharma Corporation, MSD K.K., Nihon Koden corporation, Nippon Boehringer Ingelheim Co., Ltd., Novartis Pharma K.K., Otsuka Pharmaceutical Co., Ltd., Pfizer Japan Inc., Shionogi & Co., Ltd., St. Jude Medical, Inc., Takeda Pharmaceutical Company Limited, Teijin Pharma Limited
8: Medtronic Japan Co., Ltd., OMRON HEALTHCARE Co., Ltd.
9: None
With regard to editing the content of “A Scientific Statement on Nutritional Assessment and Management in Heart Failure Patients”, the committee members and authors, as specialists and specialty physicians of the relevant fields, aimed to guarantee scientific and medical justice and validity, improve the quality of care for the target diseases, and increase healthy life expectancy and QOL of the target patients. Conflicts of interest were handled in accordance with “The Conflict-of-Interest (COI) Policy” of the Japanese Heart Failure Society.
The company names declared are as stated above (applicable period: April 1, 2017–March 31, 2018). Company names are as of March 2018 (in alphabetical order). Publishers and organizations in a neutral position were not included.
Appendix. The Japanese Heart Failure Society, Expert Consensus Writing Committee
Chair:
・ Kazuhiro Yamamoto, Department of Cardiovascular Medicine and Endocrinology and Metabolism, Faculty of Medicine, Tottori University
Vice Chair:
・ Miyuki Tsuchihashi-Makaya, School of Nursing, Kitasato University
Manager:
・ Yoshiharu Kinugasa, Department of Cardiovascular Medicine and Endocrinology and Metabolism, Faculty of Medicine, Tottori University
Members:
・ Taiki Higo, Department of Cardiovascular Medicine, Kyushu University Graduate School of Medical Sciences
・ Yuki Iida, Department of Rehabilitation Medicine, Kainan Hospital
・ Kentaro Kamiya, Department of Rehabilitation, Kitasato University
・ Yasuki Kihara, Department of Cardiovascular Medicine, Hiroshima University Graduate School of Biomedical & Health Sciences
・ Yuji Kono, Department of Rehabilitation, Fujita Health University Bantane Hospital
・ Isao Miyajima, Department of Clinical Nutrition, Chikamori Hospital
・ Yasushi Miyazawa, Department of Clinical Nutrition, Tokyo Medical University Hospital
・ Yukihito Sato, Department of Cardiovascular Medicine, Hyogo Prefectural Amagasaki General Medical Center
・ Norio Suzuki, Division of Cardiology, Department of Internal Medicine, St. Marianna University School of Medicine Yokohama City Seibu Hospital
・ Harumi Takeuchi, Department of Clinical Nutrition, Nagoya University Hospital
・ Hiroyuki Tsutsui, Department of Cardiovascular Medicine, Kyushu University Graduate School of Medical Sciences
・ Akira Yamashina, Tokyo Medical University Medical Education Promotion Center
・ Katsushi Yoshita, Department of Food and Human Health Science, Osaka City University Graduate School of Human Life Science
・ Koichi Washida, Faculty of Nursing, Kobe Women’s University / Department of Nursing, Hyogo Prefectural Amagasaki General Medical Center
Independent Assessment Committee:
・ Naoyuki Hasebe, Division of Cardiology, Nephrology, Pulmonology and Neurology, Department of Internal Medicine, Asahikawa Medical University
・ Masafumi Kuzuya, Department of Community Healthcare & Geriatrics, Nagoya University Graduate School of Medicine
・ Yutaka Nakaya, Department of Internal Medicine, Touto Kasukabe Hospital
・ Tetsuya Takahashi, Department of Physical Therapy, Faculty of Health Science, Juntendo University / Department of Rehabilitation, Juntendo University Hospital
(The affiliations of the members are as of February 2020)
References
- 1.
Goto S, Suzuki T. Nutrition and Nutrients. Contemporary Dietary Habits. Contemporary Dietary Habits. Rikogakusha Publishing, 1988.
- 2.
Nakamura T, Kuranuki S. Nutrition Management. In: Nakamura T, Kuranuki S. Nutrition Management. Watanabe R, Ito S, Takimoto H, editors. Applied Nutrition, 5th ed. Nankodo, 2015.
- 3.
Taketani Y. Nutritional Disturbance. In: Tanaka K, editor. Structures and Functions of the Human Body and Mechanisms of Diseases III, Mechanisms of Diseases. Nakayama Shoten, 2017.
- 4.
Oguchi K. Nutriology of Vitamins. In: Tachi Y, editor. Basic Nutrition, 3rd ed. Yodosha, 2016.
- 5.
Oguchi K. Nutriology of Minerals. In: Tachi Y, editor. Basic Nutrition, 3rd ed. Yodosha, 2016.
- 6.
Ministry of Health, Labour and Welfare. “Dietary Reference Intakes for Japanese (2015)” Preparation Committee Report (March 2014). https://www.mhlw.go.jp/file/05-Shingikai-10901000-Kenkoukyoku-Soumuka/0000114399.pdf [Accessed in February 2020]
- 7.
Yoshita K. Nutritional/Dietary Planning, Reference Diagrams for Improving the Understanding of Intake Assessment. In: Yoshita K, Ishida H, editors. Nutritional and Feeding Management Based on the Dietary Reference Intakes: Implementation of the PDCA Cycle. Kenpakusha, 2015.
- 8.
Kenchaiah S, Evans JC, Levy D, Wilson PW, Benjamin EJ, Larson MG, et al. Obesity and the risk of heart failure. N Engl J Med 2002; 347: 305–313. PMID: 12151467
- 9.
Anker SD, Ponikowski P, Varney S, Chua TP, Clark AL, Webb-Peploe KM, et al. Wasting as independent risk factor for mortality in chronic heart failure. Lancet 1997; 349: 1050–1053. PMID: 9107242
- 10.
Yancy CW, Jessup M, Bozkurt B, Butler J, Casey DE, Drazner MH, et al. WRITING COMMITTEE MEMBERS. 2013 ACCF/AHA guideline for the management of heart failure: A report of the American College of Cardiology Foundation/American Heart Association Task Force on practice guidelines. Circulation 2013; 128: e240–e327. PMID: 23741058
- 11.
Ponikowski P, Voors AA, Anker SD, Bueno H, Cleland JGF, Coats AJS, et al. ESC Scientific Document Group. 2016 ESC Guidelines for the diagnosis and treatment of acute and chronic heart failure: The Task Force for the diagnosis and treatment of acute and chronic heart failure of the European Society of Cardiology (ESC) Developed with the special contribution of the Heart Failure Association (HFA) of the ESC. Eur Heart J 2016; 37: 2129–2200. PMID: 27206819
- 12.
Takiguchi M, Yoshihisa A, Miura S, Shimizu T, Nakamura Y, Yamauchi H, et al. Impact of body mass index on mortality in heart failure patients. Eur J Clin Invest 2014; 44: 1197–1205. PMID: 25331191
- 13.
Matsushita M, Shirakabe A, Hata N, Shinada T, Kobayashi N, Tomita K, et al. Association between the body mass index and the clinical findings in patients with acute heart failure: Evaluation of the obesity paradox in patients with severely decompensated acute heart failure. Heart Vessels 2017; 32: 600–608. PMID: 27778068
- 14.
Hamaguchi S, Tsuchihashi-Makaya M, Kinugawa S, Goto D, Yokota T, Goto K, et al. JCARE-CARD Investigators. Body mass index is an independent predictor of long-term outcomes in patients hospitalized with heart failure in Japan. Circ J 2010; 74: 2605–2611. PMID: 21060207
- 15.
Chen LK, Woo J, Assantachai P, Auyeung TW, Chou MY, Iijima K, et al. Asian Working Group for Sarcopenia: 2019 Consensus Update on Sarcopenia Diagnosis and Treatment. J Am Med Dir Assoc 2020; 21: 300–307.e2. PMID: 32033882
- 16.
Fried LP, Tangen CM, Walston J, Newman AB, Hirsch C, Gottdiener J, et al. Cardiovascular Health Study Collaborative Research Group. Frailty in older adults: Evidence for a phenotype. J Gerontol A Biol Sci Med Sci 2001; 56: M146–M156. PMID: 11253156
- 17.
Satake S, Senda K, Hong YJ, Miura H, Endo H, Sakurai T, et al. Validity of the Kihon Checklist for assessing frailty status. Geriatr Gerontol Int 2016; 16: 709–715. PMID: 26171645
- 18.
Evans WJ, Morley JE, Argilés J, Bales C, Baracos V, Guttridge D, et al. Cachexia: A new definition. Clin Nutr 2008; 27: 793–799. PMID: 18718696
- 19.
Pasini E, Aquilani R, Dioguardi FS, D’Antona G, Gheorghiade M, Taegtmeyer H. Hypercatabolic syndrome: Molecular basis and effects of nutritional supplements with amino acids. Am J Cardiol 2008; 101 Suppl: 11E–15E. PMID: 18514619
- 20.
Rydén M, Arner P. Fat loss in cachexia: Is there a role for adipocyte lipolysis? Clin Nutr 2007; 26: 1–6. PMID: 17095126
- 21.
Polak J, Kotrc M, Wedellova Z, Jabor A, Malek I, Kautzner J, et al. Lipolytic effects of B-type natriuretic peptide 1-32 in adipose tissue of heart failure patients compared with healthy controls. J Am Coll Cardiol 2011; 58: 1119–1125. PMID: 21884948
- 22.
Nakayama H, Koyama S, Kuragaichi T, Shiba M, Fujiwara H, Takatsu Y, et al. Prognostic value of rising serum albumin during hospitalization in patients with acute heart failure. Am J Cardiol 2016; 117: 1305–1309. PMID: 27020611
- 23.
Kuzuya M. Nutritional assessment and nutritional management for the elderly. [Article in Japanese] Jpn J Geriatr 2003; 40: 199–203.
- 24.
Japanese Society for Parenteral & Enteral Nutrition. JSPEN Guideline for Parenteral and Enteral Nutrition: Guidelines for the Proper Use of Parenteral and Enteral Nutrition 3rd ed. Shorinsha, 2013.
- 25.
Japan Society for the Study of Obesity. Guideline for the Management of Obesity Disease 2016. Life Science Publishing, 2016.
- 26.
Kenchaiah S, Pocock SJ, Wang D, Finn PV, Zornoff LA, Skali H, et al. CHARM Investigators. Body mass index and prognosis in patients with chronic heart failure: Insights from the Candesartan in Heart failure: Assessment of Reduction in Mortality and morbidity (CHARM) program. Circulation 2007; 116: 627–636. PMID: 17638930
- 27.
Komukai K, Minai K, Arase S, Ogawa T, Nakane T, Nagoshi T, et al. Impact of body mass index on clinical outcome in patients hospitalized with congestive heart failure. Circ J 2012; 76: 145–151. PMID: 22094909
- 28.
Sharma A, Lavie CJ, Borer JS, Vallakati A, Goel S, Lopez-Jimenez F, et al. Meta-analysis of the relation of body mass index to all-cause and cardiovascular mortality and hospitalization in patients with chronic heart failure. Am J Cardiol 2015; 115: 1428–1434. PMID: 25772740
- 29.
Zamora E, Díez-López C, Lupón J, de Antonio M, Domingo M, Santesmases J, et al. Weight loss in obese patients with heart failure. J Am Heart Assoc 2016; 5: e002468. PMID: 27013541
- 30.
The Japanese Circulation Society. JCS Joint Working Groups for Guidelines for Diagnosis and Treatment of Cardiovascular Diseases (2009 Joint Working Groups Report). Guidelines for Treatment of Chronic Heart Failure (JCS 2010). http://www.j-circ.or.jp/guideline/pdf/JCS2010_matsuzaki_h.pdf [Accessed in February 2020]
- 31.
Miyazawa Y. Nutritional Assessment. In: Clinical Nutrition Management. Nissoken Publishing, 2010.
- 32.
Kamiya K, Masuda T, Matsue Y, Inomata T, Hamazaki N, Matsuzawa R, et al. Complementary role of arm circumference to body mass index in risk stratification in heart failure. JACC Heart Fail 2016; 4: 265–273. PMID: 26874391
- 33.
McManus ML, Churchwell KB, Strange K. Regulation of cell volume in health and disease. N Engl J Med 1995; 333: 1260–1266. PMID: 7566004
- 34.
Sergi G, Lupoli L, Volpato S, Bertani R, Coin A, Perissinotto E, et al. Body fluid distribution in elderly subjects with congestive heart failure. Ann Clin Lab Sci 2004; 34: 416–422. PMID: 15648783
- 35.
Ohashi Y, Otani T, Tai R, Tanaka Y, Sakai K, Aikawa A. Assessment of body composition using dry mass index and ratio of total body water to estimated volume based on bioelectrical impedance analysis in chronic kidney disease patients. J Ren Nutr 2013; 23: 28–36. PMID: 22406124
- 36.
Lee Y, Kwon O, Shin CS, Lee SM. Use of bioelectrical impedance analysis for the assessment of nutritional status in critically ill patients. Clin Nutr Res 2015; 4: 32–40. PMID: 25713790
- 37.
Sergi G, Lupoli L, Enzi G, Volpato S, Perissinotto E, Bertani R, et al. Reliability of bioelectrical impedance methods in detecting body fluids in elderly patients with congestive heart failure. Scand J Clin Lab Invest 2006; 66: 19–30. PMID: 16464784
- 38.
Uthamalingam S, Kandala J, Daley M, Patvardhan E, Capodilupo R, Moore SA, et al. Serum albumin and mortality in acutely decompensated heart failure. Am Heart J 2010; 160: 1149–1155. PMID: 21146671
- 39.
Lourenço P, Silva S, Friões F, Alvelos M, Amorim M, Couto M, et al. Low prealbumin is strongly associated with adverse outcome in heart failure. Heart 2014; 100: 1780–1785. PMID: 24986895
- 40.
Suzuki N, Kida K, Suzuki K, Harada T, Akashi YJ. Assessment of transthyretin combined with mini nutritional assessment on admission provides useful prognostic information in patients with acute decompensated heart failure. Int Heart J 2015; 56: 226–233. PMID: 25740580
- 41.
Rauchhaus M, Clark AL, Doehner W, Davos C, Bolger A, Sharma R, et al. The relationship between cholesterol and survival in patients with chronic heart failure. J Am Coll Cardiol 2003; 42: 1933–1940. PMID: 14662255
- 42.
The Japanese Society of Dysphagia Rehabilitation, JSDR Dysphagia Diet Committee. Dysphagia Assessment [brief version] 2015. https://www.jsdr.or.jp/wp-content/uploads/file/doc/assessment2015-announce.pdf [Accessed in February 2020]
- 43.
Rubenstein LZ, Harker JO, Salvà A, Guigoz Y, Vellas B. Screening for undernutrition in geriatric practice: Developing the short-form mini-nutritional assessment (MNA-SF). J Gerontol A Biol Sci Med Sci 2001; 56: M366–M372. PMID: 11382797
- 44.
Sargento L, Satendra M, Almeida I, Sousa C, Gomes S, Salazar F, et al. Nutritional status of geriatric outpatients with systolic heart failure and its prognostic value regarding death or hospitalization, biomarkers and quality of life. J Nutr Health Aging 2013; 17: 300–304. PMID: 23538649
- 45.
Tsai AC, Shih CL. A population-specific Mini-Nutritional Assessment can effectively grade the nutritional status of stroke rehabilitation patients in Taiwan. J Clin Nurs 2009; 18: 82–88. PMID: 18665877
- 46.
Bouillanne O, Morineau G, Dupont C, Coulombel I, Vincent JP, Nicolis I, et al. Geriatric Nutritional Risk Index: A new index for evaluating at-risk elderly medical patients. Am J Clin Nutr 2005; 82: 777–783. PMID: 16210706
- 47.
Kinugasa Y, Kato M, Sugihara S, Hirai M, Yamada K, Yanagihara K, et al. Geriatric nutritional risk index predicts functional dependency and mortality in patients with heart failure with preserved ejection fraction. Circ J 2013; 77: 705–711. PMID: 23182759
- 48.
Ignacio de Ulíbarri J, González-Madroño A, de Villar NG, González P, González B, Mancha A, et al. CONUT: A tool for controlling nutritional status. First validation in a hospital population. Nutr Hosp 2005; 20: 38–45. PMID: 15762418
- 49.
Alvares-da-Silva MR, Reverbel da Silveira T. Comparison between handgrip strength, subjective global assessment, and prognostic nutritional index in assessing malnutrition and predicting clinical outcome in cirrhotic outpatients. Nutrition 2005; 21: 113–117. PMID: 15723736
- 50.
Shirakabe A, Hata N, Kobayashi N, Okazaki H, Matsushita M, Shibata Y, et al. The prognostic impact of malnutrition in patients with severely decompensated acute heart failure, as assessed using the Prognostic Nutritional Index (PNI) and Controlling Nutritional Status (CONUT) score. Heart Vessels 2018; 33: 134–144. PMID: 28803356
- 51.
Detsky AS, McLaughlin JR, Baker JP, Johnston N, Whittaker S, Mendelson RA, et al. What is subjective global assessment of nutritional status? JPEN J Parenter Enteral Nutr 1987; 11: 8–13. PMID: 3820522
- 52.
Kondrup J, Rasmussen HH, Hamberg O, Stanga Z. Ad Hoc ESPEN Working Group. Nutritional risk screening (NRS 2002): A new method based on an analysis of controlled clinical trials. Clin Nutr 2003; 22: 321–336. PMID: 12765673
- 53.
Akinde DO. Comparative estimation of inevitable endogenous ileal flow of amino acids in Pekin ducks under varying dietary or physiological conditions and their significance to nutritional requirements for amino acids. Poult Sci 2017; 96: 3616–3625. PMID: 28938771
- 54.
Ziegler TR. L-glutamine-enriched parenteral nutrition in catabolic patients. Clin Nutr 1993; 12: 65–66. PMID: 16843288
- 55.
The Committee on Japanese Guidelines for Nutrition Support Therapy in the Adult and Pediatric Critically Ill Patients, Japanese Society of Intensive Care Medicine. Japanese Guidelines for Nutrition Support Therapy in the Adult and Pediatric Critically Ill Patients. J Jpn Soc Intensive Care Med 2016; 23: 185–281.
- 56.
Cooper RM, Bilash I, Zubek JP. The effect of age on taste sensitivity. J Gerontol 1959; 14: 56–58. PMID: 13620904
- 57.
The Japanese Circulation Society and the Japanese Heart Failure Society Joint Working Group. JCS 2017/JHFS 2017 Guideline on Diagnosis and Treatment. http://www.j-circ.or.jp/guideline/pdf/JCS2017_tsutsui_h.pdf (Accessed in February 2020)
- 58.
Saijo T. Basics of Enteral Feeding: Classification of Enteral Nutrition Products. Nutrition Care 2017; 10: 996–1000.
- 59.
Ohama O. Enteral Nutrition: Classification and Characteristics of Enteral Nutrition Products. In: Shimada S, et al, editors. Basic Practice of Parenteral and Enteral Nutrition. Elsevier Japan, 2003.
- 60.
Kodama H. Deficiency of some nutrients due to use of enteral and special milk formulas. No To Hattatsu 2014; 46: 5–9.
- 61.
Sato N, Ohtake K, Irahara T, Harima Y, Murata S, Mori T, et al. How do we select an enteral nutrition? J Jpn Soc Parenter Enteral Nutr 2015; 30: 665–668.
- 62.
Sacks FM, Svetkey LP, Vollmer WM, Appel LJ, Bray GA, Harsha D, et al. DASH-Sodium Collaborative Research Group. Effects on blood pressure of reduced dietary sodium and the Dietary Approaches to Stop Hypertension (DASH) diet. N Engl J Med 2001; 344: 3–10. PMID: 11136953
- 63.
Berger MM, Revelly JP, Cayeux MC, Chiolero RL. Enteral nutrition in critically ill patients with severe hemodynamic failure after cardiopulmonary bypass. Clin Nutr 2005; 24: 124–132. PMID: 15681110
- 64.
Berger MM, Berger-Gryllaki M, Wiesel PH, Revelly JP, Hurni M, Cayeux C, et al. Intestinal absorption in patients after cardiac surgery. Crit Care Med 2000; 28: 2217–2223. PMID: 10921543
- 65.
Leone M, Bechis C, Baumstarck K, Ouattara A, Collange O, Augustin P, et al. Outcome of acute mesenteric ischemia in the intensive care unit: A retrospective, multicenter study of 780 cases. Intensive Care Med 2015; 41: 667–676. PMID: 25731634
- 66.
McClave SA, Martindale RG, Vanek VW, McCarthy M, Roberts P, Taylor B, et al. A.S.P.E.N. Board of Directors. Guidelines for the Provision and Assessment of Nutrition Support Therapy in the Adult Critically Ill Patient: Society of Critical Care Medicine (SCCM) and American Society for Parenteral and Enteral Nutrition (A.S.P.E.N.). JPEN J Parenter Enteral Nutr 2009; 33: 277–316. PMID: 19398613
- 67.
Zannad F, Garcia AA, Anker SD, Armstrong PW, Calvo G, Cleland JG, et al. Clinical outcome endpoints in heart failure trials: A European Society of Cardiology Heart Failure Association consensus document. Eur J Heart Fail 2013; 15: 1082–1094. PMID: 23787718
- 68.
Koller M, Schütz T, Valentini L, Kopp I, Pichard C, Lochs H. Clinical Nutrition Guideline Group. Outcome models in clinical studies: Implications for designing and evaluating trials in clinical nutrition. Clin Nutr 2013; 32: 650–657. PMID: 23021606
- 69.
Ponikowski P, Voors AA, Anker SD, Bueno H, Cleland JG, Coats AJ, et al. Authors/Task Force Members. 2016 ESC Guidelines for the diagnosis and treatment of acute and chronic heart failure: The Task Force for the diagnosis and treatment of acute and chronic heart failure of the European Society of Cardiology (ESC). Developed with the special contribution of the Heart Failure Association (HFA) of the ESC. Eur J Heart Fail 2016; 18: 891–975. PMID: 27207191
- 70.
Yancy CW, Jessup M, Bozkurt B, Butler J, Casey DE, Colvin MM, et al. 2017 ACC/AHA/HFSA Focused Update of the 2013 ACCF/AHA Guideline for the Management of Heart Failure: A Report of the American College of Cardiology/American Heart Association Task Force on Clinical Practice Guidelines and the Heart Failure Society of America. Circulation 2017; 136: e137–e161. PMID: 28455343
- 71.
Milne AC, Avenell A, Potter J. Meta-analysis: Protein and energy supplementation in older people. Ann Intern Med 2006; 144: 37–48. PMID: 16389253
- 72.
Milne AC, Potter J, Vivanti A, Avenell A. Protein and energy supplementation in elderly people at risk from malnutrition. Cochrane Database Syst Rev 2009: CD003288. PMID: 19370584
- 73.
Cawood AL, Elia M, Stratton RJ. Systematic review and meta-analysis of the effects of high protein oral nutritional supplements. Ageing Res Rev 2012; 11: 278–296. PMID: 22212388
- 74.
Beck AM, Holst M, Rasmussen HH. Oral nutritional support of older (65 years+) medical and surgical patients after discharge from hospital: Systematic review and meta-analysis of randomized controlled trials. Clin Rehabil 2013; 27: 19–27. PMID: 22643726
- 75.
Bally MR, Blaser Yildirim PZ, Bounoure L, Gloy VL, Mueller B, Briel M, et al. Nutritional support and outcomes in malnourished medical inpatients: A systematic review and meta-analysis. JAMA Intern Med 2016; 176: 43–53. PMID: 26720894
- 76.
Cermak NM, Res PT, de Groot LC, Saris WH, van Loon LJ. Protein supplementation augments the adaptive response of skeletal muscle to resistance-type exercise training: A meta-analysis. Am J Clin Nutr 2012; 96: 1454–1464. PMID: 23134885
- 77.
Finger D, Goltz FR, Umpierre D, Meyer E, Rosa LH, Schneider CD. Effects of protein supplementation in older adults undergoing resistance training: A systematic review and meta-analysis. Sports Med 2015; 45: 245–255. PMID: 25355074
- 78.
Thomas DK, Quinn MA, Saunders DH, Greig CA. Protein supplementation does not significantly augment the effects of resistance exercise training in older adults: A systematic review. J Am Med Dir Assoc 2016; 17: 959.e1–959.e9. PMID: 27670605
- 79.
Beaudart C, Dawson A, Shaw SC, Harvey NC, Kanis JA, Binkley N, et al. IOF-ESCEO Sarcopenia Working Group. Nutrition and physical activity in the prevention and treatment of sarcopenia: Systematic review. Osteoporos Int 2017; 28: 1817–1833. PMID: 28251287
- 80.
Yoshimura Y, Wakabayashi H, Yamada M, Kim H, Harada A, Arai H. Interventions for treating sarcopenia: A systematic review and meta-analysis of randomized controlled studies. J Am Med Dir Assoc 2017; 18: 553.e1–553.e16. PMID: 28549707
- 81.
Stratton RJ, Hébuterne X, Elia M. A systematic review and meta-analysis of the impact of oral nutritional supplements on hospital readmissions. Ageing Res Rev 2013; 12: 884–897. PMID: 23891685
- 82.
Deutz NE, Matheson EM, Matarese LE, Luo M, Baggs GE, Nelson JL, et al. NOURISH Study Group. Readmission and mortality in malnourished, older, hospitalized adults treated with a specialized oral nutritional supplement: A randomized clinical trial. Clin Nutr 2016; 35: 18–26. PMID: 26797412
- 83.
The Committee on the Guidelines for the Management of Sarcopenia. Guidelines for the Management of Sarcopenia 2017. Life Science Publishing, 2017.
- 84.
Kim H, Osuga Y, Fujino K, Aoki T, Kojima N. Nutritional interventions in sarcopenia. Journal of Japanese Association on Sarcopenia and Frailty 2017; 1: 38–47.
- 85.
Lichtenstein AH, Appel LJ, Brands M, Carnethon M, Daniels S, Franch HA, et al. American Heart Association Nutrition Committee. Diet and lifestyle recommendations revision 2006: A scientific statement from the American Heart Association Nutrition Committee. Circulation 2006; 114: 82–96. PMID: 16785338
- 86.
Brown IJ, Tzoulaki I, Candeias V, Elliott P. Salt intakes around the world: Implications for public health. Int J Epidemiol 2009; 38: 791–813. PMID: 19351697
- 87.
Ministry of Health, Labour and Welfare. The National Health and Nutrition Survey in Japan, 2015. https://www.mhlw.go.jp/bunya/kenkou/eiyou/dl/h27-houkoku.pdf [Accessed in February 2020]
- 88.
O’Donnell M, Mente A, Rangarajan S, McQueen MJ, Wang X, Liu L, et al. PURE Investigators. Urinary sodium and potassium excretion, mortality, and cardiovascular events. N Engl J Med 2014; 371: 612–623. PMID: 25119607
- 89.
The Japanese Circulation Society. Joint Working Groups for Guidelines for Diagnosis and Treatment of Cardiovascular Diseases (2010 Joint Working Groups Report). Guidelines for Secondary Prevention of Myocardial Infarction (JCS 2011). http://www.j-circ.or.jp/guideline/pdf/JCS2011_ogawah_h.pdf [Accessed in February 2020]
- 90.
The Japanese Society of Hypertension Committee for Guidelines for the Management of Hypertension. Guidelines for the Management of Hypertension 2014. Life Science Publishing, 2014.
- 91.
World Health Organization. Obesity: Preventing and managing the global epidemic. Report of a WHO Consultation (WHO Technical Report Series 894). http://www.who.int/nutrition/publications/obesity/WHO_TRS_894/en/ [Accessed in February 2020]
- 92.
Cui R, Iso H, Toyoshima H, Date C, Yamamoto A, Kikuchi S, et al. JACC Study Group. Body mass index and mortality from cardiovascular disease among Japanese men and women: The JACC study. Stroke 2005; 36: 1377–1382. PMID: 15920029
- 93.
Chei CL, Iso H, Yamagishi K, Inoue M, Tsugane S. Body mass index and weight change since 20 years of age and risk of coronary heart disease among Japanese: The Japan Public Health Center-Based Study. Int J Obes (Lond) 2008; 32: 144–151. PMID: 17637701
- 94.
Mozaffarian D. Dietary and policy priorities for cardiovascular disease, diabetes, and obesity: A comprehensive review. Circulation 2016; 133: 187–225. PMID: 26746178
- 95.
Abete I, Romaguera D, Vieira AR, Lopez de Munain A, Norat T. Association between total, processed, red and white meat consumption and all-cause, CVD and IHD mortality: A meta-analysis of cohort studies. Br J Nutr 2014; 112: 762–775. PMID: 24932617
- 96.
Nagao M, Iso H, Yamagishi K, Date C, Tamakoshi A. Meat consumption in relation to mortality from cardiovascular disease among Japanese men and women. Eur J Clin Nutr 2012; 66: 687–693. PMID: 22333876
- 97.
Kaluza J, Akesson A, Wolk A. Processed and unprocessed red meat consumption and risk of heart failure: Prospective study of men. Circ Heart Fail 2014; 7: 552–557. PMID: 24926039
- 98.
Nakamura K, Nagata C, Oba S, Takatsuka N, Shimizu H. Fruit and vegetable intake and mortality from cardiovascular disease are inversely associated in Japanese women but not in men. J Nutr 2008; 138: 1129–1134. PMID: 18492845
- 99.
Nagura J, Iso H, Watanabe Y, Maruyama K, Date C, Toyoshima H, et al. JACC Study Group. Fruit, vegetable and bean intake and mortality from cardiovascular disease among Japanese men and women: The JACC Study. Br J Nutr 2009; 102: 285–292. PMID: 19138438
- 100.
Takachi R, Inoue M, Ishihara J, Kurahashi N, Iwasaki M, Sasazuki S, et al. JPHC Study Group. Fruit and vegetable intake and risk of total cancer and cardiovascular disease: Japan Public Health Center-Based Prospective Study. Am J Epidemiol 2008; 167: 59–70. PMID: 17928402
- 101.
Aune D, Keum N, Giovannucci E, Fadnes LT, Boffetta P, Greenwood DC, et al. Whole grain consumption and risk of cardiovascular disease, cancer, and all cause and cause specific mortality: Systematic review and dose-response meta-analysis of prospective studies. BMJ 2016; 353: i2716. PMID: 27301975
- 102.
Mayhew AJ, de Souza RJ, Meyre D, Anand SS, Mente A. A systematic review and meta-analysis of nut consumption and incident risk of CVD and all-cause mortality. Br J Nutr 2016; 115: 212–225. PMID: 26548503
- 103.
Larsson SC, Wallin A, Wolk A. Alcohol consumption and risk of heart failure: Meta-analysis of 13 prospective studies. Clin Nutr 2018; 37: 1247–1251. PMID: 28554815
- 104.
Estruch R, Ros E, Salas-Salvadó J, Covas MI, Corella D, Arós F, et al. PREDIMED Study Investigators. Primary prevention of cardiovascular disease with a Mediterranean diet supplemented with extra-virgin olive oil or nuts. N Engl J Med 2018; 378: e34. PMID: 29897866
- 105.
National Institutes of Health. Description of the DASH Eating Plan. https://www.nhlbi.nih.gov/health-topics/dash-eating-plan [Accessed in February 2020]
- 106.
Van Horn L, Carson JA, Appel LJ, Burke LE, Economos C, Karmally W, et al. American Heart Association Nutrition Committee of the Council on Lifestyle and Cardiometabolic Health; Council on Cardiovascular Disease in the Young; Council on Cardiovascular and Stroke Nursing; Council on Clinical Cardiology; and Stroke Council. Recommended dietary pattern to achieve adherence to the American Heart Association/American College of Cardiology (AHA/ACC) Guidelines: A scientific statement from the American Heart Association. Circulation 2016; 134: e505–e529. PMID: 27789558
- 107.
Japan Atherosclerosis Society. Guidelines for Prevention of Atherosclerotic Cardiovascular Diseases 2017. Japan Atherosclerosis Society, 2017.
- 108.
Maruyama K, Iso H, Date C, Kikuchi S, Watanabe Y, Wada Y, et al. JACC Study Group. Dietary patterns and risk of cardiovascular deaths among middle-aged Japanese: JACC Study. Nutr Metab Cardiovasc Dis 2013; 23: 519–527. PMID: 22410388
- 109.
Shimazu T, Kuriyama S, Hozawa A, Ohmori K, Sato Y, Nakaya N, et al. Dietary patterns and cardiovascular disease mortality in Japan: A prospective cohort study. Int J Epidemiol 2007; 36: 600–609. PMID: 17317693
- 110.
Ministry of Agriculture, Forestry and Fisheries. Forestry and Fisheries. Japanese Food Guide Spinning Top. http://www.maff.go.jp/j/balance_guide/ [Accessed in February 2020]
- 111.
Kurotani K, Akter S, Kashino I, Goto A, Mizoue T, Noda M, et al. Japan Public Health Center based Prospective Study Group. Quality of diet and mortality among Japanese men and women: Japan Public Health Center based prospective study. BMJ 2016; 352: i1209. PMID: 27005903
- 112.
Eshak ES, Iso H, Mizoue T, Inoue M, Noda M, Tsugane S. Soft drink, 100% fruit juice, and vegetable juice intakes and risk of diabetes mellitus. Clin Nutr 2013; 32: 300–308. PMID: 22917499
- 113.
Huang M, Quddus A, Stinson L, Shikany JM, Howard BV, Kutob RM, et al. Artificially sweetened beverages, sugar-sweetened beverages, plain water, and incident diabetes mellitus in postmenopausal women: The prospective Women’s Health Initiative observational study. Am J Clin Nutr 2017; 106: 614–622. PMID: 28659294
- 114.
Ministry of Agriculture, Forestry and Fisheries. Information on trans-fatty acid. http://www.maff.go.jp/j/syouan/seisaku/trans_fat/ [Accessed in March 2020]
- 115.
Chowdhury R, Warnakula S, Kunutsor S, Crowe F, Ward HA, Johnson L, et al. Association of dietary, circulating, and supplement fatty acids with coronary risk: A systematic review and meta-analysis. Ann Intern Med 2014; 160: 398–406. PMID: 24723079
- 116.
de Souza RJ, Mente A, Maroleanu A, Cozma AI, Ha V, Kishibe T, et al. Intake of saturated and trans unsaturated fatty acids and risk of all cause mortality, cardiovascular disease, and type 2 diabetes: Systematic review and meta-analysis of observational studies. BMJ 2015; 351: h3978. PMID: 26268692
- 117.
Sacks FM, Lichtenstein AH, Wu JHY, Appel LJ, Creager MA, Kris-Etherton PM, et al. American Heart Association. Dietary fats and cardiovascular disease: A presidential advisory from the American Heart Association. Circulation 2017; 136: e1–e23. PMID: 28620111
- 118.
Schwingshackl L, Hoffmann G. Monounsaturated fatty acids, olive oil and health status: A systematic review and meta-analysis of cohort studies. Lipids Health Dis 2014; 13: 154. PMID: 25274026
- 119.
Ramsden CE, Zamora D, Leelarthaepin B, Majchrzak-Hong SF, Faurot KR, Suchindran CM, et al. Use of dietary linoleic acid for secondary prevention of coronary heart disease and death: Evaluation of recovered data from the Sydney Diet Heart Study and updated meta-analysis. BMJ 2013; 346: e8707. PMID: 23386268
- 120.
Ramsden CE, Zamora D, Majchrzak-Hong S, Faurot KR, Broste SK, Frantz RP, et al. Re-evaluation of the traditional diet-heart hypothesis: Analysis of recovered data from Minnesota Coronary Experiment (1968–73). BMJ 2016; 353: i1246. PMID: 27071971
- 121.
Yokoyama M, Origasa H, Matsuzaki M, Matsuzawa Y, Saito Y, Ishikawa Y, et al. Japan EPA lipid intervention study (JELIS) Investigators. Effects of eicosapentaenoic acid on major coronary events in hypercholesterolaemic patients (JELIS): A randomised open-label, blinded endpoint analysis. Lancet 2007; 369: 1090–1098. PMID: 17398308
- 122.
Siscovick DS, Barringer TA, Fretts AM, Wu JH, Lichtenstein AH, Costello RB, et al. American Heart Association Nutrition Committee of the Council on Lifestyle and Cardiometabolic Health; Council on Epidemiology and Prevention; Council on Cardiovascular Disease in the Young; Council on Cardiovascular and Stroke Nursing; and Council on Clinical Cardiology. Omega-3 polyunsaturated fatty acid (fish oil) supplementation and the prevention of clinical cardiovascular disease: A science advisory from the American Heart Association. Circulation 2017; 135: e867–e884. PMID: 28289069
- 123.
Yamagishi K, Iso H, Kokubo Y, Saito I, Yatsuya H, Ishihara J, et al. JPHC Study Group. Dietary intake of saturated fatty acids and incident stroke and coronary heart disease in Japanese communities: The JPHC Study. Eur Heart J 2013; 34: 1225–1232. PMID: 23404536
- 124.
Wu JH, Mozaffarian D. ω-3 fatty acids, atherosclerosis progression and cardiovascular outcomes in recent trials: New pieces in a complex puzzle. Heart 2014; 100: 530–533. PMID: 24459289
- 125.
Watanabe Y, Saito I, Henmi I, Yoshimura K, Maruyama K, Yamauchi K, et al. Skipping breakfast is correlated with obesity. J Rural Med 2014; 9: 51–58. PMID: 25648986
- 126.
Uemura M, Yatsuya H, Hilawe EH, Li Y, Wang C, Chiang C, et al. Breakfast skipping is positively associated with incidence of type 2 diabetes mellitus: Evidence from the Aichi Workers’ Cohort Study. J Epidemiol 2015; 25: 351–358. PMID: 25787236
- 127.
Kubota Y, Iso H, Sawada N, Tsugane S. JPHC Study Group. Association of breakfast intake with incident stroke and coronary heart disease: The Japan Public Health Center-Based Study. Stroke 2016; 47: 477–481. PMID: 26732562
- 128.
Kutsuma A, Nakajima K, Suwa K. Potential association between breakfast skipping and concomitant late-night-dinner eating with metabolic syndrome and proteinuria in the Japanese population. Scientifica (Cairo) 2014; 2014: 253581. PMID: 24982814
- 129.
Harvey SE, Parrott F, Harrison DA, Bear DE, Segaran E, Beale R, et al. CALORIES Trial Investigators. Trial of the route of early nutritional support in critically ill adults. N Engl J Med 2014; 371: 1673–1684. PMID: 25271389
- 130.
Simpson F, Doig GS. Parenteral vs. enteral nutrition in the critically ill patient: A meta-analysis of trials using the intention to treat principle. Intensive Care Med 2005; 31: 12–23. PMID: 15592814
- 131.
Peter JV, Moran JL, Phillips-Hughes J. A metaanalysis of treatment outcomes of early enteral versus early parenteral nutrition in hospitalized patients. Crit Care Med 2005; 33: 213–220. PMID: 15644672
- 132.
Louie BE, Noseworthy T, Hailey D, Gramlich LM, Jacobs P, Warnock GL. 2004 MacLean-Mueller prize enteral or parenteral nutrition for severe pancreatitis: A randomized controlled trial and health technology assessment. Can J Surg 2005; 48: 298–306. PMID: 16149365
- 133.
Thibault R, Pichard C, Wernerman J, Bendjelid K. Cardiogenic shock and nutrition: Safe? Intensive Care Med 2011; 37: 35–45. PMID: 21086113
- 134.
Casaer MP, Mesotten D, Hermans G, Wouters PJ, Schetz M, Meyfroidt G, et al. Early versus late parenteral nutrition in critically ill adults. N Engl J Med 2011; 365: 506–517. PMID: 21714640
- 135.
Doig GS, Simpson F, Sweetman EA, Finfer SR, Cooper DJ, Heighes PT, et al. Early PN Investigators of the ANZICS Clinical Trials Group. Early parenteral nutrition in critically ill patients with short-term relative contraindications to early enteral nutrition: A randomized controlled trial. JAMA 2013; 309: 2130–2138. PMID: 23689848
- 136.
Poehlman ET, Scheffers J, Gottlieb SS, Fisher ML, Vaitekevicius P. Increased resting metabolic rate in patients with congestive heart failure. Ann Intern Med 1994; 121: 860–862. PMID: 7772113
- 137.
Wooley JA. Indirect calorimetry: Applications in practice. Respir Care Clin N Am 2006; 12: 619–633. PMID: 17150435
- 138.
Walker RN, Heuberger RA. Predictive equations for energy needs for the critically ill. Respir Care 2009; 54: 509–521. PMID: 19327188
- 139.
Arabi YM, Tamim HM, Dhar GS, Al-Dawood A, Al-Sultan M, Sakkijha MH, et al. Permissive underfeeding and intensive insulin therapy in critically ill patients: A randomized controlled trial. Am J Clin Nutr 2011; 93: 569–577. PMID: 21270385
- 140.
Rice TW, Mogan S, Hays MA, Bernard GR, Jensen GL, Wheeler AP. Randomized trial of initial trophic versus full-energy enteral nutrition in mechanically ventilated patients with acute respiratory failure. Crit Care Med 2011; 39: 967–974. PMID: 21242788
- 141.
Zusman O, Theilla M, Cohen J, Kagan I, Bendavid I, Singer P. Resting energy expenditure, calorie and protein consumption in critically ill patients: A retrospective cohort study. Crit Care 2016; 20: 367. PMID: 27832823
- 142.
Larsson J, Lennmarken C, Mårtensson J, Sandstedt S, Vinnars E. Nitrogen requirements in severely injured patients. Br J Surg 1990; 77: 413–416. PMID: 2111195
- 143.
Tamura T, Yatabe T, Yokoyama M, Nishimura M. Current status of studies about optimal composition of amino acid and protein for ICU patients: Systematic review. Anaesth Intensive Care 2014; 42: 806–807. PMID: 25342421
- 144.
Aliti GB, Rabelo ER, Clausell N, Rohde LE, Biolo A, Beck-da-Silva L. Aggressive fluid and sodium restriction in acute decompensated heart failure: A randomized clinical trial. JAMA Intern Med 2013; 173: 1058–1064. PMID: 23689381
- 145.
Drakulovic MB, Torres A, Bauer TT, Nicolas JM, Nogué S, Ferrer M. Supine body position as a risk factor for nosocomial pneumonia in mechanically ventilated patients: A randomised trial. Lancet 1999; 354: 1851–1858. PMID: 10584721
- 146.
The Japanese Respiratory Society. NPPV (Noninvasive Positive Pressure Ventilation) Guidelines (Second revised edition). Nankodo, 2015.
- 147.
McClave SA, Lukan JK, Stefater JA, Lowen CC, Looney SW, Matheson PJ, et al. Poor validity of residual volumes as a marker for risk of aspiration in critically ill patients. Crit Care Med 2005; 33: 324–330. PMID: 15699835
- 148.
Metheny NA, Schallom L, Oliver DA, Clouse RE. Gastric residual volume and aspiration in critically ill patients receiving gastric feedings. Am J Crit Care 2008; 17: 512–520. PMID: 18978236
- 149.
Martindale RG, McClave SA, Vanek VW, McCarthy M, Roberts P, Taylor B, et al. American College of Critical Care Medicine. Guidelines for the provision and assessment of nutrition support therapy in the adult critically ill patient: Society of Critical Care Medicine and American Society for Parenteral and Enteral Nutrition: Executive Summary. Crit Care Med 2009; 37: 1757–1761. PMID: 19373044
- 150.
Dhaliwal R, Cahill N, Lemieux M, Heyland DK. The Canadian critical care nutrition guidelines in 2013: An update on current recommendations and implementation strategies. Nutr Clin Pract 2014; 29: 29–43. PMID: 24297678
- 151.
MacLeod JB, Lefton J, Houghton D, Roland C, Doherty J, Cohn SM, et al. Prospective randomized control trial of intermittent versus continuous gastric feeds for critically ill trauma patients. J Trauma 2007; 63: 57–61. PMID: 17622869
- 152.
Hiebert JM, Brown A, Anderson RG, Halfacre S, Rodeheaver GT, Edlich RF. Comparison of continuous vs intermittent tube feedings in adult burn patients. JPEN J Parenter Enteral Nutr 1981; 5: 73–75. PMID: 6785478
- 153.
Ciocon JO, Galindo-Ciocon DJ, Tiessen C, Galindo D. Continuous compared with intermittent tube feeding in the elderly. JPEN J Parenter Enteral Nutr 1992; 16: 525–528. PMID: 1494208
- 154.
Heyland DK, Dhaliwal R, Suchner U, Berger MM. Antioxidant nutrients: A systematic review of trace elements and vitamins in the critically ill patient. Intensive Care Med 2005; 31: 327–337. PMID: 15605227
- 155.
Miró Ò, Estruch R, Martín-Sánchez FJ, Gil V, Jacob J, Herrero-Puente P, et al. ICA-SEMES Research Group. Adherence to Mediterranean diet and all cause mortality after an episode of acute heart failure: Results of the MEDIT-AHF study. JACC Heart Fail 2018; 6: 52–62. PMID: 29226819
- 156.
Ministry of Health, Labour and Welfare. Study group on the system to provide medical care for patients with stroke, heart disease, and other cardiovascular diseases. The system to provide medical care for patients with stroke, heart disease, and other cardiovascular diseases (July 2017). http://www.mhlw.go.jp/file/05-Shingikai-10901000-Kenkoukyoku-Soumuka/0000173149.pdf [Accessed in February 2020]
- 157.
Cruz-Jentoft AJ, Baeyens JP, Bauer JM, Boirie Y, Cederholm T, Landi F, et al. European Working Group on Sarcopenia in Older People. Sarcopenia: European consensus on definition and diagnosis: Report of the European Working Group on Sarcopenia in Older People. Age Ageing 2010; 39: 412–423. PMID: 20392703
- 158.
Fülster S, Tacke M, Sandek A, Ebner N, Tschöpe C, Doehner W, et al. Muscle wasting in patients with chronic heart failure: Results from the studies investigating co-morbidities aggravating heart failure (SICA-HF). Eur Heart J 2013; 34: 512–519. PMID: 23178647
- 159.
Chaudhry SI, McAvay G, Chen S, Whitson H, Newman AB, Krumholz HM, et al. Risk factors for hospital admission among older persons with newly diagnosed heart failure: Findings from the Cardiovascular Health Study. J Am Coll Cardiol 2013; 61: 635–642. PMID: 23391194
- 160.
Khan H, Kalogeropoulos AP, Georgiopoulou VV, Newman AB, Harris TB, Rodondi N, et al. Frailty and risk for heart failure in older adults: The health, aging, and body composition study. Am Heart J 2013; 166: 887–894. PMID: 24176445
- 161.
von Haehling S, Anker SD. Prevalence, incidence and clinical impact of cachexia: Facts and numbers-update 2014. J Cachexia Sarcopenia Muscle 2014; 5: 261–263. PMID: 25384990
- 162.
von Haehling S, Lainscak M, Springer J, Anker SD. Cardiac cachexia: A systematic overview. Pharmacol Ther 2009; 121: 227–252. PMID: 19061914
- 163.
von Haehling S, Anker SD. Treatment of cachexia: An overview of recent developments. J Am Med Dir Assoc 2014; 15: 866–872. PMID: 25455531
- 164.
Weir JB. New methods for calculating metabolic rate with special reference to protein metabolism. J Physiol 1949; 109: 1–9. PMID: 15394301
- 165.
Harris JA, Benedict FG. A biometric study of human basal metabolism. Proc Natl Acad Sci U S A 1918; 4: 370–373. PMID: 16576330
- 166.
Japanese Society for Parenteral & Enteral Nutrition. Parenteral and Enteral Nutrition Guidelines for healthcare providers. Nankodo, 2000.
- 167.
Krenitsky J. Adjusted body weight, pro: Evidence to support the use of adjusted body weight in calculating calorie requirements. Nutr Clin Pract 2005; 20: 468–473. PMID: 16207686
- 168.
ASPEN Bored Director. Nutrition Support Dietetics Core Curriculum. Second Edition. ASPEN, 1993.
- 169.
Deutz NE, Bauer JM, Barazzoni R, Biolo G, Boirie Y, Bosy-Westphal A, et al. Protein intake and exercise for optimal muscle function with aging: Recommendations from the ESPEN Expert Group. Clin Nutr 2014; 33: 929–936. PMID: 24814383
- 170.
Bauer J, Biolo G, Cederholm T, Cesari M, Cruz-Jentoft AJ, Morley JE, et al. Evidence-based recommendations for optimal dietary protein intake in older people: A position paper from the PROT-AGE Study Group. J Am Med Dir Assoc 2013; 14: 542–559. PMID: 23867520
- 171.
Japanese Society of Nephrology. Dietary Recommendations for Chronic Kidney Diseases, 2014. https://cdn.jsn.or.jp/guideline/pdf/CKD-Dietaryrecommendations2014.pdf [Accessed in February 2020]
- 172.
Aquilani R, Viglio S, Iadarola P, Opasich C, Testa A, Dioguardi FS, et al. Oral amino acid supplements improve exercise capacities in elderly patients with chronic heart failure. Am J Cardiol 2008; 101 Suppl: 104E–110E. PMID: 18514618
- 173.
Scognamiglio R, Negut C, Palisi M, Dioguardi FS, Coccato M, Iliceto S. Effects of oral amino acid supplements on cardiac function and remodeling in patients with type 2 diabetes with mild-to-moderate left ventricular dysfunction. Am J Cardiol 2008; 101 Suppl: 111E–115E. PMID: 18514620
- 174.
Yancy CW, Jessup M, Bozkurt B, Butler J, Casey DE, Drazner MH, et al. American College of Cardiology Foundation. 2013 ACCF/AHA guideline for the management of heart failure: A report of the American College of Cardiology Foundation/American Heart Association Task Force on Practice Guidelines. J Am Coll Cardiol 2013; 62: e147–e239. PMID: 23747642
- 175.
Witte KK, Clark AL, Cleland JG. Chronic heart failure and micronutrients. J Am Coll Cardiol 2001; 37: 1765–1774. PMID: 11401109
- 176.
Morisco C, Trimarco B, Condorelli M. Effect of coenzyme Q10 therapy in patients with congestive heart failure: A long-term multicenter randomized study. Clin Investig 1993; 71 Suppl: S134–S136. PMID: 8241697
- 177.
Flanagan JL, Simmons PA, Vehige J, Willcox MD, Garrett Q. Role of carnitine in disease. Nutr Metab (Lond) 2010; 7: 30. PMID: 20398344
- 178.
Beyranvand MR, Khalafi MK, Roshan VD, Choobineh S, Parsa SA, Piranfar MA. Effect of taurine supplementation on exercise capacity of patients with heart failure. J Cardiol 2011; 57: 333–337. PMID: 21334852
- 179.
Ghatak A, Brar MJ, Agarwal A, Goel N, Rastogi AK, Vaish AK, et al. Oxy free radical system in heart failure and therapeutic role of oral vitamin E. Int J Cardiol 1996; 57: 119–127. PMID: 9013263
- 180.
Genth-Zotz S, Zotz R, Geil S, Voigtländer T, Meyer J, Darius H. Recombinant growth hormone therapy in patients with ischemic cardiomyopathy: Effects on hemodynamics, left ventricular function, and cardiopulmonary exercise capacity. Circulation 1999; 99: 18–21. PMID: 9884373
- 181.
Moruzzi P, Doria E, Agostoni PG, Capacchione V, Sganzerla P. Usefulness of L-thyroxine to improve cardiac and exercise performance in idiopathic dilated cardiomyopathy. Am J Cardiol 1994; 73: 374–378. PMID: 8109552
- 182.
The Japanese Circulation Society. Joint Working Groups for Guidelines for Diagnosis and Treatment of Cardiovascular Diseases (2011 Joint Working Groups Report). Guidelines for Rehabilitation in Patients with Cardiovascular Disease (JCS 2012). http://www.j-circ.or.jp/guideline/pdf/JCS2012_nohara_h.pdf [Accessed in February 2020]
- 183.
Boirie Y. Physiopathological mechanism of sarcopenia. J Nutr Health Aging 2009; 13: 717–723. PMID: 19657556
- 184.
Kim HK, Suzuki T, Saito K, Yoshida H, Kobayashi H, Kato H, et al. Effects of exercise and amino acid supplementation on body composition and physical function in community-dwelling elderly Japanese sarcopenic women: A randomized controlled trial. J Am Geriatr Soc 2012; 60: 16–23. PMID: 22142410
- 185.
Doukky R, Avery E, Mangla A, Collado FM, Ibrahim Z, Poulin MF, et al. Impact of dietary sodium restriction on heart failure outcomes. JACC Heart Fail 2016; 4: 24–35. PMID: 26738949
- 186.
Abshire M, Xu J, Baptiste D, Almansa JR, Xu J, Cummings A, et al. Nutritional interventions in heart failure: A systematic review of the literature. J Card Fail 2015; 21: 989–999. PMID: 26525961
- 187.
The Japanese Heart Failure Society Guidelines Committee. Chapter VII Guidelines for end-of-life care for older patients with heart failure. In: Statement on treatment of older patients with heart failure. 2016: 51–55. http://www.asas.or.jp/jhfs/pdf/Statement_HeartFailurel.pdf [Accessed in February 2020]
- 188.
Badia T, Formiga F, Ferrer A, Sanz H, Hurtos L, Pujol R. Multifactorial assessment and targeted intervention in nutritional status among the older adults: A randomized controlled trial: The Octabaix study. BMC Geriatr 2015; 15: 45. PMID: 25887312
- 189.
Ministry of Health, Labour and Welfare. Promotion of team medical care (Report of a conference on the promotion of team medical care) (March 19, 2010). http://www.mhlw.go.jp/shingi/2010/03/dl/s0319-9a.pdf [Accessed in February 2020]
- 190.
Takamasu T. Skill mix in the care of children with allergies (multidisciplinary cooperation). The Japanese Journal of Pediatric Intractable Asthma and Allergic Diseases 2015; 13: 199–202.
- 191.
Hamaguchi S, Kinugawa S, Tsuchihashi-Makaya M, Goto D, Yamada S, Yokoshiki H, et al. Characteristics, management, and outcomes for patients during hospitalization due to worsening heart failure-A report from the Japanese Cardiac Registry of Heart Failure in Cardiology (JCARE-CARD). J Cardiol 2013; 62: 95–101. PMID: 23672787
- 192.
Ministry of Health, Labour and Welfare, Health Policy Bureau. Promotion of team medical care with cooperation and collaboration of medical staff (Announcement No.0430-1 by the MHLW Health Policy Bureau, April 30, 2010). http://www.mhlw.go.jp/shingi/2010/05/dl/s0512-6h.pdf [Accessed in February 2020]
- 193.
McAlister FA, Stewart S, Ferrua S, McMurray JJ. Multidisciplinary strategies for the management of heart failure patients at high risk for admission: A systematic review of randomized trials. J Am Coll Cardiol 2004; 44: 810–819. PMID: 15312864
- 194.
Donovan HS, Ward SE, Song MK, Heidrich SM, Gunnarsdottir S, Phillips CM. An update on the representational approach to patient education. J Nurs Scholarsh 2007; 39: 259–265. PMID: 17760800
- 195.
Tsuchihashi T, Kai H, Kusaka M, Kawamura M, Matsuura H, Miura K, et al. Assessment and application of salt intake in the management of hypertension. In: Salt Reduction Committee, Japanese Society of Hypertension, editor. Report of the Salt Reduction Committee, Japanese Society of Hypertension 2012. The Japanese Society of Hypertension, 2012: 39–50.
- 196.
Muscaritoli M, Anker SD, Argilés J, Aversa Z, Bauer JM, Biolo G, et al. Consensus definition of sarcopenia, cachexia and pre-cachexia: Joint document elaborated by Special Interest Groups (SIG) “cachexia-anorexia in chronic wasting diseases” and “nutrition in geriatrics”. Clin Nutr 2010; 29: 154–159. PMID: 20060626
- 197.
Andreae C, Strömberg A, Sawatzky R, Årestedt K. Psychometric evaluation of two appetite questionnaires in patients with heart failure. J Card Fail 2015; 21: 954–958. PMID: 26497759
- 198.
Kodama H, Itakura H, Omori H, Sasaki M, Sando K, Takamura T, et al. Practice Guideline for Zinc Deficiency. J Jpn Soc Clin Nutr 2016; 38: 104–148.
- 199.
Puts MTE, Toubasi S, Andrew MK, Ashe MC, Ploeg J, Atkinson E, et al. Interventions to prevent or reduce the level of frailty in community-dwelling older adults: A scoping review of the literature and international policies. Age Ageing 2017; 46: 383–392. PMID: 28064173
- 200.
Frost R, Belk C, Jovicic A, Ricciardi F, Kharicha K, Gardner B, et al. Health promotion interventions for community-dwelling older people with mild or pre-frailty: A systematic review and meta-analysis. BMC Geriatr 2017; 17: 157. PMID: 28728570
- 201.
Volpi E, Kobayashi H, Sheffield-Moore M, Mittendorfer B, Wolfe RR. Essential amino acids are primarily responsible for the amino acid stimulation of muscle protein anabolism in healthy elderly adults. Am J Clin Nutr 2003; 78: 250–258. PMID: 12885705
- 202.
Katsanos CS, Kobayashi H, Sheffield-Moore M, Aarsland A, Wolfe RR. A high proportion of leucine is required for optimal stimulation of the rate of muscle protein synthesis by essential amino acids in the elderly. Am J Physiol Endocrinol Metab 2006; 291: E381–E387. PMID: 16507602
- 203.
Paddon-Jones D, Rasmussen BB. Dietary protein recommendations and the prevention of sarcopenia. Curr Opin Clin Nutr Metab Care 2009; 12: 86–90. PMID: 19057193
- 204.
Houston DK, Nicklas BJ, Ding J, Harris TB, Tylavsky FA, Newman AB, et al. Health ABC Study. Dietary protein intake is associated with lean mass change in older, community-dwelling adults: The Health, Aging, and Body Composition (Health ABC) Study. Am J Clin Nutr 2008; 87: 150–155. PMID: 18175749
- 205.
Bartali B, Frongillo EA, Stipanuk MH, Bandinelli S, Salvini S, Palli D, et al. Protein intake and muscle strength in older persons: Does inflammation matter? J Am Geriatr Soc 2012; 60: 480–484. PMID: 22283208
- 206.
Beasley JM, LaCroix AZ, Neuhouser ML, Huang Y, Tinker L, Woods N, et al. Protein intake and incident frailty in the Women’s Health Initiative observational study. J Am Geriatr Soc 2010; 58: 1063–1071. PMID: 20487071