Abstract
Background:
There is limited evidence for pulmonary arterial hypertension (PAH)-targeted therapy in patients with pulmonary hypertension associated with respiratory disease (R-PH). Therefore, we conducted a multicenter prospective study of patients with R-PH to examine real-world characteristics of responders by evaluating demographics, treatment backgrounds, and prognosis.
Methods and Results:
Among the 281 patients with R-PH included in this study, there was a treatment-naïve cohort of 183 patients with normal pulmonary arterial wedge pressure and 1 of 4 major diseases (chronic obstructive pulmonary diseases, interstitial pneumonia [IP], IP with connective tissue disease, or combined pulmonary fibrosis with emphysema); 43% of patients had mild ventilatory impairment (MVI), whereas 52% had a severe form of PH. 68% received PAH-targeted therapies (mainly phosphodiesterase-5 inhibitors). Among patients with MVI, those treated initially (i.e., within 2 months of the first right heart catheterization) had better survival than patients not treated initially (3-year survival 70.6% vs. 34.2%; P=0.01); there was no significant difference in survival in the group with severe ventilatory impairment (49.6% vs. 32.1%; P=0.38). Responders to PAH-targeted therapy were more prevalent in the group with MVI.
Conclusions:
This first Japanese registry of R-PH showed that a high proportion of patients with MVI (PAH phenotype) had better survival if they received initial treatment with PAH-targeted therapies. Responders were predominant in the group with MVI.
In patients with respiratory disease, the frequency of pulmonary hypertension (PH) could increase with the degree of hypoxemia and ventilatory impairment, although there are some patients who have severe PH regardless of ventilatory impairment or hypoxemia.1
The use of pulmonary arterial hypertension (PAH)-targeted therapy has not been approved for PH associated with respiratory disease (R-PH), despite the existence of PAH phenotypes and the fact that it is permissible to treat these patients according to the algorithm for PAH.2
A study of severe chronic obstructive pulmonary disease (COPD)-PH in the ASPIRE (Assessing the Spectrum of Pulmonary hypertension Identified at a REferral centre) registry showed that there was no difference in survival with or without pulmonary vasodilators, although prognosis was significantly better in responders.3
In a retrospective study from Japan, patients with severe R-PH, defined as a mean pulmonary arterial pressure (mPAP) ≥35 mmHg, who took phosphodiesterase-5 inhibitors (PDE-5I) had a better prognosis during follow-up than those not taking these drugs.4
These findings suggested the presence of responders. We conducted the first prospective registry in Japan to determine the current status and prognosis of R-PH in respiratory and PH expert centers. We also investigated the frequency of the use of PAH-targeted therapies, including PDE-5I, and the real-world characteristics of patients who responded to these therapies.
Editorial p ????
Methods
Study Design
The Japan Respiratory PH Study (JRPHS) is the first Japanese observational and prospective registry for R-PH and was conducted between September 2013 and December 2017 at 21 specialized respiratory and PH centers. This study was registered with the University Hospital Medical Information Network (UMIN) Clinical Trials Registry (ID: UMIN000011541).
Subjects
We enrolled treatment-naïve and previously treated patients aged ≥18 years with R-PH who had mPAP ≥25 mmHg, confirmed by right heart catheterization (RHC). The underlying diseases were COPD, interstitial pneumonia (IP), IP with connective tissue disease (CTD-IP), combined pulmonary fibrosis and emphysema (CPFE), and PH associated with all other respiratory diseases. COPD was defined as a ratio of post-bronchodilator forced expiratory volume in 1 s (FEV1) to forced vital capacity (FVC) ≤0.7, which confirmed the presence of airflow limitation.5
IP, CPFE, and CTD-IP were diagnosed by individual physicians at the specialized respiratory centers, although the extent of fibrosis measured by computed tomography (CT) was not assessed. Idiopathic IP was classified according to the American Thoracic Society guidelines as either idiopathic pulmonary fibrosis, idiopathic non-specific pneumonia, or idiopathic unclassifiable IP.6
CTD-IP was diagnosed on the basis of the following diagnostic criteria: scleroderma, rheumatoid arthritis, dermatomyositis or polymyositis, mixed connective tissue disease, Sjögren’s syndrome, or systemic lupus erythematosus. For CPFE, the extent of emphysema was assessed visually (on a scale of 0–100%, with 5% increments) at each institution, although a standardized method was not used. The extent of emphysema ranged from 10% to 80%, with a median extent of 40%.
Measurements
The following parameters were recorded using an electric data capture (EDC) system: World Health Organization functional classification (WHO-FC), Brinkman index, pulmonary hemodynamics using the Swan-Ganz catheter method, which included pulmonary arterial pressure, pulmonary arterial wedge pressure (PAWP), cardiac output (CO; thermodilution method or indirect Fick method), cardiac index (CardI; calculated as CO divided by body surface area), and pulmonary vascular resistance (PVR; calculated as 80 × [mPAP − PAWP] / CO), 6-min walk distance (6MWD) and Borg score, respiratory function, diffusing capacity of the lung for carbon monoxide (DLCO), arterial blood gas test findings, percutaneous oxygen saturation (SPO2), electrocardiographic findings, laboratory findings (including serum creatinine, total bilirubin, uric acid, B-type natriuretic peptide [BNP], N-terminal pro BNP [NT-proBNP], thyroid function, and markers for connective tissue diseases), and IP treatment, including PAH-targeted therapies, anticoagulants, oxygen therapy, non-invasive positive pressure ventilation, diuretics, digitalis, angiotensin II receptor blockers, angiotensin-converting enzyme inhibitors, respiratory therapeutic agents, and immune suppressants.
In principle, follow-up examinations were conducted every 3–6 months and included adverse events, newly performed 6MWD, respiratory function, SPO2
or blood gas, pulmonary hemodynamics, biomarkers, and other test results.
Ethical Considerations
The registry was approved initially by the Ethics Committee at Chiba University School of Medicine (Approval no. 1569), and then by the institutional review boards of all the participating centers. All participants provided written informed consent to take part in the study, which was conducted in accordance with the Declaration of Helsinki.
Subcategories
Patients were divided into different groups: (1) those with PAWP ≤15 or >15 mmHg; (2) those with severe (mean PAP ≥35 mmHg or CardI <2.5L·min−1·m−2) or mild (mean PAP <35 mmHg and CardI ≥2.5L·min−1·m−2) R-PH;7
and (3) those with mild (percent predicted FVC [%FVC] ≥70% and percent predicted FEV1
[%FEV1] ≥60) or severe (%FVC <70 or %FEV1
<60) ventilatory impairment.2
Survival Analysis
Survival was calculated from the date of baseline RHC until the occurrence of either death (from all causes) or lung transplantation within 1,095 days or by December 31, 2017. Survival rates in the treatment-naïve cohort with a PAWP ≤15 mmHg were compared between those patients treated initially (defined as within 2 months from the date of baseline RHC) by PAH-targeted therapies and those not treated initially (late treated and untreated). Four patients that had previously been treated with beraprost alone were included in the treatment-naïve cohort because beraprost has not been approved for PAH in most countries other than Japan and is used mainly for Raynaud’s phenomenon.
Survival was assessed separately in patients with mild ventilatory impairment (PAH phenotype) and severe ventilatory impairment (pure Group 3 PH phenotype). In addition, survival was compared between patients with the severe form of PH who were treated initially with PAH-targeted therapies as well as PDE-5I as the first drug and patients who were not treated initially.
Analysis of Responders
Patient responses to PAH-targeted therapies were evaluated among the initially treated patients with 1 of the 4 major diseases and who were treatment naïve at baseline. Responders were defined as having either an improvement in WHO-FC, a decrease in PVR >15%, or an increase in 6MWD >15% at the first follow-up visit (mean [±SD] 332±274 days, median 258 days; n=88). The clinical characteristics and survival of responders were compared to those of non-responders.
Statistical Analysis
Data are expressed as the mean±SD for continuous variables and as the number and percentage for categorical variables. Background characteristics of the patients were compared across subgroups using Pearson’s Chi-squared test for categorical variables and 1-way analysis of variance or unpaired t-tests for continuous variables. Kaplan-Meier curves were constructed and survival was compared across subgroups using the log-rank test. Bonferroni correction was used to adjust for multiple comparisons. Univariate and multivariate Cox proportional hazard models were used to examine prognostic factors. As primary analyses, Cox proportional hazard regression analyses were conducted using the complete cases. As a sensitivity analysis to address missing data regarding PH severity, DLCO, and mild or severe ventilatory impairment, multiple imputation (n=25) was conducted with the Markov chain Monte Carlo method and Rubin’s rule8
was used to derive estimates for hazard ratios (HRs) and the standard error.
All tests were 2 tailed and P<0.05 was considered statistically significant. All statistical analyses were performed using SAS 9.4 (SAS Institute, Cary, NC, USA).
Results
Baseline Characteristics
In all, 281 patients were enrolled in the study. Mean patient age was 67±11 years. The major underlying diseases were IP (29%), COPD (21%), CTD-IP (19%), CPFE (13%), and others (other obstructive pulmonary disease, n=10; pulmonary emphysema other than COPD, 5; developmental lung diseases, 5; sleep-disordered breathing or alveolar hypoventilation disorders, 5; bronchiectasis, 3). The details of the subcategorized patients with IP and CTD-IP are given in
Supplementary Table 1. Most patients were categorized as WHO-FC Class III or IV (72.6%). Mean mPAP was 33±8 mmHg, with 43% of patients classified as having mild ventilatory impairment and 52% of patients classified as having a severe form of R-PH. At baseline, 69% of patients were receiving ambulatory oxygen therapy. Among the patients, 19% had received treatment before the baseline RHC, with 68% having used PAH-targeted therapy at some stage and 162 (58% of total; 85% of treated patients) having received PDE-5I; 40% of patients had received monotherapy (Table 1).
Table 1.
Baseline Characteristics of Patients With PH Associated With Respiratory Disease (n=281)
| Age (years) |
67±11 |
%DLCO (n=195) |
37±20 |
| No. females/males |
85/196 |
BNP (pg/mL; n=225) |
215±391 |
| Classification of disease |
6MWD (m; n=228) |
290±141 |
| COPD |
60 (21) |
Treatment |
| CPFE |
36 (13) |
Oxygen therapy |
194 (69) |
| CTD-IP |
53 (19) |
Diuretics (n=214) |
73 (34) |
| IP |
82 (29) |
Oral anticoagulation |
43 (15) |
| OtherA |
50 (18) |
PAH-targeted therapy at baseline |
54 (19) |
| WHO-FC I/II/III/IV (n) |
10/67/150/54 |
Monotherapy at baseline |
32 (11) |
| Pra (mmHg; n=278) |
6±4 |
Double therapy at baseline |
15 (5) |
| mPAP (mmHg) |
33±8 |
Triple therapy at baseline |
7 (3) |
| mPAP ≥35 mmHg |
86 (31) |
Prostanoid at baseline |
23 (8) |
| Severe form of PH (n=280) |
146 (52) |
Epoprostenol or treprostinil at
baseline |
2 (1) |
| PAWP (mmHg; n=278) |
10±5 |
ERA at baseline |
27 (10) |
| PAWP ≤15 mmHg (n=278) |
257 (93) |
PDE-5I at baseline |
33 (12) |
| CardI (L·min−1·m−2; n=280) |
2.9±0.9 |
Others at baseline |
4 (1) |
| PVR (dyn·s·cm−5; n=271) |
466±311 |
PAH-targeted therapy at any time |
191 (68) |
| PaO2 (torr) room air (n=183) |
64±13 |
Monotherapy at anytime |
111 (40) |
| PaCO2 (torr) room air (n=183) |
41±8 |
Double therapy at anytime |
55 (20) |
| %FVC (n=217) |
68±20 |
Triple therapy at anytime |
25 (9) |
| FEV1/FVC (%; n=229) |
70±22 |
Prostanoid at any time |
44 (16) |
| %FEV1 (n=235) |
66±25 |
ERA at any time |
83 (30) |
| Mild ventilatory impairment (n=235) |
100 (43) |
PDE-5I at any time |
162 (58) |
Unless indicated otherwise, data are expressed as the mean±SD or n (%). AOther obstructive pulmonary disease, n=10; pulmonary emphysema other than chronic obstructive pulmonary disease (COPD), 5; developmental lung diseases, 5; sleep-disordered breathing or alveolar hypoventilation disorders, 5; bronchiectasis, 3; pulmonary lymphoangiomiomatosis, 2. 6MWD, 6-min walk distance; BNP, B-type natriuretic peptide; CardI, cardiac index; CPFE, combined pulmonary fibrosis and emphysema; CTD-IP, interstitial pneumonia with connective tissue disease; %DLCO, percent predicted diffusing capacity of the lung for carbon monoxide; ERA, endothelin receptor antagonist; FEV1, forced expiratory volume in 1 s; %FEV1, percent predicted FEV1; FVC, forced vital capacity; %FVC, percent predicted FVC; IP, interstitial pneumonia; mPAP, mean pulmonary arterial pressure; PAH, pulmonary arterial hypertension; PAWP, pulmonary arterial wedge pressure; PDE-5I, phosphodiesterase-5 inhibitors; PH, pulmonary hypertension; Pra, right atrial pressure; PVR, pulmonary vascular resistance; WHO-FC, World Health Organization functional class.
In all, 115 patients died during follow-up. Overall 1-, 3-, and 5-year survival from the date of baseline RHC was 79.0%, 61.9%, and 54.3%, respectively (Supplementary Figure 1). The mean follow-up period was 682±362 days. The cause of death was PH or right heart failure (n=44), respiratory failure or progression or acute exacerbation of underlying disease (n=10), pneumonia or infection (n=9), lung cancer (n=3), others (n=9), and unknown (n=7).
Characteristics and Survival of Patients According to the 4 Major Underlying Diseases
The baseline characteristics of the 4 groups (n=215) with a normal PAWP are given in
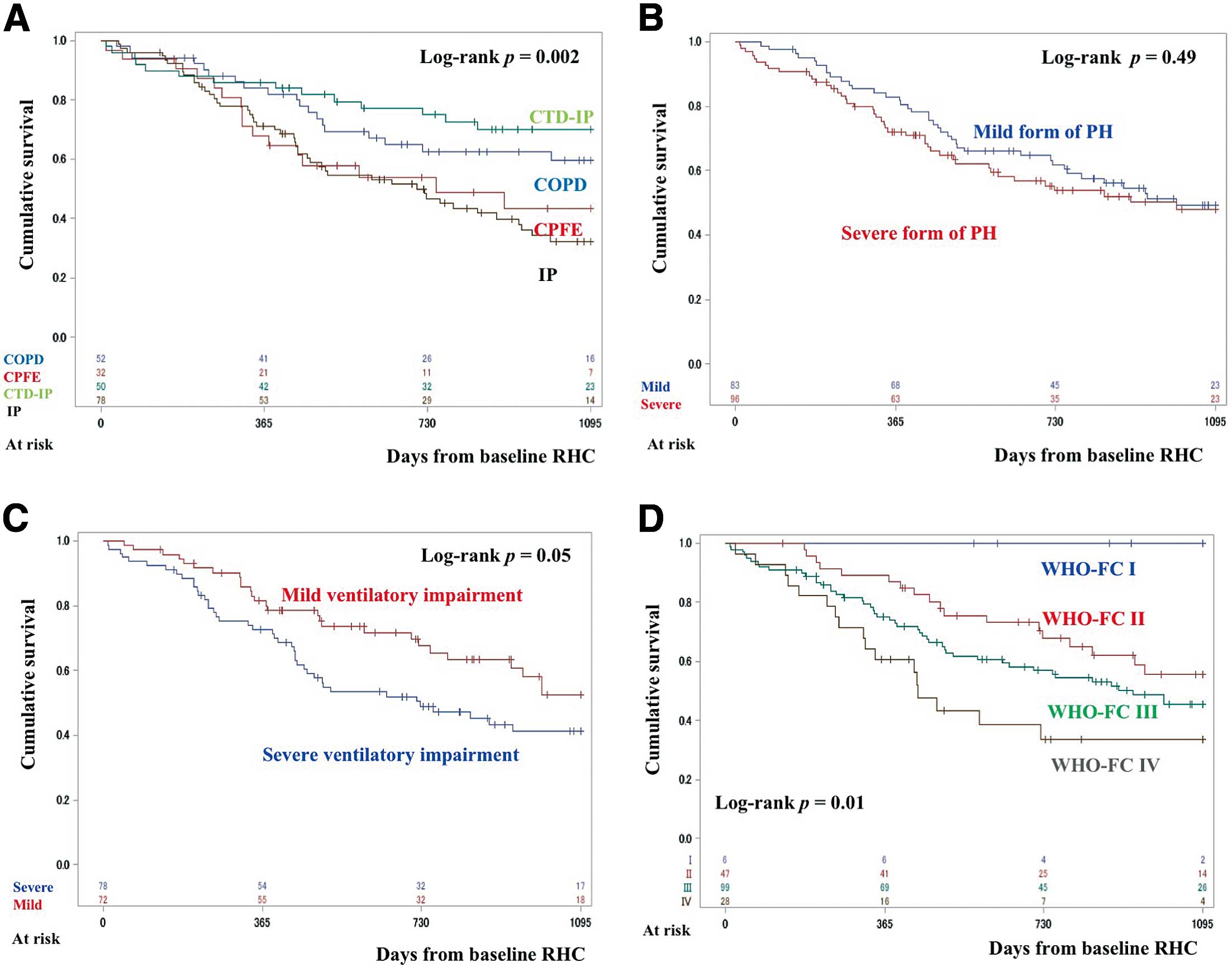
Table 2. CTD-IP predominantly occurred in females, whereas CPFE was more common in the group with mild ventilatory impairment. In all, 179 patients were classified as treatment naïve at baseline and 36 patients were classified as having received prior treatment. The patients with IP received combination therapies less often. Endothelin receptor antagonists (ERA) were used less often in the IP group. The 3-year survival rates were 59.5% for COPD, 43.4% for CPFE, 70.1% for CTD-IP, and 32.2% for IP (Figure 1A). Survival was significantly better in the CTD-IP than IP group (P=0.02). Details of the cause of death in patients with a normal PAWP grouped by the 4 major underlying diseases are given in
Supplementary Table 2. In COPD and CTD-IP, the leading cause of death was PH or right heart failure (57% and 42%, respectively). In contrast, these causes of deaths only accounted for 17% and 33% of deaths in the IP and CPFE groups, respectively. The major cause of death in the IP and CPFE groups was respiratory failure or progression or acute exacerbation of underlying disease (59% and 53%, respectively).
Table 2.
Baseline Characteristics of Patients With a Normal Pulmonary Arterial Wedge Pressure Categorized According to the 4 Major Underlying Diseases (n=215)
| |
COPD
(n=54) |
CPFE
(n=33) |
CTD-IP
(n=50) |
IP
(n=78) |
P value |
| Age (years) |
71±8 |
72±7 |
65±12 |
69±10 |
0.001 |
| Female sex |
3 (6) |
0 (0) |
28 (56) |
24 (31) |
<0.001 |
| WHO-FC III/IV |
39 (72) |
26 (79) |
41 (82) |
51 (65) |
0.18 |
| mPAP (mmHg) |
34±8 |
34±7 |
32±7 |
32±8 |
0.24 |
| CardI (L·min−1·m−2) |
2.8±0.8 |
2.6±1.0 |
2.9±0.9 |
2.8±0.7 |
0.44 |
| PVR (dyn·s·cm−5) |
513±336 |
488±244 |
501±325 |
410±251 |
0.18 |
| Severe form of PH |
31 (57) |
22 (67) |
25 (50) |
35 (45) |
0.19 |
| PaO2 (torr) room air |
63±15 |
59±10 |
66±15 |
63±14 |
0.44 |
| PaCO2 (torr) room air |
40±7 |
38±5 |
39±7 |
42±8 |
0.10 |
| %FVC |
84±17 |
80±15 |
62±18 |
64±17 |
<0.001 |
| %FEV1 |
59±26 |
84±20 |
69±24 |
73±21 |
<0.001 |
| Mild ventilatory impairment |
20 (46) |
22 (82) |
16 (42) |
26 (37) |
0.001 |
| No. patients with data |
44 |
27 |
38 |
70 |
|
| %DLCO |
36±22 |
29±11 |
35±21 |
35±13 |
0.33 |
| BNP (pg/mL) |
291±589 |
278±365 |
188±368 |
161±274 |
0.33 |
| 6MWD (m) |
343±118 |
242±125 |
284±150 |
316±150 |
0.03 |
| PAH-targeted therapy |
| At baseline |
10 (18) |
3 (9) |
16 (32) |
7 (9) |
0.004 |
| At any time |
40 (74) |
25 (76) |
37 (74) |
44 (56) |
0.06 |
| Monotherapy at any time |
20 (37) |
14 (42) |
17 (34) |
35 (45) |
|
| Double therapy at anytime |
13 (24) |
7 (21) |
13 (26) |
8 (10) |
0.02 |
| Triple therapy at any time |
7 (13) |
4 (12) |
7 (14) |
1 (1) |
|
| ERA at any time |
23 (43) |
12 (36) |
20 (40) |
10 (13) |
<0.001 |
| PDE-5I at any time |
36 (67) |
22 (67) |
30 (60) |
40 (51) |
0.26 |
Unless indicated otherwise, data are expressed as the mean±SD or n (%). Abbreviations as in Table 1.
Of the 183 treatment-naïve patients, including 4 patients who had been treated with beraprost only, 92 had received initial treatment (i.e., treatment within 2 months of baseline RHC) and 91 had not (22 were late treated and 69 were untreated;
Supplementary Table 3). The group that was initially treated included more patients with either COPD or CPFE. These patients had a higher prevalence of WHO-FC III or IV and a severe form of PH with higher BNP concentrations and lower 6MWD. In addition, they were more often treated by PDE-5I as the first drug and less often by ERA.
Baseline Characteristics of Treatment-Naïve Patients With Mild or Severe Ventilatory Impairment, With or Without Initial PAH-Targeted Therapy
In the mild ventilatory impairment group, 33 patients had received initial treatment, whereas 39 had not (late treated, n=8; untreated, n=31). The initially treated group included fewer patients with IP and had a higher prevalence of the severe form of PH with higher mPAP, PVR, and BNP.
In the severe ventilatory impairment group, 41 patients were initially treated and 37 were not (late treated, n=10, untreated, n=27). All patients with CPFE received initial treatment. The initially treated group had higher mPAP, PVR, and BNP and lower 6 MWD than the non-initially treated group (Table 3).
Table 3.
Comparison of Baseline Characteristics of Treatment-Naïve Patients With a Normal Pulmonary Arterial Wedge Pressure Who Were Treated Initially or Not Grouped According to the Severity of Ventilatory Impairment Associated With the 4 Major Underlying Diseases (n=150)
| |
Mild ventilatory impairment |
Severe ventilatory impairment |
Treated initially
(n=33) |
Not treated initially
(n=39; late
treatment n=8) |
P value |
Treated initially
(n=41) |
Not treated initially
(n=37; late
treatment n=10) |
P value |
| Age (years) |
72±9 |
74±6 |
0.22 |
66±9 |
67±12 |
0.83 |
| Female sex |
4 (12) |
6 (15) |
0.75 |
11 (27) |
10 (27) |
0.98 |
| COPD |
10 (30) |
6 (15) |
0.03 |
10 (24) |
8 (22) |
0.17 |
| CPFE |
11 (33) |
9 (23) |
|
5 (12) |
0 (0) |
|
| CTD-IP |
7 (21) |
5 (13) |
|
7 (17) |
8 (22) |
|
| IP |
5 (15) |
19 (49) |
|
19 (46) |
21 (57) |
|
| WHO-FC III/IV |
24 (73) |
22 (56) |
0.15 |
33 (81) |
23 (62) |
0.07 |
| mPAP (mmHg) |
34±6 |
30±6 |
0.01 |
35±10 |
29±4 |
<0.001 |
| PAWP (mmHg) |
9±4 |
9±3 |
0.61 |
8±4 |
9±4 |
0.28 |
| CardI (L·min−1·m−2) |
2.5±0.8 |
3.0±0.9 |
0.02 |
2.8±0.8 |
3.0±0.7 |
0.13 |
| PVR (dyn·s·cm−5) |
512±220 |
341±148 |
<0.001 |
551±413 |
331±118 |
0.003 |
| Severe form of PH |
23 (72) |
17 (44) |
0.02 |
23 (56) |
13 (35) |
0.06 |
Oxygen therapy at
baseline |
27 (82) |
25 (64) |
0.09 |
38 (93) |
14 (38) |
<0.001 |
| PaO2 (torr) |
62±13 |
64±11 |
0.47 |
61±11 |
66±16 |
0.13 |
| PaCO2 (torr) |
39±4 |
37±4 |
0.06 |
43±7 |
43±9 |
0.86 |
| %FVC |
86±11 |
85±11 |
0.54 |
59±17 |
58±15 |
0.82 |
| %FEV1 |
84±14 |
91±16 |
0.06 |
55±18 |
58±21 |
0.55 |
| %DLCO |
37±19 |
39±17 |
0.77 |
30±14 |
38±16 |
0.06 |
| BNP (pg/mL) |
197±328 |
48±50 |
0.01 |
268±473 |
61±59 |
0.02 |
| 6MWD (m) |
277±120 |
337±135 |
0.08 |
217±125 |
303±153 |
0.01 |
Unless indicated otherwise, data are expressed as the mean±SD or n (%). Abbreviations as in Table 1.
In treatment-naïve patients with 1 of the 4 major underlying diseases and a normal PAWP, there was no significant difference in survival between those with the severe and mild forms of PH (Figure 1B). Conversely, the 3-year survival rate tended to be better for those with mild than severe ventilatory impairment (Figure 1C). The 3-year survival rates for patients with WHO-FC I, II, III, and IV were 100%, 55.5%, 45.5%, and 33.7%, respectively. Patients with WHO-FC I or II had better survival than those with WHO-FC III or IV (P=0.01;
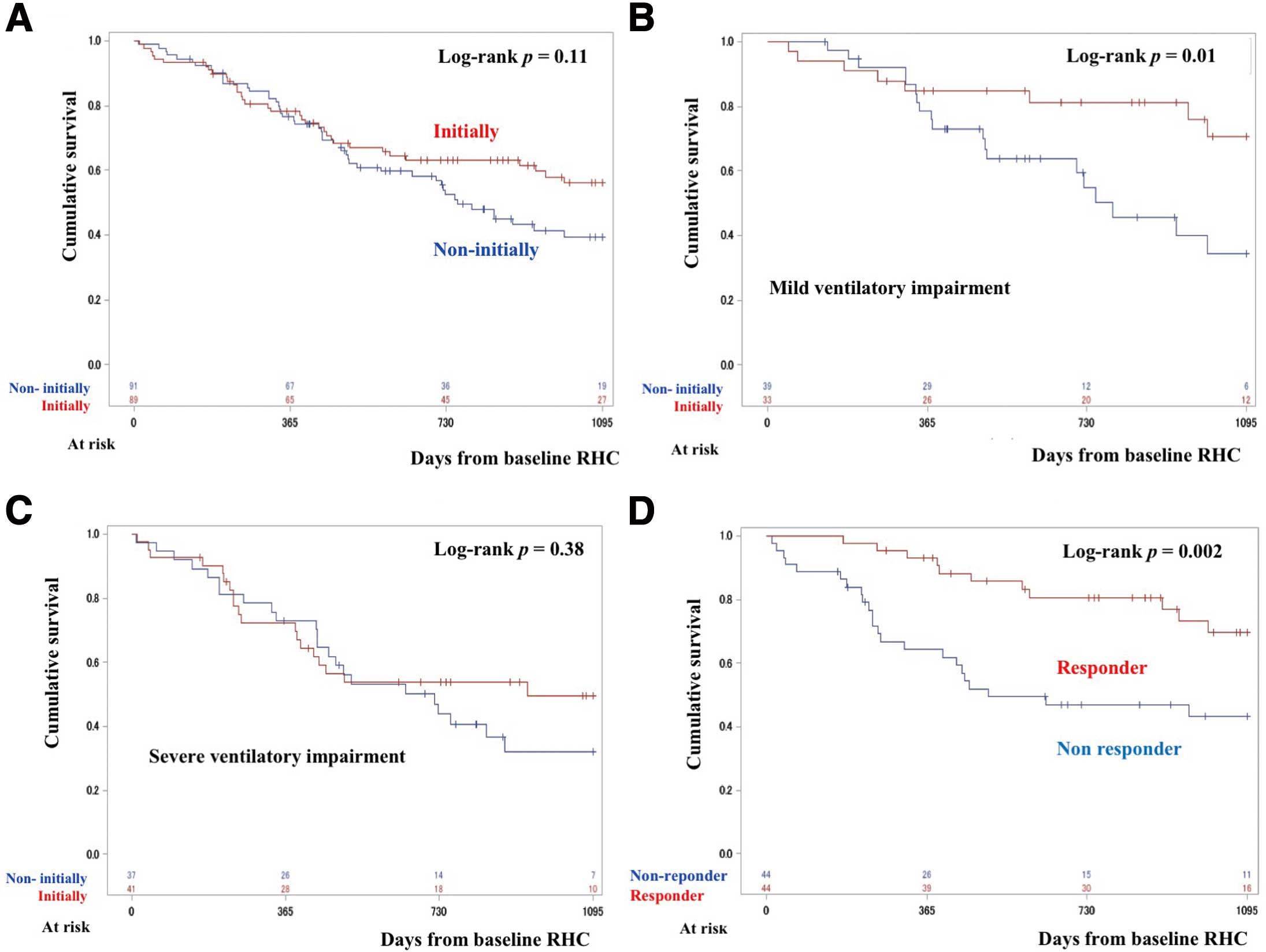
Figure 1D). The 3-year survival rate of patients who were and were not treated initially was 56.0% and 39.4%, respectively (P=0.11;
Figure 2A). There was also no significant difference in survival between these 2 treatment groups among patients with the severe form of PH (3-year survival 51.5% vs. 40.9%, respectively; P=0.64;
Supplementary Figure 2A). Similarly, survival was not better in patients with the severe form of PH in the initially treated group who were administered PDE-5I as the first drug compared with non-initially treated group (3-year survival 49.0% vs. 40.3%; P=0.71;
Supplementary Figure 2B).
In the univariate Cox proportional hazard model, IP, WHO-FC III or IV, low DLCO, and oxygen therapy at baseline were predictors of poor prognosis. Although oxygen therapy at baseline was a strong predictor of poor prognosis, all deceased patients were treated by oxygen therapy during early follow-up, and we therefore considered that the importance of other predictors may be lost if they were included in the analysis. Multivariable analysis showed that initial treatment and mild ventilatory impairment were good prognostic factors when adjusted for the other factors (Table 4). The estimates from the multiply imputed datasets were similar to those in the complete case analyses (Supplementary Table 4).
Table 4.
Univariate and Multivariable Analyses of Prognostic Factors for Treatment-Naïve Patients With a Normal Pulmonary Arterial Wedge Pressure, Grouped by the 4 Major Underlying Diseases (n=180)
| Factor |
Univariate HR
(95% CI) |
P value |
Multivariate HR
(95% CI) |
P value |
| IP vs. CTD-IP |
2.19 (1.15–4.17) |
0.02 |
1.99 (0.89–4.43) |
0.09 |
| CPFE vs. CTD-IP |
1.98 (0.93–4.24) |
0.08 |
2.31 (0.81–6.60) |
0.12 |
| COPD vs. CTD-IP |
1.03 (0.48–2.23) |
0.94 |
1.11 (0.42–2.97) |
0.83 |
| Age |
1.006 (0.984–1.029) |
0.58 |
1.017 (0.990–1.044) |
0.22 |
| WHO-FC III–IV vs. I–II |
1.88 (1.11–3.16) |
0.02 |
2.12 (1.14–3.93) |
0.02 |
| Severe form of PH |
1.17 (0.76–1.80) |
0.49 |
1.03 (0.59–1.81) |
0.91 |
| mPAP |
1.022 (0.995–1.049) |
0.10 |
|
|
| PVR |
1.000 (1.000–1.001) |
0.39 |
|
|
| BNP |
1.000 (1.000–1.001) |
0.57 |
|
|
| 6MWD |
0.998 (0.997–1.000) |
0.05 |
|
|
| PaO2 (room air) |
0.99 (0.97–1.01) |
0.3 |
|
|
| %DLCO |
0.983 (0.968–0.999) |
0.03 |
0.988 (0.969–1.006) |
0.19 |
| %FVC |
0.990 (0.980–1.001) |
0.08 |
|
|
| %FEV1 |
1.005 (0.995–1.015) |
0.32 |
|
|
| Mild ventilatory impairment |
0.62 (0.38–1.01) |
0.054 |
0.46 (0.24–0.89) |
0.02 |
| Oxygen therapy at baseline |
2.53 (1.50–4.27) |
<0.001 |
|
|
| Initial treatment |
0.70 (0.46–1.09) |
0.11 |
0.46 (0.26–0.82) |
0.01 |
CI, confidence interval; HR, hazard ratio. Other abbreviations as in Table 1.
In the group with mild ventilatory impairment, survival was significantly better in patients who were initially treated than in those who were not (3-year survival 70.6% vs. 34.2%, respectively; P=0.01;
Figure 2B). Multivariable analysis showed that initial treatment was an independent factor for a good prognosis (Supplementary Table 5). Similarly, the estimates from the multiply imputed datasets were similar to those from the complete case analysis (Supplementary Table 6).
In the group with severe ventilatory impairment, there was no significant difference in survival between patients who were or were not initially treated (3-year survival 49.6% vs. 32.1%, respectively; P=0.38;
Figure 2C). Multivariable analysis revealed that CPFE and WHO-FC III–IV were predictive of poor prognosis (Supplementary Table 7).
Characteristics of Responders
In the initially treated group, there were 44 responders and 44 non-responders. Responders were more common in the group with mild ventilatory impairment. In addition, many patients with COPD and CTD-IP were classified as responders (Table 5). Responders to PAH-targeted therapy had a significantly better prognosis than non-responders (3-year survival 69.6% vs. 43.3%, respectively; log-rank test, P=0.002;
Figure 2D).
Table 5.
Characteristics of Responders vs. Non-Responders to Pulmonary Arterial Hypertension-Targeted Therapy in the Initially Treated Group
| |
Non-responders
(n=44) |
Responders
(n=44) |
P value |
| Age (years) |
67±9 |
71±10 |
0.07 |
| Female sex |
9 (21) |
12 (27) |
0.45 |
| COPD |
9 (21) |
17 (39) |
<0.001 |
| CPFE |
13 (30) |
5 (11) |
|
| CTD-IP |
3 (7) |
16 (36) |
|
| IP |
19 (43) |
6 (14) |
|
| WHO-FC III/IV |
34 (77) |
37 (84) |
0.42 |
| mPAP (mmHg) |
35±9 |
35±8 |
1.0 |
| mPAP ≥35 mmHg |
17 (39) |
21 (48) |
0.39 |
| CardI (L·min−1·m−2) |
2.7±0.8 |
2.5±0.9 |
0.34 |
| PVR (dyn·s·cm−5) |
526±317 |
631±410 |
0.20 |
| Severe form of PH |
37 (63) |
33 (66) |
0.72 |
| PaO2 (torr) room air |
59±10 |
64±13 |
0.13 |
| PaCO2 (torr) room air |
42±6 |
39±6 |
0.07 |
| %FVC |
65±19 |
75±19 |
0.02 |
| %FEV1 |
66±22 |
69±21 |
0.58 |
| Mild ventilatory impairment |
10 (28) |
22 (60) |
0.01 |
| No. patients with data |
36 |
37 |
|
| %DLCO |
32±12 |
36±20 |
0.37 |
| BNP (pg/mL) |
294±504 |
331±541 |
0.77 |
| 6MWD (m) |
245±129 |
227±118 |
0.54 |
Unless indicated otherwise, data are expressed as the mean±SD or n (%). Responders were defined as those with a decrease in PVR >15%, an increase in the 6MWD >15%, or an improvement in WHO-FC. Abbreviations as in Table 1.
Discussion
This is the first prospective registry of PH associated with respiratory diseases in Japan. We found that a high proportion (43%) of patients had mild ventilatory impairment, which may be classified as Group 1 PH. In the mild ventilatory impairment group, 33 patients (46%) who were treated initially with PAH-targeted therapy survived longer than those patients who did not receive initial treatment. Initial treatment was also a good prognostic factor when adjusted for other factors, including disease category, WHO-FC, and severe form of PH. Conversely, in the group with severe ventilatory impairment (pure Group 3), there was no significant difference in survival between patients who were and were not treated initially. Furthermore, the prognosis of responders to PAH-targeted therapy was better than that of non-responders, with the number of responders greater in the mild ventilatory impairment group. Several issues need to be considered when interpreting these results.
First, the summary of the 6th NICE conference divided patients into either limited chronic lung disease (%FEV1
>60, %FVC >70, and minimal parenchymal CT changes) and extensive lung disease, and allowed the use of PAH-targeted therapy in patients with a PAH phenotype and limited chronic lung disease.2
Recently Peacock et al reported that patients with PAH coexisting with lung disease confirmed by CT and mild ventilatory impairment (%FEV1
>60, %FVC >60, percent predicted total lung capacity >60) had a good response to PAH-targeted therapy but poor survival (1-year survival 72%) compared with patients with PAH and no lung disease.9
The present study focused on PH with lung disease instead of PAH, resulting in only 41 patients (57%), including 8 in the late-treated group, receiving PAH-targeted therapy, considerably less than the number of patients in the study of Peacock et al9
(97.3%) and the PAH registry in Japan (100%).10
Although in the present study the attending physician determined whether a patient met the criteria for R-PH and we could not examine the extent of lung disease by CT, we may have included patients with more severe lung disease on imaging in our Group 3 PH with mild ventilatory impairment than in the study of Peacock et al.9
In particular, CPFE includes both restrictive and obstructive ventilatory impairment, but shows mild ventilatory impairment.11
In these cases, CPFE is likely to be classified as PAH with lung disease. Therefore, it may be difficult to completely differentiate PAH with lung disease from Group 3 PH associated with mild ventilatory impairment and extensive lung disease.
Second, in the group with severe ventilatory impairment, there was no significant difference in survival between patients who received initial treatment and those who did not, and only CPFE and WHO-FC III-IV were identified as poor prognostic factors. It is well known that CPFE shows poor prognosis, even compared with IP.4,12
Because ventilatory dysfunction of CPFE is usually mild,4,11
if patients have severe ventilatory impairment, the extent of the abnormal area could be larger than that observed in other diseases. In addition, in this study, no patient with CPFE and severe ventilatory impairment responded to treatment (data not shown). Therefore, early treatment is needed when these patients show mild ventilatory impairment.
Third, in the ASPIRE registry, responders to PAH-targeted therapy, defined as those having an improvement in ∆PVR >20% or WHO-FC, had better survival than non-responders with severe PH due to COPD (mPAP ≥40 mmHg).3
Hoeper et al also reported that patients with PH associated with idiopathic IP whose 6MWD improved by ≥20 m had a better prognosis than patients with no improvement in 6MWD, indicating the possibility of responders.13
However, there are no data on the responders in terms of their characteristics and the prevalence of each category of Group 3 PH. Consistent with previous reports, the present study showed that the prognosis of responders was better than that of non-responders, and that responders had an increased prevalence of CTD-IP and COPD, with significantly more patients classified as having mild ventilatory impairment.
Fourth, the 3-year survival rate (32.2–71.1%) in the present study was worse than that reported for Japanese patients with PAH (88.2%),10
suggesting insufficient treatment for R-PH. However, the 3-year survival rate of 59.5% for COPD was better than the rate of 41% reported in the ASPIRE registry.14
Similarly, the 3-year survival rate of 32.2% for patients with IP was better than the rate of 16% reported for the ASPIRE registry,14
and almost the same as the rate of 34.4% in the Comparative, Prospective Registry of Newly Initiated Therapies for Pulmonary Hypertension (COPMERA) registry, which included only treated patients.13
The prevalence of the PAH phenotype and the existence of some responders to initial PAH targeted-therapy may lead to improved prognosis in the registry.
Fifth, the major cause of death was respiratory failure or progression or acute exacerbation of underlying disease in CPFE (59%) and IP (53%). Similarly, Hoeper et al reported that the leading cause of death in PH associated with idiopathic IP was respiratory failure (58.9%), whereas death due to right heart failure was recorded in 28.6% of cases.13
Worsening of respiratory failure in R-PH may be caused by tissue hypoxia due to increased pulmonary arterial pressure and low CO.15
Therefore, PH could also contribute to death even in these patients. In addition, effective treatment for the underlying fibrosing lung disease will be more important to improve the prognosis in these patients.
Finally, we divided patients treated with PAH-targeted therapy into those receiving initial and late treatment, with the late-treated patients included in the group of untreated patients to minimize the immortal bias for the treated group. A retrospective study in a Japanese cohort with severe PH and mPAP ≥35 mmHg reported that prognosis was better for patients treated with than without a PDE-5I.4
In the present prospective study, there was no difference in survival between patients receiving initial treatment and those not receiving this treatment, even for patients with the severe form of PH. These inconsistencies may be explained by the selection of patients or reporting bias resulting from exclusion of untreated sick patients in the previous retrospective study.4
In addition, immortal bias existed in that retrospective study, because early deaths were included only in the untreated group, whereas late-treated patients were included in the treated group.4
In addition, based on the results of that retrospective study, the aggressive use of PDE-5I in Japan for critically ill patients with an expected poor prognosis may be a factor in the early deaths in patients with severe R-PH treated by PAH-targeted therapy, mainly PDE-5I. In patients with the severe form of PH with severe ventilatory impairment, even PDE-5I may exacerbate gas exchange and lead to poor a prognosis. This suggests that PDE-51 should not be used in critically ill patients with a severe form of PH and severe ventilatory impairment.
Study Limitations
A limitation of the present study was that it was a nationwide registry and did not include a sufficient number of cases for each disease to be analyzed. Therefore, a greater number of studies on each disease from this registry is required. As we have already discussed, R-PH may include Group 1 (PAH) and pure Group 3 PH phenotypes. It may be acceptable that the mild ventilatory impairment group was classified as having PAH. Furthermore, and more importantly, selection bias could not be discounted in our registry because any decision to use PAH-targeted therapy was made solely by the physicians at each institution. Randomized placebo-controlled studies are therefore needed to demonstrate the efficacy of PAH-targeted therapy for patients with the PAH phenotype as well as pure Group 3 PH.
Conclusions
This first Japanese prospective registry of R-PH showed a high proportion of patients with mild ventilatory impairment (PAH phenotype) who had a better survival rate following initial PAH-targeted therapies. The patients who responded to this treatment primarily had mild ventilatory impairment.
Acknowledgments
We thank all colleagues of the JRPHS group listed in
Appendix
for the contribution to this study.
Sources of Funding
This study was supported, in part, by a grant from the Intractable Respiratory Diseases and Pulmonary Hypertension Research Group of the Ministry of Health, Labor, and Welfare of Japan (No. 27280401) and grants from the Japan Agency for Medical Research and Development (AMED; No. 16ek0109127 h0002 and No. JP18lk1601003 h0001).
Disclosures
N.T. has received remuneration from Nippon Shinyaku, Actelion Pharmaceuticals Japan, Bayer Yakuhin, and Daiichi Sankyo, and scholarship funds from Nippon Shinyaku. N.T. belongs to a department endowed by Actelion Pharmaceuticals Japan. H. Kumamaru has received consultation fees from Mitsubishi Tanabe Pharma and speaker fees from Pfizer Japan Inc., and is affiliated with the Department of Healthcare Quality Assessment at the University of Tokyo. This department is a social collaboration department supported by the National Clinical Database, Johnson & Johnson K.K., and Nipro Cooperation. N.E. has received remuneration from Actelion Pharmaceuticals Japan, Bayer Yakuhin, and Nippon Shinyaku; research funds from Actelion Pharmaceuticals Japan; and scholarship funds from Actelion Pharmaceuticals Japan, Bayer Yakuhin, and Nippon Shinyaku. Y.Y. has received remuneration from Actelion Pharmaceuticals Japan. I.T. has received research funds from Actelion Pharmaceuticals Japan and belongs to a department endowed by Nippon Shinyaku, Nippon, Boehringer Ingelheim, and Mochida Pharmaceutical. H. Kimura belongs to an department endowed by Actelion Pharmaceuticals Japan. T.S. has received remuneration from Actelion Pharmaceuticals Japan; research funds from Actelion Pharmaceuticals Japan; and scholarship funds from Nippon Shinyaku, Bayer Yakuhin, and Mochida Pharmaceutical. T.H. has received research funds from FUJIFILM and belongs to a department endowed by TEIJIN PHARMA. H.M. is affiliated with the Department of Healthcare Quality Assessment at the University of Tokyo, which is a social collaboration department supported by the National Clinical Database, Johnson & Johnson K.K., and Nipro Cooperation. K. Tatsumi has received remuneration from Actelion Pharmaceuticals Japan and research funds from Astellas Pharma Inc.
IRB Information
This study was initially approved by the Ethics Committee at Chiba University, School of Medicine (Approval no. 1569), and then by the institutional review boards of all the participating centers.
Data Availability
The deidentified participant data will not be shared.
Appendix
The members of the Japan Respiratory PH Study (JRPHS) Group are listed below:
N. Tanabe, S. Sakao, K. Tatsumi, A. Shigeta, R. Suda, Y. Ichimura, T. Sugiura, C. Handa, and T. Hirano (Chiba University, Chiba, Japan); H. Kumamaru and M. Hatano (The University of Tokyo, Tokyo, Japan); Y. Tamura (International University of Health and Welfare Mita Hospital, Tokyo, Japan); H. Taniguchi, Y. Kondoh, T. Kimura, M. Yagi, R. Teramachi, and T. Satoh (Tosei General Hospital, Seto, Japan); N. Emoto (Kobe Pharmaceutical University, Kobe, Japan); K. Nakayama, Y. Taniguchi, K. Hirata, Y. Nishimura, and T. Nagano (Kobe University Graduate School of Medicine, Kobe, Japan); K. Monden (Shinko Hospital, Kobe, Japan); Y. Yamada and K. Tanaka (Japan Railway Tokyo General Hospital, Tokyo, Japan); O. Nishiyama (Kindai University, Osaka, Japan); I. Tsujino, H. Ohira, and M. Nishimrua (Hokkaido University Hospital, Sapporo, Japan); H. Kuraishi (Nagano Red Cross Hospital, Nagano, Japan); H. Kimura, R. Uyama, A. Nakamura, M. Yoshikawa, and S. Muro (Nara Medical University, Kashihara, Japan); H. Kimura, K. Atsumi, and Y. Tanaka (Nippon Medical School Graduate School of Medicine, Tokyo, Japan); Y. Inoue, H. Matsui, S. Minomo, and M. Sekiguchi (National Hospital Organization Kinki-Chuo Chest Medical Center, Osaka, Japan); T. Nagaoka (Juntendo University Graduate School of Medicine, Tokyo, Japan); Y. Morio and Kei Kusaka (National Hospital Organization Tokyo National Hospital, Kiyose, Japan); Y. Nakatsumi (Kanazawa Municipal Hospital, Kanazawa, Japan); T. Satoh and M. Sekino (Kyorin University Hospital, Mitaka, Japan); M. Hanaoka, Y. Yada, and K. Sonehara (Shinshu University School of Medicine, Matsumoto, Japan); M. Sumitani (Osaka City General Hospital, Osaka, Japan); T. Handa, S. Satoh, H. Kinoshita, and T. Hirai (Kyoto University, Kyoto, Japan); H. Miyata (Keio University School of Medicine, Tokyo, Japan); F. Sakamaki (Tokai University, Hachioji Hospital, Hachioji, Japan); H. Matsubara and A. Ogawa (National Hospital Organization Okayama Medical Centre, Okayama, Japan); H. Shimizu (JCHO Tokyo Shinjuku Medical Center, Tokyo, Japan); N. Hayama (Tokai University Hospital, Isehara, Japan); M. Fujita and M. Shiraishi (Fukuoka University Hospital, Fukuoka, Japan); K. Takamura (Obihiro Kosei Hospital, Obihiro, Japan); T. Saitoh and K. Hyoudo (National Hospital Organization Ibarakihigashi National Hospital, Ibaraki, Japan); S. Imai, T. Itoh, and O. Ono (Saiseikai Narashino Hospital, Narashino, Japan); T. Ogura (Kanagawa Cardiovascuiar and Respiratory Center, Yokohama, Japan); Y. Matsuzawa (Toho University Sakura Hospital, Sakura, Japan); N. Nakanishi and T. Ogo (National Cerebral and Cardiovascular Center, Osaka, Japan)
Supplementary Files
Please find supplementary file(s);
http://dx.doi.org/10.1253/circj.CJ-20-0939
References
- 1.
Chaouat A, Naeije R, Weitzenblum E. Pulmonary hypertension in COPD. Eur Respir J 2008; 32: 1371–1385.
- 2.
Nathan SD, Barbera JA, Gaine SP, Harari S, Martinez FJ, Olschewski H, et al. Pulmonary hypertension in chronic lung disease and hypoxia. Eur Respir J 2019; 53: 1801914.
- 3.
Hurdman J, Condliffe R, Elliot CA, Swift A, Rajaram S, Davies C, et al. Pulmonary hypertension in COPD: Results from the ASPIRE registry. Eur Respir J 2013; 41: 1292–1301.
- 4.
Tanabe N, Taniguchi H, Tsujino I, Sakamaki F, Emoto N, Kimura H, et al. Multi-institutional retrospective cohort study of patients with severe pulmonary hypertension associated with respiratory diseases. Respirology 2015; 20: 805–812.
- 5.
Vestbo J, Hurd SS, Agustí AG, Jones PW, Vogelmeier C, Anzueto A, et al. Global strategy for the diagnosis, management, and prevention of chronic obstructive pulmonary disease: GOLD executive summary. Am J Respir Crit Care Med 2013; 187: 347–365.
- 6.
Travis WD, Costabel U, Hansell DM, King TE Jr, Lynch DA, Nicholson AG, et al. An official American Thoracic Society/European Respiratory Society statement: Update of the international multidisciplinary classification of the idiopathic interstitial pneumonias. Am J Respir Crit Care Med 2013; 188: 733–748.
- 7.
Galiè N, Humbert M, Vachiery JL, Gibbs S, Lang I, Torbicki A, et al. 2015 ESC/ERS guidelines for the diagnosis and treatment of pulmonary hypertension: The Joint Task Force for the Diagnosis and Treatment of Pulmonary Hypertension of the European Society of Cardiology (ESC) and the European Respiratory Society (ERS): Endorsed by: Association for European Pediatric and Congenital Cardiology (AEPC), International Society for Heart and Lung Transplantation (ISHLT). Eur Heart J 2016; 37: 67–119.
- 8.
Rubin DB, Schenker N. Multiple imputation in healthcare databases: An overview and some applications. Stat Med 1991; 10: 585–598.
- 9.
Peacock AJ, Ling Y, Johnson MK, Kiely DG, Condliffe R, Elliot CA, et al. Idiopathic pulmonary arterial hypertension and co-existing lung disease: Is this a new phenotype? Pulm Circ 2020; 10: 2045894020914851.
- 10.
Tamura Y, Kumamaru H, Satoh T, Miyata H, Ogawa A, Tanabe N, et al. Effectiveness and outcome of pulmonary arterial hypertension-specific therapy in Japanese patients with pulmonary arterial hypertension. Circ J 2017; 82: 275–282.
- 11.
Cottin V, Le Pavec J, Prévot G, Mal H, Humbert M, Simonneau G, et al. Pulmonary hypertension in patients with combined pulmonary fibrosis and emphysema syndrome. Eur Respir J 2010; 35: 105–111.
- 12.
Mejía M, Carrillo G, Rojas-Serrano J, Estrada A, Suárez T, Alonso D, et al. Idiopathic pulmonary fibrosis and emphysema: Decreased survival associated with severe pulmonary arterial hypertension. Chest 2009; 136: 10–15.
- 13.
Hoeper MM, Behr J, Held M, Grunig E, Vizza CD, Vonk-Noordegraaf A, et al. Pulmonary hypertension in patients with chronic fibrosing idiopathic interstitial pneumonias. PLoS One 2015; 10: e0141911.
- 14.
Hurdman J, Condliffe R, Elliot CA, Davies C, Hill C, Wild JM, et al. ASPIRE registry: Assessing the spectrum of pulmonary hypertension identified at a referral centre. Eur Respir J 2012; 39: 945–955.
- 15.
Suda R, Tanabe N, Terada J, Naito A, Kasai H, Nishimura R, et al. Pulmonary hypertension with a low cardiac index requires a higher PaO2 level to avoid tissue hypoxia. Respirology 2020; 25: 97–103.



