論文ID: CJ-20-1062
論文ID: CJ-20-1062
Background: It is unclear whether catheter ablation is beneficial for frail elderly patients with atrial fibrillation (AF). This study evaluated the effect of ablation on outcomes in frail elderly patients with AF.
Methods and Results: From the Korean National Health Insurance Service database, 194,928 newly diagnosed AF patients were treated with ablation or medical therapy (rhythm or rate control) between 2005 and 2015. Among these patients, the study included 1,818 (ablation; n=119) frail and 1,907 (ablation; n=230) non-frail elderly (≥75 years) patients. Propensity score matching was used to correct for differences between groups. During 28 months (median) follow up, the risk of all-cause death, composite outcome (all-cause death, heart failure admission, stroke/systemic embolism, and sudden cardiac arrest), and each outcome did not change after ablation in frail elderly patients. However, in non-frail elderly patients, ablation was associated with a lower risk of all-cause death (3.5 and 6.2 per 100 person-years; hazard ratio [HR] 0.48; 95% confidence interval [CI] 0.30–0.79; P=0.004), and composite outcome (6.9 and 11.2 per 100 person-years; HR 0.54; 95% CI 0.38–0.75; P<0.001).
Conclusions: Ablation may be associated with a lower risk of death and composite outcome in non-frail elderly, but the beneficial effect of ablation was not significant in frail elderly patients with AF. The effect of frailty on the outcome of ablation should be evaluated in further studies.
Atrial fibrillation (AF) is increasingly prevalent among older adults and is a major contributor to their morbidity from strokes, heart failure, and impairment of their quality of life.1 The age distribution of AF among the populations of developed countries is predicted to shift in the coming years, with an expected increase in AF cases among the elderly. Frailty is a clinical state of vulnerability caused by an age-related decline in the ability of the body’s physiological systems to respond to stressor events. A diagnosis of AF is associated with a loss of independence in the performance of daily activities, and there is a 4-fold increase in the potential for AF patients to be classified as frail compared to non-AF patients.2,3 Frailty is associated with many adverse clinical outcomes (e.g., delirium and functional decline) in older individuals admitted to hospitals.
Because an optimal strategy for the management of AF in frail elderly patients is not presently known, consensus statements and guidelines recommend a patient-centered and individually tailored approach.4 In clinical trials, catheter ablation for the treatment of AF has been shown to be superior to antiarrhythmic medication for the maintenance of sinus rhythm and the improvement of the patient’s quality of life in symptomatic patients who are unresponsive to drug treatment.5,6 A limitation of the previously published randomized controlled trials of catheter ablation for the treatment of AF is that frail elderly patients have been largely excluded from the study cohorts.7,8 This study aimed to evaluate whether ablation reduces death, heart failure admission, and ischemic stroke in real-world frail and non-frail elderly patients with AF using data from the Korean National Health Insurance Service database.
This study was a retrospective cohort analysis using the National Health Insurance Service (NHIS) claims database (NHIS-2016-4-009) established by the NHIS of Korea. The NHIS is the single insurer managed by the Korean government. The majority (97.1%) of Korean citizens are mandatory subscribers to the NHIS, and the remaining 3% of the population are under the Medical Aid program. As the NHIS database contains the information of Medical Aid users, it is based on the entire Korean population.1,9 This study was approved by the institutional review board of the Yonsei University Health System (reference number: 4-2016-0179), and the requirement for informed consent was waived.
All data and materials have been made publicly available from the NHIS of Korea. The data can be accessed from the National Health Insurance Data Sharing Service homepage of the NHIS (http://nhiss.nhis.or.kr). Applications to use the NHIS data will be reviewed by the inquiry committee of research support and, once approved, raw data will be provided to the authorized researcher with a fee at several permitted sites.
Study PopulationFrom the entire Korean population (51.5 million inhabitants) in the Korean NHIS database, we identified 194,928 patients with AF who were aged ≥18 years and treated with ablation or medical therapy (antiarrhythmic drugs or rate control drugs) from 1 January 2006 to 31 December 2015. AF was diagnosed using the International Classification of Disease 10th revision code, I48. To ensure diagnostic accuracy, AF was defined as being present only when it was a discharge diagnosis or confirmed at least twice in the outpatient department. The AF diagnosis has previously been validated in the NHIS database with a positive predictive value of 94.1%.9–11 The exclusion criteria for both groups were patients with valvular AF (moderate to severe mitral stenosis or mechanical valve), previous arrhythmia surgery (Maze and similar procedures), those with an implanted cardiac electric device, or those age <75 years. Among medical therapy patients, patients who had taken oral anticoagulants (OACs) <30 days or antiarrhythmic drugs <90 days during the same period were additionally excluded. After exclusions, 1,818 (ablation; n=119) frail and 1,907 (ablation; n=230) non-frail elderly (≥75 years) patients remained for the analysis (Figure 1).

Flowchart of the enrollment and analysis of the study population. AAD, antiarrhythmic drug; AF, atrial fibrillation; ICD, implantable cardioverter–defibrillator; OAC, oral anticoagulant; PS, propensity score.
For each patient, the Hospital Frailty Risk Score was calculated retrospectively using all available ICD-10 diagnostic codes that were documented for the particular admission, as recommended by Gilbert et al.12,13 The score was an aggregate of the 109 ICD-10 diagnostic codes that were found to be associated with frailty-based risk (Supplementary Table 1). Each of these ICD-10 diagnostic codes was given a specific value proportional to how strongly it predicted frailty. According to the aggregate score, patients were divided into 3 frailty-based risk categories: low-risk (<5 points), intermediate-risk (5–15 points), and high-risk (>15 points).12 Non-frail patients were defined as those with low Hospital Frailty Risk categorization. Frail patients were defined as those with intermediate or high Hospital Frailty Risk categorizations.
CovariatesInformation regarding comorbidity conditions was obtained from inpatient and outpatient hospital diagnoses. Baseline comorbidities were defined using medical claims and prescription medications before the index date. The patients were considered to have comorbidities when the condition was a discharge diagnosis or was confirmed at least twice in an outpatient setting, similar to previous studies using NHIS data (Supplementary Table 2).1,9–11 Baseline economic status was determined on the basis of the relative economic levels categorized into 10 levels according to their health insurance premiums in the index year. Prescription medication use was verified by identifying NHIS database claims within 90 days before the index date.
Clinical Outcomes and AssessmentsThe primary clinical outcomes were all-cause death and a composite of all-cause death, heart failure admission, ischemic stroke/systemic embolism (SE), and sudden cardiac arrest. The secondary outcomes were each of these outcomes considered separately. Patients were followed until the end of the study period (31 December 2016) or death. Data on vital status and date of death were confirmed from the National Population Registry of the Korea National Statistical Office, with the use of a unique personal identification number, in which central registration of death was conducted on the basis of death certificates. This approach provides a complete event ascertainment because the NHIS and National Statistical Office are national organizations covering all Korean subjects. The definitions of clinical outcomes are presented in Supplementary Table 2. It must be noted that the same patient could have more than one study outcome during the study duration, but only the first event of each outcome was considered in the study.
For both the ablation group and the medical therapy group, the time at risk was counted from the index date of the first medical therapy. In patients who underwent AF ablation without medical therapy, the time at risk was counted from the index date of the first ablative procedure. The effect of ablation was analyzed as a time-varying exposure.
Statistical MethodsOne-to-two propensity score matching was used to account for the differences in baseline characteristics between patients who underwent ablation and those who were treated with medical therapy alone. A propensity score, the probability of undergoing ablation, was estimated using logistic regression based on sociodemographics, medical history, concurrent medication use, and AF duration (variables in Table 1). The balance between the treatment populations was evaluated by standardized differences of all baseline covariates using a threshold of 0.1 to indicate imbalance.
| Before propensity score matching | After propensity score matching | |||||
|---|---|---|---|---|---|---|
| Ablation (N=119) |
Medical therapy (N=1,699) |
SMD | Ablation (N=82) |
Medical therapy (N=149) |
SMD | |
| Demographics | ||||||
| Age, years | 77 (76–79) | 79 (77–82) | 69.9 | 78 (76–80) | 78 (76–80) | 0.9 |
| Male | 58.0 | 45.7 | 24.8 | 54 | 49 | 9.3 |
| High income status | 68.1 | 52.1 | 32.9 | 66 | 66 | 1.2 |
| AF duration, months | 49.0 (9.3–90.5) | 23.0 (3.0–61.7) | 34.7 | 38.4 (6.8–67.0) | 32.4 (4.0–67.1) | 1.0 |
| Risk scores | ||||||
| CHA2DS2-VASc score | 6.0 (5.0–7.0) | 7.0 (5.0–7.0) | 47.4 | 6.0 (5.0–7.0) | 6.0 (5.0–7.0) | 7.7 |
| mHAS-BLED score† | 4.0 (3.0–5.0) | 4.0 (3.0–5.0) | 10.3 | 4.0 (4.0–5.0) | 3.0 (3.0–5.0) | 17.2 |
| Charlson comorbidity index | 6.0 (4.0–9.0) | 7.0 (5.0–10.0) | 61.6 | 6.0 (4.0–9.0) | 7.0 (5.0–9.0) | 9.9 |
| Hospital Frailty Risk score | 8.5 (6.0–11.6) | 11.2 (7.5–17.9) | 186.6 | 9.9 (7.2–2.7) | 9.5 (6.6–13.4) | 6.3 |
| Comorbidities | ||||||
| Heart failure | 47.1 | 70.3 | 48.7 | 54 | 58 | 9.5 |
| Hypertension | 92.4 | 95.6 | 13.6 | 94 | 96 | 9.4 |
| Diabetes | 34.5 | 39.4 | 10.3 | 38 | 37 | 1.8 |
| Dyslipidemia | 93.3 | 89.9 | 12.3 | 92 | 88 | 11.6 |
| Ischemic stroke | 54.6 | 63.3 | 17.7 | 59 | 56 | 5.7 |
| Hemorrhagic stroke | 5.0 | 5.8 | 3.5 | 5 | 6 | 5.1 |
| Myocardial infarction | 23.5 | 25.4 | 4.4 | 22 | 30 | 17.3 |
| Peripheral arterial disease | 27.7 | 27.1 | 1.5 | 31 | 30 | 0.6 |
| Chronic kidney disease | 14.3 | 16.5 | 6.2 | 17 | 18 | 2.7 |
| End stage renal disease | 0.0 | 1.5 | 17.3 | 0 | 0 | <0.1 |
| Proteinuria | 6.7 | 5.8 | 3.7 | 9 | 6 | 9.6 |
| Hyperthyroidism | 21.8 | 17.0 | 12.3 | 21 | 22 | 3.4 |
| Hypothyroidism | 16.8 | 17.4 | 1.6 | 15 | 18 | 9.4 |
| Malignancy | 30.3 | 36.6 | 13.4 | 33 | 30 | 7.3 |
| COPD | 49.6 | 55.8 | 12.5 | 55 | 45 | 19.8 |
| HCMP | 3.4 | 3.5 | 0.6 | 4 | 4 | 1.9 |
| History of any bleeding | 55.5 | 50.7 | 9.6 | 52 | 45 | 14.9 |
| Osteoporosis | 55.5 | 61.4 | 12.0 | 59 | 58 | 0.3 |
| Sleep apnea | 0.8 | 0.4 | 5.4 | 1 | 0 | 15.6 |
| Medication (Treatment) | ||||||
| OAC | 65.5 | 78.5 | 29.2 | 72 | 78 | 13.6 |
| Antiplatelet agents | 90.8 | 78.0 | 35.6 | 90 | 87 | 11.4 |
| ACE-inhibitor/ARB | 79.0 | 75.9 | 7.5 | 78 | 79 | 1.1 |
| Dihydropyridine CCB | 63.0 | 58.2 | 10.0 | 31 | 34 | 11.1 |
| Diuretics | 63.0 | 77.3 | 31.5 | 71 | 70 | 2.0 |
| K sparing diuretics | 11.8 | 30.3 | 46.7 | 16 | 14 | 4.9 |
| Statin | 56.3 | 56.0 | 0.5 | 59 | 59 | 1.1 |
| β-blocker | 74.8 | 68.0 | 15.1 | 73 | 77 | 9.2 |
| Nondihydropyridine CCB | 37.0 | 24.8 | 26.5 | 67 | 62 | 8.0 |
| Digoxin | 19.3 | 32.8 | 31.2 | 26 | 26 | 0.2 |
Values are presented as median (Q1, Q3, quartiles [25th and 75th percentiles]) or %. †Modified HAS-BLED: hypertension, 1 point: >65 years old, 1 point: stroke history, 1 point: bleeding history or predisposition, 1 point: liable international normalized ratio, not assessed: ethanol or drug abuse, 1 point: drug predisposing to bleeding, 1 point. ACE, angiotensin-converting enzyme; AF, atrial fibrillation; ARB, angiotensin receptor blocker; CCB, calcium channel blocker; COPD, chronic obstructive pulmonary disease; HCMP, hypertrophic cardiomyopathy; OAC, oral anticoagulant; SMD, standardized mean difference.
Incidence rates of events were calculated by dividing the number of events by person-times at risk, with the 95% confidence intervals (CI) estimated by exact Poisson distributions. We plotted cumulative incidence curves of clinical outcomes and compared the incidences using the log-rank test. Cox proportional hazards regressions were used to compare those patients treated with ablation and medical therapy. The Fine and Gray method was used to consider death as a competing risk when assessing non-fatal outcomes (i.e., heart failure admission and ischemic stroke/SE when considered separately). The proportional hazards assumption was tested on the basis of Schoenfeld residuals.
For sensitivity analysis, inverse probability of treatment (IPT) propensity-score weighting was used to account for the differences in baseline characteristics between patients who underwent ablation and those who were treated with medical therapy alone. We assigned patients who underwent ablation a weight of 1 / (propensity score) and those who were treated with medical therapy alone a weight of 1 / (1-propensity score). The balance between the treatment populations was evaluated by standardized differences of all baseline covariates using a threshold of 0.1 to indicate imbalance.
We used “falsification analysis” to determine whether ablation was associated with lower rates of urinary tract infections, Varicella-zoster, and fall accidents that should not be lower with ablation and would indicate that the population receiving ablation was different in ways that would result in reduced mortality or stroke that had nothing to do with ablation.
A 2-sided P value of <0.05 was considered significant. Statistical analyses were conducted using SAS version 9.3 (SAS Institute, Cary, NC, USA) and R version 3.3.2 (The R Foundation, www.R-project.org).
Before propensity score matching, compared to patients with medical therapy alone, patients with ablation were younger, more often male, healthy, earned a higher income, and had longer AF duration in both the frail (Table 1) and non-frail groups (Supplementary Table 3). After propensity score matching, all baseline characteristics were similar between ablation and medical therapy groups in both frail (Table 1) and non-frail patients (Supplementary Table 3). In multivariable analysis, the factors independently associated with the likelihood of undergoing catheter ablation were younger age, income in the highest quartile, and higher HAS-BLED score in both frail and non-frail patients. Among frail patients, the probability of undergoing AF ablation decreased as the Hospital Frailty Risk score increased (Supplementary Table 4).
All-Cause MortalityIn frail elderly patients, during a median (25th, 75th percentiles) follow up of 28 (16, 48) months, the cumulative incidence of all-cause death was not significantly different between the ablation and medical therapy groups (P=0.669, Figure 2A Upper panel). The incidence and risk of all-cause death were similar between the 2 groups (8.3 and 10.2 per 100 person-years; hazard ratio [HR] 0.83; 95% CI 0.48–1.44; P=0.506) (Table 2).

Propensity score-matched cumulative incidence curves of all-cause death (A) and composite outcome (B) for frail (Upper panel) and non-frail (Lower panel) AF elderly patients according to whether ablation was performed. AF, atrial fibrillation.
| Number of events |
Person- years |
Event rate (100 person- years) |
Number of events |
Person- years |
Event rate (100 person- years) |
Absolute reduction in event rate (95% CI) |
Adjusted HR (95% CI)† |
P value | |
|---|---|---|---|---|---|---|---|---|---|
| Medical therapy (N=149) | Ablation (N=82) | ||||||||
| Frail patients | |||||||||
| All-cause death | 56 | 548 | 10.2 | 22 | 264 | 8.3 | 1.9 (−2.7~0.6) | 0.83 (0.48~1.44) | 0.506 |
| Composite outcome‡ | 99 | 370 | 26.8 | 38 | 209 | 18.2 | 8.6 (3.3~16.8) | 0.71 (0.48~1.04) | 0.076 |
| Heart failure admission | 28 | 479 | 5.8 | 8 | 244 | 3.3 | 2.6 (−0.9~6.0) | 0.67 (0.28~1.61) | 0.449 |
| Ischemic stroke/SE | 31 | 414 | 7.5 | 14 | 217 | 6.5 | 1.0 (−3.4~5.4) | 0.96 (0.47~1.88) | 0.714 |
| Sudden cardiac arrest | 8 | 545 | 1.5 | 3 | 263 | 1.1 | 0.3 (−1.4~−2.0) | 0.88 (0.18~4.21) | 0.875 |
| Medical therapy (N=329) | Ablation (N=184) | ||||||||
| Non-frail patients | |||||||||
| All-cause death | 99 | 1,601 | 6.2 | 26 | 745 | 3.5 | 2.7 (0.7~4.7) | 0.48 (0.30~0.79) | 0.004 |
| Composite outcome‡ | 148 | 1,326 | 11.2 | 47 | 679 | 6.9 | 4.2 (1.4~7.1) | 0.54 (0.38~0.75) | <0.001 |
| Heart failure admission | 48 | 1,453 | 3.3 | 17 | 698 | 2.4 | 0.9 (−0.7~2.4) | 0.66 (0.37~1.19) | 0.166 |
| Ischemic stroke/SE | 57 | 1,428 | 4.0 | 17 | 718 | 2.4 | 1.6 (−0.04~3.3) | 0.60 (0.34~1.05) | 0.075 |
| Sudden cardiac arrest | 14 | 1,574 | 0.9 | 1 | 745 | 0.1 | 0.8 (0.1~1.5) | 0.12 (0.01~1.06) | 0.056 |
†Adjusted for age, sex, income, AF duration, CHA2DS2-VASc score, modified HAS-BLED score, Hospital Frailty Risk score, Charlson comorbidity index, hypertension, diabetes, ischemic stroke/TIA, myocardial infarction, peripheral arterial disease, HCMP, chronic kidney disease, end-stage renal disease, malignancy, hyperthyroidism, hypothyroidism, history of venous thromboembolism, COPD, history of intracranial bleeding, previous cardioversion, history of any bleeding, baseline use of warfarin, non-vitamin K antagonist OAC, aspirin, clopidogrel, β-blocker, ACE-inhibitor/ARB, dihydropyridine/nondihydropyridine CCB, statin, diuretics, and digoxin, and OAC coverage rate of time at risk. ‡Composite outcome was a composite of all-cause death, heart failure admission, ischemic stroke/SE, and sudden cardiac arrest. CI, confidence interval; HR, hazard ratio; SE, systemic embolism. Other abbreviations are as per Table 1.
In non-frail elderly patients, during a median (25th, 75th percentiles) follow up of 41 (21, 71) months, the cumulative incidence of all-cause death was significantly lower in the ablation group compared to the medical therapy group (P=0.019, Figure 2A Lower panel). Ablation was related to lower incidence and 52% lower risk of all-cause death (3.5 and 6.2 per 100 person-years, HR 0.48, 95% CI 0.30–0.79, P=0.004) compared to the medical therapy alone (Table 2). Other factors associated with an increased risk of all-cause death included: older age (per 10 increase: HR 2.40, 95% CI 1.96–2.94, P<0.001), and higher Hospital Frailty Risk scores (per 1 increase: HR 1.09, 95% CI 1.03–1.16, P=0.002).
Subgroup analyses for all-cause death in frail elderly AF patients did not show relative variations in the treatment effect of ablation large enough to be clinically significant in most examined subgroups, except in the subgroup stratified by an OAC rate (Figure 3). The risk of all-cause death was lower in the ablation group compared to the medical therapy group in frail elderly AF patients with non-optimal anticoagulation (HR 0.30, 95% CI 0.16–0.57 for non-optimal anticoagulation [proportion of days covered by OAC <80%] and HR 1.35, 95% CI 0.62–2.91 for optimal anticoagulation [proportion of days covered by OAC ≥80%], P interaction=0.021). In subgroup analyses for the risk of all-cause death in non-frail elderly AF patients, the benefit of ablation compared to medical therapy was consistent across all of the examined subgroups.

Subgroup analyses for the risk of all-cause death and composite outcome in frail elderly AF patients. AF, atrial fibrillation; HR, hazard ratio; PYs, person-years; TIA, transient ischemic attack; OAC, oral anticoagulant.
In frail elderly patients, the cumulative incidence of the composite outcome (all-cause death, heart failure admission, ischemic stroke/SE, and sudden cardiac arrest) was not significantly different in the ablation group compared to the medical therapy alone group (P=0.115, Figure 2B Upper panel). The risk of the composite outcome was not significantly reduced by ablation compared to medical therapy alone (18.2 and 26.8 per 100 person-years; HR 0.71, 95% CI 0.48–1.04, P=0.076) (Table 2).
In non-frail elderly patients, the cumulative incidence of the composite outcome was significantly lower in the ablation group compared to the medical therapy group (P=0.006 Figure 2B Lower panel). Compared to patients with medical therapy, the risk of composite outcome was reduced by 46% in patients with ablation (HR 0.54, 95% CI 0.38–0.75, P<0.001) (Table 2). Other factors associated with the increased risk of the composite outcome included: older age (per 10 increase: HR 1.70, 95% CI 1.44–2.01, P<0.001), diagnosed heart failure (HR 1.50, 95% CI 1.14–1.96, P=0.004), chronic obstructive pulmonary disease (HR 1.23, 95% CI 1.06–1.44, P=0.007) and higher Hospital Frailty Risk scores (per one increase: HR 1.14, 95% CI 1.09–1.19, P<0.001).
Subgroup analyses for the risk of the composite outcome in frail elderly AF patients did not show relative variations in the treatment effect of ablation large enough to be clinically significant in all examined subgroups (Figure 3). In subgroup analyses for the risk of the composite outcome in non-frail elderly AF patients, the benefit of ablation compared to medical therapy was consistent across all of the examined subgroups.
Secondary OutcomesBoth in frail and non-frail elderly AF patients, the cumulative incidences of heart failure admission, ischemic stroke/SE, and sudden cardiac arrest were not significantly different between ablation and medical therapy groups (Figure 4). Likewise, the incidence and risk of heart failure admission, ischemic stroke/SE, and sudden cardiac arrest were not significantly different between ablation and medical therapy groups (Table 2).
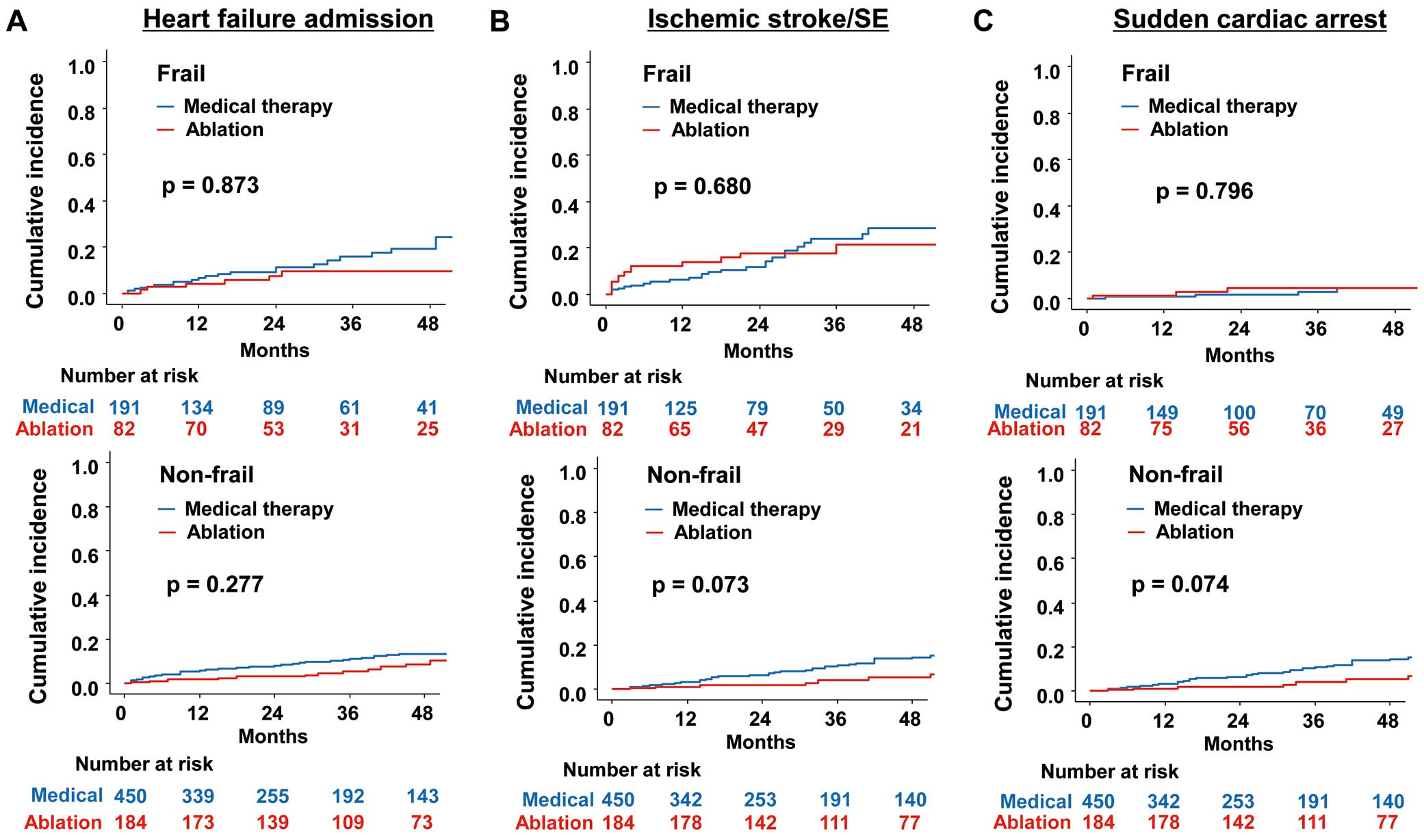
Propensity score-matched cumulative incidence curves of heart failure admission (A), ischemic stroke/SE (B), and sudden cardiac arrest (C) for frail (Upper panel) and non-frail (Lower panel) AF elderly patients according to whether ablation was performed. AF, atrial fibrillation; SE, systemic embolism.
The results using propensity score weighted AF patients were very similar to the primary results (Table 3). For all-cause death, the HR of ablation group compared to the medical therapy group was 0.91 (95% CI 0.51–1.67, P=0.781) for frail patients, and 0.55 (95% CI 0.34–0.88, P=0.013) for non-frail patients. For the composite outcome, the HR of the ablation group compared to the medical therapy group was 0.97 (95% CI 0.67–1.41, P=0.870) for frail patients, and 0.50 (95% CI 0.36–0.72, P<0.001) for non-frail patients. Ablation was also related to a lower risk of heart failure admission and ischemic stroke/SE in non-frail elderly AF patients. However, these outcomes were not changed by ablation in frail elderly AF patients.
| Number of events |
Person- years |
Event rate (100 person- years) |
Number of events |
Person- years |
Event rate (100 person- years) |
Absolute reduction in event rate (95% CI) |
Adjusted HR (95% CI)† |
P value | |
|---|---|---|---|---|---|---|---|---|---|
| Medical therapy (N=1,699) | Ablation (N=119) | ||||||||
| Frail patients | |||||||||
| All-cause death | 805 | 5,163 | 15.6 | 32 | 283 | 11.3 | 4.3 (−0.4~9.0) | 0.91 (0.51~1.67) | 0.781 |
| Composite outcome‡ | 871 | 3,497 | 24.9 | 45 | 224 | 20.2 | 4.8 (−1.9~11.5) | 0.97 (0.67~1.41) | 0.870 |
| Heart failure admission | 329 | 4,173 | 7.9 | 13 | 253 | 5.1 | 2.7 (−0.7~6.3) | 0.60 (0.32~1.11) | 0.105 |
| Ischemic stroke/SE | 315 | 4,205 | 7.5 | 17 | 221 | 7.7 | −0.2 (−3.9~3.5) | 0.74 (0.44~1.26) | 0.272 |
| Sudden cardiac arrest | 90 | 5,096 | 1.8 | 11 | 281 | 4.1 | −2.1 (−3.8~0.5) | 3.08 (0.69~1.37) | 0.139 |
| Medical therapy (N=1,677) | Ablation (N=230) | ||||||||
| Non-frail patients | |||||||||
| All-cause death | 583 | 7,656 | 7.6 | 33 | 786 | 4.2 | 3.4 (1.4~5.4) | 0.55 (0.34~0.88) | 0.013 |
| Composite outcome‡ | 872 | 6,108 | 14.8 | 54 | 712 | 7.5 | 6.7 (3.8~9.6) | 0.50 (0.36~0.72) | <0.001 |
| Heart failure admission | 306 | 6,680 | 4.6 | 18 | 730 | 2.5 | 2.1 (0.5~3.7) | 0.61 (0.38~0.96) | 0.031 |
| Ischemic stroke/SE | 294 | 6,866 | 4.3 | 19 | 763 | 2.5 | 1.8 (0.3~3.3) | 0.50 (0.31~0.82) | 0.006 |
| Sudden cardiac arrest | 56 | 7,604 | 0.7 | 1 | 786 | 0.1 | 0.6 (0.01~1.2) | 0.16 (0.02~1.24) | 0.079 |
†Adjusted for age, sex, income, AF duration, CHA2DS2-VASc score, modified HAS-BLED score, Hospital Frailty Risk score, Charlson comorbidity index, hypertension, diabetes, ischemic stroke/TIA, myocardial infarction, peripheral arterial disease, HCMP, chronic kidney disease, end-stage renal disease, malignancy, hyperthyroidism, hypothyroidism, history of venous thromboembolism, COPD, history of intracranial bleeding, previous cardioversion, history of any bleeding, baseline use of warfarin, non-vitamin K antagonist OAC, aspirin, clopidogrel, β-blocker, ACE-inhibitor/ARB, dihydropyridine/nondihydropyridine CCB, statin, diuretics, and digoxin, and OAC coverage rate of time at risk. ‡Composite outcome was a composite of all-cause death, heart failure admission, ischemic stroke/SE, and sudden cardiac death. Abbreviations are as per Tables 1,2.
Both in frail and non-frail elderly AF patients, no significant differences were observed between the ablation and medical therapy groups for all falsification endpoints: urinary tract infections, influenza, varicella-zoster virus infections, and fall accidents (Supplementary Table 5).
The main finding of this study was that the risk of all-cause death was significantly lower in non-frail patients, but there was no statistically significant mortality reduction in frail elderly AF patients with ablation than those with medical therapy. Second, non-frail patients who underwent AF ablation had a lower risk of composite outcome than those with medical therapy. However, the risk of a composite outcome was not significantly changed by ablation in frail patients. The effect of frailty risk on the outcome of ablation should be considered when deciding about ablation in elderly AF patients; this should also be evaluated in further studies.
No Significant Improvement of the Hard Outcome by Ablation in Frail Elderly AF PatientsAlthough several non-randomized follow-up studies have reported favorable outcomes such as the reduction of mortality and heart failure admission, the improvement of hard outcomes after catheter ablation of AF was not confirmed in other populations.14,15
In the real-world Korean population, catheter ablation was associated with a lower risk of all-cause death and composite outcome including all-cause death, heart failure admission, ischemic stroke/SE, and sudden cardiac arrest in non-frail elderly patients, but not in frail elderly patients with AF. The complications of catheter ablation may be even higher in a population with a high risk of frailty. Bhargava et al16 reported a higher complication rate with catheter ablation in the treatment of AF in an older population. In contrast, other studies reported that radiofrequency or cryoballoon ablation for paroxysmal AF is a feasible and safe procedure in elderly patients, with similar success and complication rates when compared to when it is used in a younger population.17,18 These differences of outcome might be related to different degrees of frailty of an elderly population. The peri-procedural major complication rate of AF catheter ablation in the meta-analysis of heart failure patients was 6.3%.19
Effect of Ablation on Other OutcomesThe risks of heart failure admission, ischemic stroke/SE, and sudden cardiac arrest were similar between patients who received ablations and those who did not. In many studies including a randomized controlled study, catheter ablation for AF consistently improved left ventricular ejection fraction and complication rates, including heart failure readmissions for heart failure patients.20 However, improvement of heart failure was not demonstrated in patients without heart failure. Although more than half of the study population had heart failure, the significant reduction of heart failure admission was not observed in this study. The prevention of ischemic stroke was observed in several non-randomized follow-up studies,14,15 but was not demonstrated in randomized control studies.5,6,20 Although this study was not a randomized control trial, the reduction of ischemic stroke or hemorrhage was not observed. This discrepancy might be related to the strict inclusion criteria for the control group having antiarrhythmic medications for >90 days, or insufficient statistical power due to the small sample size of the study population after exclusion.
Study LimitationsThe present study has several limitations. First, studies using administrative databases might be susceptible to errors arising from coding inaccuracies. To minimize this problem, we applied the definition that was validated in previous studies using the Korean NHIS sample cohort.1,10,11 As we defined AF cases only with ICD-10 codes, we could not analyze paroxysmal, persistent, and permanent AF subgroups separately. Second, the number of elderly frail patients with AF after matching was low, especially patients with ablation, and relatively underpowered. The present study findings need to be confirmed in prospective studies with larger sample sizes. Finally, causal relationships cannot be established by a retrospective registry study such as this one, and only associations can be reported. Although propensity score matching and weighting were performed to match the 2 groups, unknown confounding cannot be addressed.
Compared to medical therapy alone, ablation may be associated with a lower risk of all-cause death and composite outcome in non-frail patients, but the beneficial effect of ablation was not significant in frail elderly patients with AF. The effect of frailty risk on the outcome of ablation should be evaluated in further studies.
The National Health Information Database was provided by the National Health Insurance Service (NHIS) of Korea. The authors would like to thank the NHIS for their cooperation.
This study was supported by a research grant from the Korean Healthcare Technology R&D project funded by the Ministry of Health & Welfare, Republic of Korea (HI15C1200, HC19C0130).
B.J. has served as a speaker for Bayer, BMS/Pfizer, Medtronic, and Daiichi-Sankyo, and received research funds from Medtronic and Abbott. None of the other authors have any disclosures to make. No fees are directly received personally. The other authors have nothing to declare.
This study was approved by the institutional review board of the Yonsei University Health System (reference number: 4-2016-0179), and the requirement for informed consent was waived.
Please find supplementary file(s);
http://dx.doi.org/10.1253/circj.CJ-20-1062