論文ID: CJ-20-0703
論文ID: CJ-20-0703
Vaccines are well-known therapies for infectious disease and cancer; however, recently, we and others have developed vaccines for other chronic diseases, such as hypertension, diabetes and dyslipidemia. Although we have many treatment options for hypertension, including angiotensin II type 1 receptor blockers, calcium-channel blockers, and diuretics, a substantial portion of the hypertensive population has uncontrolled blood pressure due to poor medication adherence. When these vaccines are established in the future as therapeutic options for chronic diseases, their administration regimen, such as several times per year, will replace daily medication use. Thus, therapeutic vaccines might be a novel option to control the progression of cardiovascular diseases. Importantly, regarding the development of vaccines against self-antigens (i.e., angiotensin II), the vaccine should efficiently induce a blocking antibody response against the self-antigen without provoking cytotoxic T cells. Therefore, to address the safety and efficiency of therapeutic vaccines, we have developed an original B-cell vaccine to induce antibody production and used carrier proteins, which include exogenous T-cell epitopes through the major histocompatibility complex. In this review, we will introduce the challenges in developing therapeutic vaccines for chronic diseases and describe the therapeutic potential for cardiovascular diseases.
Vaccines are common preventive medicines against infectious diseases and have recently been developed as therapies against hypertension, dyslipidemia, Alzheimer’s disease, cancer, and inflammatory diseases by targeting self-antigens.1–7 If the efficacy and safety of vaccines is equivalent to that of medication, vaccines may be an alternative to daily medication for lifestyle diseases. Here, we describe our concept of therapeutic vaccines and summarize the current knowledge of vaccines for cardiovascular diseases (CVD).
Vaccines are commonly used as preventive medicine for infectious diseases, such as those caused by viruses or bacteria. These vaccines mimic the immune response against pathogens and induce the activation of T cells and antibody production. Namely, coactivation of innate immunity and antigen presentation induces T-cell activation. By contrast, therapeutic vaccines target a self-antigen (i.e., angiotensin (Ang) II). Importantly, vaccines against self-antigens should efficiently induce a blocking antibody response against the self-antigen without provoking cytotoxic T cells.8 Thus, the immune reaction against self-antigens must be regulated by blocking the activation of self-reactive T cells, but self-reactive B cells should still be active and be provoked by efficient T-cell activation (Figure 1).9
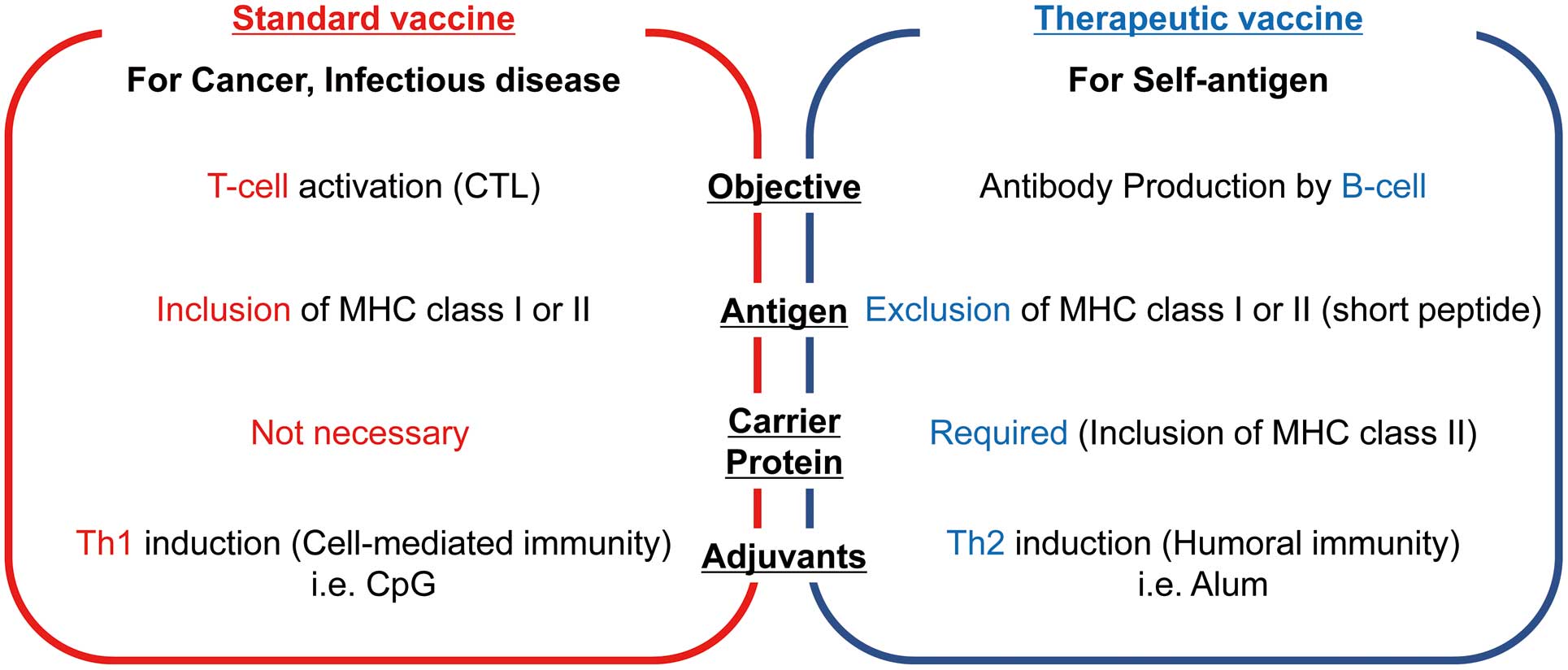
Comparison of standard and therapeutic vaccines. The goal of standard vaccines targeting cancer or infectious diseases is activation of cytotoxic T cells. Their antigens must include major histocompatibility complex (MHC) class I or II. Consequently, the antigens present a T-cell epitope through the MHC and, therefore, carrier proteins (exogenous T-cell epitopes) are not required. These vaccines favor the use of adjuvants that activate the Th1 pathway, which involves the production of interferon-γ. By contrast, the goal of therapeutic vaccines targeting chronic diseases is the induction of antibodies without cytotoxic T cells. Therefore, antigens should not include a T-cell epitope, and a carrier is required to provide the foreign T-cell epitope. Adjuvants that activate the Th2 pathway are preferable for therapeutic vaccines. CTL, cytotoxic T lymphocyte.
In the human immune tolerance system, the major mechanism of immune tolerance is T-cell tolerance, which includes central and peripheral tolerance. Central tolerance is known as “negative selection”. During the development of T cells in the thymus, the T cells carrying self-peptides on the major histocompatibility complex (MHC) are removed. Following central tolerance, peripheral tolerance, known as “anergy”, is the second branch of immunological tolerance. In particular, during the interaction of T cells and antigen-presenting cells (APCs), T cells cannot be activated without the interaction of CD28-B7.10 To activate B cells to produce antibodies, CD-4-positive cells are required for their differentiation into plasma and memory cells. Due to immune tolerance, self-reactive B cells cannot function without stimulation of CD-4-positive cells (Figure 2). Therefore, as we cannot include T-cell epitopes in antigens, peptide antigens are commonly used in combination with foreign T-cell epitopes as carrier proteins (Figure 1).9

Immune T-cell tolerance can be classified as central and peripheral tolerance. Central tolerance, called negative selection, inhibits the release of self-reactive T cells from the thymus. Peripheral tolerance inactivates T cells by inducing anergy. Cotreatment with adjuvants in vaccines effectively induces the CD28-B7 interaction through activation of innate immunity. APC, antigen-presenting cell.
Based on the immune system, we designed a therapeutic vaccine for Ang II (self-antigen) (Figure 3). We conjugated keyhole limpet hemocyanin (KLH), which is a standard carrier protein that is immunogenic and contains a strong T-cell epitope,11 and Ang II using glutaraldehyde. After immunization, APCs phagocytose KLH and the self-antigen and present a KLH-derived T-cell epitope to T cells through the MHC. In particular, it is important to avoid presentation of Ang II itself through the MHC. Next, T cells recognize the antigen through the T-cell epitope and are activated. However, as mentioned before, T cells cannot activate without the interaction of CD28-B7 from APCs due to anergy. Therefore, cotreatment with adjuvants effectively induces CD28-B7 interaction through the activation of innate immunity. Thus, the combination of Ang II-KLH and adjuvants induces the proliferation and differentiation of T cells specific to Ang II-KLH. Ang II-specific B cells internalize Ang II-KLH and present T-cell epitopes to the T cells. After that, B cells differentiate into plasmacytes and produce antibodies with the assistance of T cells. We confirmed T-cell activation not by Ang II but KLH from T-cell proliferation and enzyme-linked immunospot assays.12 Furthermore, we did not observe a “booster effect” of the antibody following Ang II infusion. Importantly, we commonly select short antigen peptides without including a T-cell epitope. Because MHC class I and II epitopes usually consist of 8–10 and 10–20 amino acids, respectively, short peptides with fewer than 8 amino acids are preferred as antigens from a safety point of view (Figure 1).13 Consequently, antibodies targeting Ang II can be produced successfully and safely.
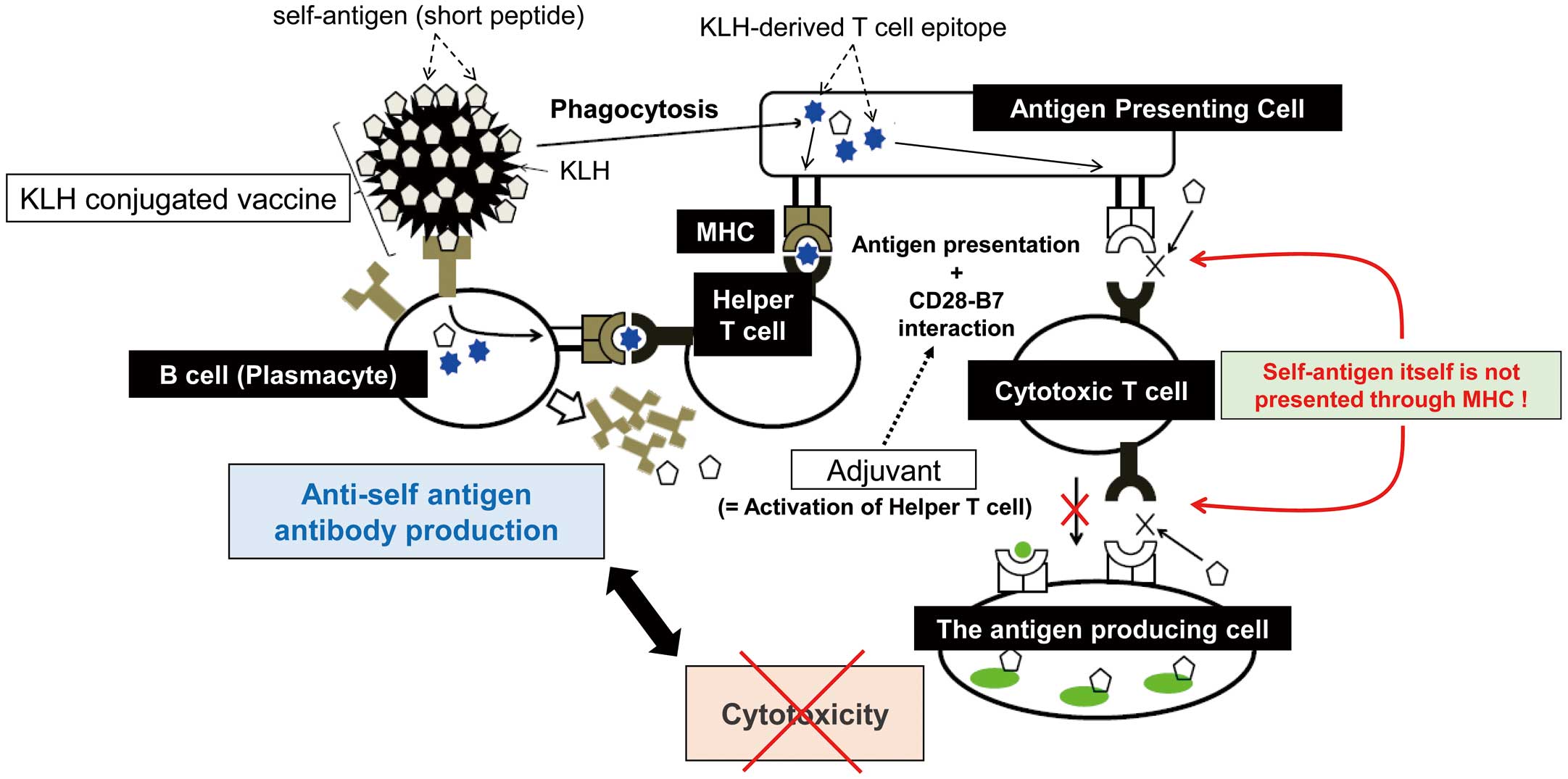
Schematic of therapeutic vaccines against self-antigens. Antigen-presenting cells phagocytose antigen-carrier protein conjugates and present a T-cell epitope of the carrier proteins to T cells through the major histocompatibility complex (MHC). Cotreatment with adjuvants effectively induces the CD28-B7 interaction through activation of innate immunity. Thus, T cells recognize the epitope through T-cell receptors and become activated. B cells recognizing the target antigen differentiate into plasmacytes and proliferate with the help of activated T cells. Consequently, B cells produce antibodies targeting the antigen. As the target antigen does not include a T-cell epitope, cytotoxic T cells are not activated for the target antigen and do not attack the antigen-producing cells. KLH, keyhole limpet hemocyanin.
In this section, we describe the history of developments in vaccines against hypertension. The preclinical investigations are summarized in Table 1.
| Target / Design of vaccine | Antibody titer | Blood pressure | Year | Reference no. |
|---|---|---|---|---|
| Renin | ||||
| KLH-conjugate | Increased | Significant decrease in SHRs | 2013 | 18 |
| Ang I | ||||
| TT-conjugate | Increased | Significant decrease in rats with Ang I infusion | 2000 | 19 |
| KLH-conjugate/TT-conjugate | Increased | Significant decrease in rats with Ang I infusion | 2003 | 21 |
| Ang II | ||||
| VLP-conjugate | Increased | Significant decrease in SHRs | 2007 | 22 |
| KLH-conjugate | Increased | Significant decrease in mice with Ang II infusion | 2013 | 12 |
| DNA vaccine | Increased | Significant decrease in SHRs | 2015 | 23 |
| AT1R | ||||
| KLH-conjugate | Increased | Significant decrease in SHRs | 2012 | 32 |
| VLP-conjugate | Increased | Significant decrease in SHRs | 2013 | 28 |
| VLP-conjugate | Increased | Significant decrease in rats with streptozotocin-induced diabetes | 2016 | 33 |
| L-type calcium-channel | ||||
| VLP-conjugate | Increased | Significant decrease in mice with Ang II infusion/SHRs | 2019 | 42 |
Ang, angiotensin; AT1R, angiotensin type 1 receptor; KLH, keyhole limpet hemocyanin; SHR, spontaneously hypertensive rat; TT, tetanus toxoid; VLP, virus-like particle.
The history of vaccines for hypertension can be traced back to the 1950s.14,15 The first investigation reported the efficacy of vaccines targeting a renin.16,17 Although renin vaccines contributed to a reduction in blood pressure (BP) in animal models, an immunological renal disease characterized by the presence of immunoglobulin and macrophage infiltration colocalizing with renin was observed at autopsy, which could be a severe hazardous risk of a renin vaccine. However, Qiu et al recently reported the efficacy of a KLH-conjugated renin vaccine for reducing high BP without an autoimmune response.18 Importantly, they designed the target peptide with only 7–10 amino acids, which is shorter than the T-cell epitope, but was not the case in the primitive vaccines. As mentioned before, it is important to inhibit the activation of cytotoxic T cells, and this is the one of the major causes of discrepancy regarding the autoimmune responses.
Angiotensin IAn Ang I vaccine conjugated with KLH also reduced BP in animal models,19,20 but did not reduce BP in a randomized double-blind placebo-controlled clinical study, even though the anti-Ang I antibody titer was increased (Table 2).19,21
| Target / Design of vaccine |
Phase/sample size | Antibody titer |
Blood pressure | Safety evaluation |
Year | Reference no. |
|---|---|---|---|---|---|---|
| Ang I | ||||||
| KLH-conjugate (PMD3117) |
Phase 1/ 50 healthy men |
Increased | No change | No problems | 2003 | 19 |
| KLH-conjugate (PMD3117) |
Phase 2/ 27 hypertensive patients |
Increased | No change | No problems | 2004 | 21 |
| Ang II | ||||||
| VLP-conjugate with alum (AngQb) |
Phase 1/ 16 healthy men |
Increased | No change | No problems | 2006 | 22 |
| VLP-conjugate with alum (AngQb) |
Phase 2/ 72 hypertensive patients |
Increased | Significant decrease only in high-dose vaccine group |
No problems | 2008 | 27 |
| DNA vaccine (AGMG0201) |
Phase 1, 2a/ 36 hypertensive patients |
Ongoing | Ongoing | Ongoing | Ongoing | – |
Ang, angiotensin; KLH, keyhole limpet hemocyanin; VLP, virus-like particle.
Ambühl et al designed an Ang II vaccine (named AngQb-Cyt006), which was conjugated to a virus-like peptide (VLP).22 The VLP was derived from the coat protein of bacteriophage Qb and behaves as a carrier protein. The systolic BP was significantly lower in vaccinated spontaneous hypertensive rats (SHRs) than in those not vaccinated (159±2 vs. 180±5 mmHg) after 3 injections. In addition, the BP was almost equivalent to that of rats treated with ramipril. As mentioned before, we also investigated the efficacy of an Ang II-KLH vaccine.12 Following Ang II infusion, the systolic BP was significantly lower in immunized mice than in those without immunization. We also designed a DNA vaccine against Ang II.23 Briefly, we inserted the DNA sequence corresponding to Ang II into the B-cell epitope of the hepatitis B core (HBc), which functions as a carrier protein,24 in a plasmid construct. It allowed the display of Ang II on the surface of the sphere formed via self-assembly. Consequently, T cells were activated by HBc instead of Ang II. The antibody response against Ang II was sustained for at least 6 months after 3 injections. The systolic BP was consistently lower in vaccinated SHRs. Furthermore, survival time was significantly prolonged in the vaccination group.23
An Ang II vaccine showed not only BP reduction but also organ-protective effects. Ang II-induced perivascular fibrosis in the heart was significantly attenuated in immunized mice or rats.12,23 Watanabe et al reported that an Ang II vaccine was effective for preventing heart failure in a rat model of myocardial infarction with permanent left anterior descending artery ligation.25 They found that immunized mice exhibited attenuation of cardiac dysfunction, inflammation, and fibrosis. Wakayama et al investigated whether pre-exposure to Ang II vaccination leads to cerebroprotective effects after middle cerebral artery occlusion in rats.26 They found that the infarction volume was not reduced in the plasma low-titer group but was reduced in the high-titer group. Anti-Ang II antibodies led to a reduction in infarct volume and induced the penetration of anti-Ang II antibodies in the ischemic hemisphere, with suppressed expression of AT1R mRNA.
Several clinical trials of vaccines against Ang II have already been performed (Table 2). In a placebo-controlled randomized phase I trial, 12 healthy volunteers were injected with AngQb-Cyt006 (100 µg), which had been effective in reducing BP in SHRs.22 The titer of anti-Ang II antibodies was raised in all subjects. Subsequently, a double-blind, randomized, placebo-controlled phase IIa trial was performed to investigate the effect of AngQb-Cyt006 on ambulatory BP after 3 injections at weeks 0, 4, and 12 in 72 patients with mild-to-moderate hypertension.27 In the 300 µg (high dose, n=24) group, the mean ambulatory daytime systolic BP was significantly lower than that in the placebo group (n=24) at week 14 (−9.0 mmHg). Furthermore, the high dose reduced the early morning BP surge compared with that with a placebo (−25 mmHg for systolic, −13 mmHg for diastolic). By contrast, there was no significant difference between the 100 µg (low dose) and placebo groups. Although 5 serious adverse events (2 in the low-dose group, 2 in the high-dose group, and 1 in the placebo group) were reported, none were deemed to be treatment related. Most side effects were mild, transient reactions at the injection site. Regrettably, this study is the first and last report to reveal the efficacy of vaccines against Ang II for a reduction in BP at this point in time.
A clinical double-blind, randomized, placebo-controlled phase I/IIa trial using the Ang II DNA vaccine (AGMG0201) started in 2018 in Australia (Table 2). The purpose of this study is to evaluate the safety, tolerability, pharmacokinetic and pharmacodynamic effects, and exploratory efficacy of AGMG0201 administered in patients with moderate essential hypertension at 2 dose levels. As AGMG0201 possibly induces hypotension, rescue therapy will be applied. For example, salt and norepinephrine administration will be performed for emergencies. Other side effects may occur at the injection sites, as with other commercially available vaccines. This trial is ongoing and may provide us with important information regarding the development of therapeutic vaccines.
Angiotensin II Type 1 ReceptorThe efficacy of a therapeutic vaccine against the AT1R has been also investigated. Chen et al designed a peptide (named AT1R-001) derived from AT1R conjugated with VLPs.28 AT1R-001 was derived from the AT1R second extracellular loop, with the sequence C-A-F-H-Y-E-S-Q, which is a ligand-binding site.29 They reported that BP was significantly decreased in SHRs with immunization compared with rats without immunization. They also revealed that the anti-AT1R-001 antibodies specifically bound to AT1R, using western blot and immunostaining, and inhibited Ca2+-dependent signal transduction events, including extracellular signal-regulated kinase 1/2 phosphorylation. Interestingly, the anti-AT1R-001 antibodies and Ang II did not competitively bind to AT1R, whereas Ang II receptor blockers (ARBs) exhibited competitive inhibition against AT1R.30,31 Azegami et al reported that an AT1R vaccination prevented NG-nitro-L-arginine methyl ester-induced nephropathy.32 They presented the finding of reduced glomerular injury in SHRs with immunization. Ding et al also revealed that vaccination prevented streptozotocin-induced diabetic nephropathy.33 Furthermore, the vaccination also prevented cardiac dysfunction and remodeling after myocardial infarction,34 and progression of calcium phosphate-induced abdominal aortic aneurysm.35 Therefore, the anti-AT1R second extracellular loop antibodies may have organ-protective effects, as with ARB.
Several investigations have reported interesting characteristics of autoantibodies against AT1R. Wallukat et al36 investigated 25 pre-eclamptic patients in whom they detected autoantibodies against AT1R extracellular second loop. Immunoglobulin from these patients with pre-eclampsia induced an increase in the beat number of cultured neonatal rat cardiomyocytes. Furthermore, they analyzed the possible epitopes on the AT1R extracellular second loop using affinity column-purified materials. Consequently, only the amino acid sequence A-F-H-Y-E-S-Q among the second loop residues abolished the increase in beat number. Dragun et al37 studied kidney transplant recipients who had refractory vascular rejection, and they also found autoantibodies against AT1R extracellular second loop in all patients with malignant hypertension and without anti-HLA antibodies. Interestingly, the autoantibodies induced phosphorylation of extracellular signal-regulated kinase 1/2, whereas, as mentioned before, the vaccination-induced antibodies inhibited phosphorylation. In addition, transfer of passive antibodies induced vasculopathy and hypertension in a rat kidney transplantation model.37 Moreover, multiple studies have reported detection of autoantibodies against AT1R in patients with primary aldosteronism, a major cause of secondary hypertension, using enzyme-linked immunosorbent assays.38–41 Piazza et al investigated the bioactivity of the autoantibodies. Aldosterone synthase expression in human adrenocortical carcinoma cells increased after stimulation with IgG purified from the sera of patients with aldosterone-producing adenoma, which is the major cause of primary aldosteronism.41
Therefore, autoantibodies against AT1R may activate AT1R, leading to hypertension or increasing aldosterone synthase expression. It is poorly understood whether autoantibodies are the cause or consequence of these pathologies. Although severe adverse effects of vaccines against AT1R were not reported in multiple investigations, we cannot deny that antibodies against the extracellular second loop induce not only blockade but also activation of AT1R (Figure 4). For clinical application, further investigations are needed.

Comparison of vaccination-induced antibodies and autoantibodies against angiotensin II type 1 receptor. Several studies have designed a target peptide derived from the AT1R second extracellular loop. The antibodies may inhibit the activity of AT1R. Consequently, blood pressure was decreased in animal models. Furthermore, organ-protective effects in the heart, kidney and vascular system were also observed. Multiple studies have revealed that autoantibodies against AT1R also target the AT1R second extracellular loop. However, autoantibodies may induce activation of AT1R, leading to hypertension, pre-eclampsia, or increased aldosterone synthase expression; the mechanisms of these differences are still poorly understood. AT1R, angiotensin II type 1 receptor; pERK, phosphorylation of extracellular signal-regulated kinase 1/2.
Wu et al reported the development of vaccines against the L-type calcium channel for treatment of hypertension in SHRs.42 The peptide (named CE12) was derived from the epitope of the third extracellular region of domain IV of the human L-type calcium channel, which plays an important role in allosteric modulation after binding to 1,4-dihydropyridines.43,44 They used a VLP as a carrier protein. The systolic BP was significantly decreased in vaccinated SHRs compared with that in rats without vaccination (199±4 vs. 212±3 mmHg) after 4 injections. In addition, a bivalent vaccine against CE12 and AT1R also decreased BP in SHRs and reduced the glomerular injury induced by NG-nitro-L-arginine methyl ester. A monoclonal antibody against CE specifically bound to the L-type calcium channels according to immunostaining and inhibited calcium-channel activation, as with nifedipine, in an electrophysiologic analysis. Furthermore, severe adverse events were not observed in these experiments.
For the prevention of CVD, it is important to treat chronic diseases, including diabetes mellitus (DM) and dyslipidemia, for a long time. Because dipeptidyl peptidase-4 (DDP-4) inhibitors are commonly used in patients with type 2 DM, we developed a therapeutic vaccine against DPP-4.45 Among several candidate epitopes within DPP4, one of them produced a significant decrease in plasma DPP4 activity and the glucose level was significantly lower than that of the control group. Furthermore, we confirmed that the DPP4 vaccine did not induce cell death using cell cytotoxicity-mediated processes.
Dyslipidemia is also a major risk factor for CVD. In particular, precise control of low-density lipoprotein cholesterol (LDL-C) is required for secondary prevention in patients with CVD. Proprotein convertase subtilisin/kexin type 9 (PCSK9) inhibitors, including monoclonal antibodies, are used in patients with heterozygous or homozygous familial hypercholesterolemia or statin-resistant atherosclerotic CVD.46,47 To tackle the issues of patient adherence and cost of treatment, we and others developed a therapeutic vaccine against PCSK9.7,48,49 Landlinger et al reported that a PCSK9 vaccine caused a significant reduction in total cholesterol and LDL-C levels in immunized mice. The levels of plasma inflammatory markers, including serum amyloid A, were also significantly reduced. Consequently, the vaccine resulted in a decrease in atherosclerotic lesion area and aortic inflammation.49 In our experiments, anti-PCSK9 antibody titers were maintained for up to 24 weeks after 3 injections. Furthermore, decreased plasma levels of total cholesterol, very low-density lipoprotein, and chylomicron were also maintained for up to 24 weeks. Thus, the PCSK9 vaccine could become a new option for long-acting treatment of dyslipidemia.
Recently, we developed a vaccine against CD153, which is one of the cell surface molecules in senescent T cells.50 Senescent T cells accumulate in visceral adipose tissues and produce proinflammatory and matrix-degrading molecules, which harm the surrounding nonsenescent cells.51–55 We confirmed that senescent T-cell accumulation was significantly reduced in visceral adipose tissue of CD153-vaccinated mice, accompanied by glucose tolerance and insulin resistance in high-fat diet-induced obese C57Bl/6J mice.
Therapeutic vaccines against self-antigens, including Ang II, DPP4 and PCSK9, use Th2-directed adjuvants (i.e., alum) to induce mouse IgG1 (human IgG2) antibody production without a cytotoxic immune response. The efficacy of vaccines against self-antigens can be explained by the fact that the antibodies have neutralizing molecular functions. By contrast, we aimed to prevent the accumulation of senescent cells by inducing apoptosis/cell death. Therefore, we selected the CpG adjuvant, which is commonly used as a Th1-directing adjuvant. The mouse IgG2 (human IgG1) antibody accompanied by component-dependent cytotoxicity was produced mainly not in CD153-alum but CD153-CpG-vaccinated mice and successfully reduced the number of senescent T cells (Figure 5). The novel concept of reducing “harmful cells” through vaccines may be an optional tool for senolytic therapy, including for CVD, as aging is associated with senescent cell accumulation.56 We hope that this novel tool to remove harmful cells will revolutionize the fight against CVD in the future.

Concept of therapeutic vaccines with complement-dependent cytotoxicity. (A) Complement-dependent cytotoxicity. (B) Comparison of IgG subtype functions. CpG, which is a Th1-directed adjuvant, or alum, which is a Th2-directed adjuvant, induces the production of mouse IgG2 (human IgG1) or mouse IgG1 (human IgG2) in B cells, respectively. Mouse IgG2 or IgG1 has cytotoxic effects or neutralizing molecular functions, respectively. MAC, membrane attack complex.
The present review summarizes the current knowledge of therapeutic vaccines for chronic diseases. As described in the Introduction, the development of novel therapies independent of patient adherence may contribute to overcoming lifestyle diseases. Therapeutic vaccines may have potential as alternative treatments. Although there are some issues to be solved before clinical application, we believe that therapeutic vaccines will contribute to improving human health.
R.M. is a founder and consultant of AnGes, and received a lecture fee from AnGes. The Department of Health Development and Medicine is an endowed department supported by Daicel, AnGes and Funpep and also has research funding from AnGes, Funpep and Bayel. The Department of Clinical Gene Therapy is an endowed department supported by Novartis, AnGes, Shionogi, Boeringher, Fancl, Saisei Mirai Clinics, Rohto and Funpep, and also has research funding from AnGes.