Abstract
Background:
Coronavirus disease 2019 (COVID-19) reportedly causes venous thromboembolism (VTE), but the status of this complication in Japan was unclear.
Methods and Results:
The VTE and COVID-19 in Japan Study is a retrospective, multicenter cohort study enrolling hospitalized patients with COVID-19 who were evaluated with contrast-enhanced computed tomography (CT) examination at 22 centers in Japan between March 2020 and October 2020. Among 1,236 patients with COVID-19, 45 (3.6%) were evaluated with contrast-enhanced CT examination. VTE events occurred in 10 patients (22.2%), and the incidence of VTE in mild, moderate, and severe COVID-19 was 0%, 11.8%, and 40.0%, respectively. COVID-19 patients with VTE showed a higher body weight (81.6 vs. 64.0 kg, P=0.005) and body mass index (26.9 vs. 23.2 kg/m2, P=0.04), and a higher proportion had a severe status for COVID-19 compared with those without. There was no significant difference in the proportion of patients alive at discharge between patients with and without VTE (80.0% vs. 88.6%, P=0.48). Among 8 pulmonary embolism (PE) patients, all were low-risk PE.
Conclusions:
Among a relatively small number of patients undergoing contrast-enhanced CT examination in Japanese real-world clinical practice, there were no VTE patients among those with mild COVID-19, but the incidence of VTE seemed to be relatively high among severe COVID-19 patients, although all PE events were low-risk without significant effect on mortality risk.
The coronavirus disease 2019 (COVID-19) was first reported in Wuhan, China, in December 2019, and has become a huge threat worldwide as a pandemic.1,2
The main pathophysiology of COVID-19 is a respiratory infectious disease caused by the severe acute respiratory syndrome coronavirus 2, but it can also cause thromboembolic complications through coagulopathy.3,4
In particular, several studies reported a high prevalence of venous thromboembolism (VTE), including pulmonary embolism (PE) and deep vein thrombosis (DVT) in hospitalized patients with COVID-19.5–11
However, the prevalence of VTE varied widely among these reports, which might be due to the different study populations, different diagnostic strategies, and different thromboprophylaxis management.12
Furthermore, ethnic differences and distinct resource availability may have notable implications in the presentation and diagnosis of VTE. A recent surveillance questionnaire reported that the number of patients diagnosed as VTE in COVID-19 in Japan could be small compared with reports from other countries, although it is unknown whether these results suggest under-diagnosis of VTE in COVID-19 or an actual lower incidence of VTE in Japan.13,14
Further studies were warranted to clarify the current status of VTE in Japan among patients with COVID-19 who were evaluated with imaging examination, whereby under-diagnosis of VTE could be avoided. Thus, we conducted a multicenter retrospective cohort study to investigate the incidence and clinical features of VTE among hospitalized patients with COVID-19 who were evaluated with contrast-enhanced computed tomography (CT) examination during hospitalization in Japan.
Methods
Study Population
The VTE and COVID-19 in Japan Study (UMIN: 000042235) is a physician-initiated, retrospective, multicenter cohort study enrolling consecutive hospitalized patients with COVID-19 who were evaluated with contrast-enhanced CT examination during hospitalization at 22 centers in Japan between March 2020 and October 2020. The current study was conducted by the Taskforce of VTE and COVID-19 in Japan Study in a collaborative effort with the Japanese Society of Phlebology and Japanese Society of Pulmonary Embolism Research.13
We searched for consecutive patients who were diagnosed as COVID-19 with a positive polymerase chain reaction test through the hospitals’ databases, and enrolled patients with COVID-19 who were evaluated with contrast-enhanced CT examination during their hospitalization. We divided the entire cohort into 2 groups according to the presence or absence of VTE diagnosis during hospitalization and compared the patients’ characteristics, thromboprophylaxis management, and outcomes of the 2 groups.
All the procedures followed were in accordance with the Declaration of Helsinki. The relevant review boards or ethics committees in participating centers approved the research protocol. Written informed consent from each patient was waived, because we used clinical information obtained in routine clinical practice. This method concurred with the Guidelines for Epidemiological Studies issued by the Ministry of Health, Labor, and Welfare in Japan.
Data Collection and Definitions
Data on the patients’ characteristics, thromboprophylaxis management, and outcomes were collected from the hospital chart or hospital database and input by physicians at each institution using an electronic case report form and prespecified definitions. The data were also manually checked at the central office for missing or contradictory input and values out of the expected range.
Patients with mild COVID-19 were defined as those who did not require oxygen, patients with moderate COVID-19 were defined as those who required oxygen, and patients with severe COVID-19 were defined as those who required mechanical ventilation. VTE was defined as PE and/or DVT objectively confirmed by contrast-enhanced CT examination during hospitalization after the patient had tested positive for COVID-19. Heart disease was defined as heart disorders such as heart failure (HF), angina pectoris, and history of myocardial infarction. HF was diagnosed if the patient had a history of hospitalization for HF, had symptoms due to HF (New York Heart Association functional class ≥2), or the left ventricular ejection fraction was <40%. Respiratory disease was defined as persistent lung disorders such as asthma, chronic obstructive pulmonary disease, and restrictive lung diseases. History of major bleeding was diagnosed if the patient had a history of International Society of Thrombosis and Hemostasis (ISTH) major bleeding, which consisted of a reduction in the hemoglobin level by at least 2 g/dL, transfusion of at least 2 units of blood or symptomatic bleeding in a critical area or organ.15
Active cancer was defined as cancer diagnosed within the previous 6 months; recurrent, regionally advanced, or metastatic cancer; cancer for which treatment had been administered within 6 months before diagnosis; or hematologic cancer that was not in complete remission.16
Transient risk factors for VTE included recent surgery (within 2 months prior to diagnosis), recent immobilization (defined as non-surgical bed-ridden patient with bathroom privileges for >4 days within 2 months prior to diagnosis), long-distance travel (lasting ≥6 h in the previous 3 weeks), central venous catheter use, pregnancy or puerperium, recent leg trauma, a fracture or a burn (any event requiring immobilization in the past 2 months), and estrogen use. The Padua risk score was calculated for each patient as a risk assessment model of hospitalized patients at risk for VTE.17
Unfractionated heparin at therapeutic dose was defined as administration of unfractionated heparin targeting the therapeutic range with reference to the activated partial thromboplastin time (APTT) (i.e., dosing guided by the APTT until therapeutic range achieved). Unfractionated heparin at prophylactic dose was defined as administration of unfractionated heparin at a fixed dose without a reference APTT. Based on the classification in the American Heart Association guideline,18
PE was classified according to clinical severity: (1) massive PE: shock and/or hypotension (systolic blood pressure <90 mmHg or a pressure drop ≥40 mmHg for >15 min if not caused by a new-onset arrhythmia, hypovolemia or sepsis); (2) submassive PE: hemodynamically stable with right ventricular (RV) dysfunction; and (3) low-risk PE: hemodynamically stable without RV dysfunction.
Clinical outcomes evaluated in the current study were VTE events, including recurrent VTE events, bleeding events and all-cause death. Bleeding events were classified as major bleeding and clinically relevant non-major (CRNM) bleeding according to ISTH definitions.15
Major bleeding was as defined above, and CRNM bleeding was defined as overt bleeding that did not meet the criteria for major bleeding but was associated with medical intervention, unscheduled contact with a physician, interruption or discontinuation of anticoagulation therapy, or discomfort or impairment of activities of daily life.
Statistical Analysis
Categorical variables are presented as numbers and percentages. Continuous variables are presented as the mean and standard deviation or the median and interquartile range based on their distributions. Categorical variables were compared with the chi-square test when appropriate; otherwise, Fisher’s exact test was used. Continuous variables were compared using Student’s t-test or Wilcoxon’s rank sum test based on their distributions. All statistical analyses were performed with JMP version 14.0.0 (SAS Institute Inc., Cary, NC, USA). All reported P values are 2-tailed, and P<0.05 was considered statistically significant.
Results
Prevalence of Contrast-Enhanced CT Examination and Incidence of VTE
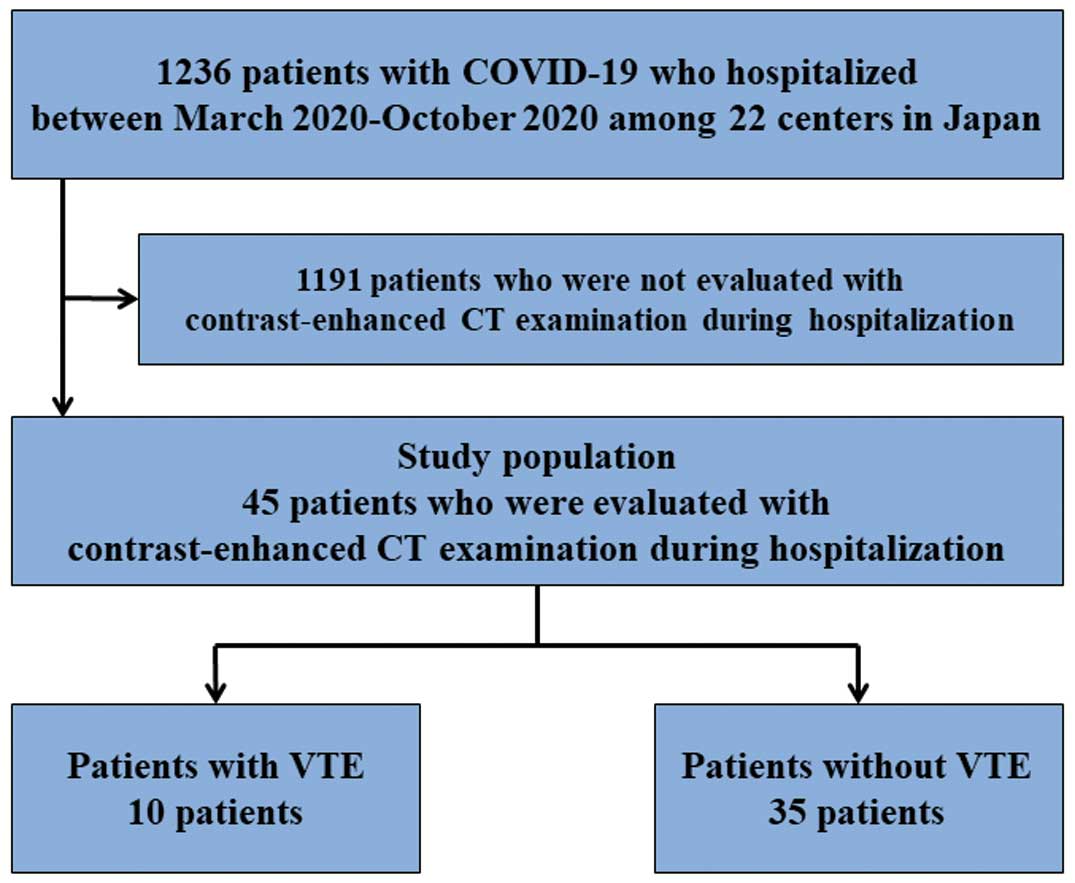
We identified 1,236 consecutive patients hospitalized with COVID-19 during the study period and of them, 45 (3.6%) were evaluated with contrast-enhanced CT examination during their hospitalization (Figure 1). Among the 45 patients, 28 (62.2%) were evaluated with contrast-enhanced CT examination due to suspicion of VTE development. In the current study population, the mean age was 67 years, 84.4% were male, and the mean body weight and body mass index (BMI) were 67.6 kg and 24.0 kg/m2, respectively. During hospitalization, VTE events occurred in 10 patients (22.0%), and the incidence of VTE in mild, moderate, and severe COVID-19 was 0%, 11.8%, and 40.0%, respectively (Figure 2).
Differences in the Characteristics of Patients With and Without VTE
There were no significant differences in the baseline characteristics or comorbidities between the patients with and without VTE, except for higher body weight, height, and BMI in the patients with VTE (81.6 vs. 64.0 kg, P=0.005; 172.3 vs. 164.7 cm, P=0.01; 26.9 vs. 23.2 kg/m2, P=0.04, respectively) (Table 1). The proportions of patients who required mechanical ventilation and extracorporeal membrane oxygenation were significantly higher among the patients with VTE (80.0% vs. 34.3%, P=0.01; 40.0% vs. 2.9%, P=0.006, respectively).
Table 1.
Patients’ Characteristics, Thromboprophylaxis Managements, and Outcomes
| |
Total
(n=45) |
Patients with
VTE (n=10) |
Patients without
VTE (n=35) |
P value |
| Baseline characteristics |
| Age (years) |
67.2±13.2 |
62.6±11.2 |
68.5±13.6 |
0.21 |
| >70 years |
21 (46.6%) |
2 (20.0%) |
19 (54.3%) |
0.08 |
| Male |
38 (84.4%) |
10 (100%) |
28 (80.0%) |
0.32 |
| Body weight (kg) |
67.6±17.2 |
81.6±24.1 |
64.0±13.0 |
0.005 |
| Height (cm) |
166.5±8.6 |
172.3±6.3 |
164.7±8.5 |
0.01 |
| Body mass index (kg/m2) |
24.0±4.9 |
26.9±7.0 |
23.2±3.8 |
0.04 |
| >30 kg/m2 |
2 (4.4%) |
1 (10.0%) |
1 (2.9%) |
0.40 |
| D-dimer level at admission (μg/mL) |
1.9 (1.2–3.2) |
1.4 (1.1–2.3) |
2.2 (1.4–4.2) |
0.13 |
| Hospitalization |
| ICU |
25 (55.6%) |
7 (70.0%) |
18 (51.4%) |
0.47 |
| General ward |
20 (44.4%) |
3 (30.0%) |
17 (48.6%) |
|
| Comorbidities |
| Hypertension |
25 (55.6%) |
5 (50.0%) |
20 (57.1%) |
0.73 |
| Diabetes mellitus |
17 (37.8%) |
6 (60.0%) |
11 (31.4%) |
0.14 |
| Heart disease |
10 (22.2%) |
1 (10.0%) |
9 (25.7%) |
0.42 |
| Respiratory disease |
8 (17.8%) |
1 (10.0%) |
7 (20.0%) |
0.66 |
| Active cancer |
3 (6.7%) |
1 (10.0%) |
2 (5.7%) |
0.54 |
| History of major bleeding |
1 (2.2%) |
0 (0%) |
1 (2.9%) |
1.00 |
| History of VTE |
1 (2.2%) |
0 (0%) |
1 (2.9%) |
1.00 |
| VTE risk at admission |
| Transient risk factors |
19 (42.2%) |
7 (70.0%) |
12 (34.3%) |
0.04 |
| Padua risk score |
2 (2–5) |
4 (2–4) |
2 (1–5) |
0.61 |
| ≥4 |
17 (37.8%) |
6 (60.0%) |
11 (31.4%) |
0.14 |
| Severity of COVID-19 |
| Need oxygen |
37 (82.2%) |
10 (100%) |
27 (77.1%) |
0.17 |
| Need mechanical ventilation |
20 (44.4%) |
8 (80.0%) |
12 (34.3%) |
0.01 |
| Need ECMO |
5 (11.1%) |
4 (40.0%) |
1 (2.9%) |
0.006 |
| Thromboprophylaxis management |
| Compression stockings/intermittent pneumatic compression |
24 (53.3%) |
9 (90.0%) |
15 (42.9%) |
0.01 |
| Anticoagulants |
30 (66.7%) |
8 (80.0%) |
22 (62.9%) |
0.31 |
| Unfractionated heparin at prophylactic dose |
14/30 (46.7%) |
3/8 (37.5%) |
11/22 (50.0%) |
0.86 |
| Unfractionated heparin at therapeutic dose |
9/30 (30.0%) |
3/8 (37.5%) |
6/22 (27.3%) |
|
| Low-molecular-weight heparin |
4/30 (13.3%) |
1/8 (12.5%) |
3/22 (13.6%) |
|
| Direct oral anticoagulants |
2/30 (6.7%) |
1/8 (12.5%) |
1/22 (4.6%) |
|
| Warfarin |
1/30 (3.3%) |
0/8 (0%) |
1/22 (4.6%) |
|
| CT examination for suspicion of VTE |
28 (62.2%) |
9 (90.0%) |
19 (54.3%) |
0.04 |
| Bleeding events |
8 (17.8%) |
5 (50.0%) |
3 (8.6%) |
0.003 |
| Major bleeding |
5 (11.1%) |
3 (30.0%) |
2 (5.7%) |
0.06 |
| Alive at discharge |
39 (86.7%) |
8 (80.0%) |
31 (88.6%) |
0.48 |
Transient risk factors for VTE included recent surgery (within 2 months prior to diagnosis), recent immobilization (non-surgical bed-ridden patients with bathroom privileges for >4 days within 2 months prior to diagnosis), long-distance travel (lasting ≥6 h in the previous 3 weeks), central venous catheter use, pregnancy or puerperium, recent leg trauma, a fracture or a burn (any event requiring immobilization in the past 2 months), and estrogen use. Unfractionated heparin at therapeutic dose was defined as administration of unfractionated heparin targeting the therapeutic range with reference to the APTT (i.e., dosing guided by the APTT until therapeutic range achieved). Unfractionated heparin at prophylactic dose was defined as administration of unfractionated heparin at a fixed dose without a reference of APTT. APTT, activated partial thromboplastin time; COVID-19, coronavirus disease 2019; CT, computed tomography; ECMO, extracorporeal membrane oxygenation; ICU, intensive care unit; VTE, venous thromboembolism.
In the current study population, 24 patients (53.3%) received compression stockings or intermittent pneumatic compression, and 30 patients (66.7%) received anticoagulants (Table 1). Among the patients with anticoagulant treatment, 46.7% received unfractionated heparin at a prophylactic dose, 30.0% of patients received unfractionated heparin at a therapeutic dose, and 13.3% of patients received low-molecular-weight heparin. Among patients with VTE, 80.0% of received anticoagulants, and 37.5% received unfractionated heparin at a therapeutic dose.
There were no recurrent VTE events during hospitalization. Among the 45 patients, there were 8 bleeding events (17.8%) including 5 major bleeding events (11.1%). There was no significant difference in the proportion of patients alive at discharge between those with and without VTE (80.0% vs. 88.6%, P=0.48).
Clinical Features of VTE in COVID-19 Patients
The median time from admission to VTE onset was 18 days: 50.0% of patients were diagnosed as VTE in the intensive care unit (ICU) and 50.0% were diagnosed as VTE in the general ward (Table 2). Among 10 VTE patients, 8 (80.0%) were diagnosed as PE with or without DVT, and all cases were low-risk PE. As antiviral therapies for COVID-19, 7 patients received favipiravir, 4 patients received remdesivir, 2 patients received ivermectin, and 1 patient received tocilizumab. For the treatment of VTE, 7 patients received with unfractionated heparin at a therapeutic dose or increased dose targeting a higher therapeutic range with a reference of APTT, 2 patients received rivaroxaban as an initial intensive regimen, and 1 patient received edoxaban at the normal dose.
Table 2.
Detailed Presentation of VTE Cases
| |
Patients (n=10) |
| Days from admission to VTE onset |
18 (9–31) |
| VTE diagnosis |
| ICU |
5 (50.0%) |
| General ward |
5 (50.0%) |
| D-dimer level at diagnosis (μg/mL) |
10.0 (7.4–19.5) |
| VTE type |
| PE with DVT |
2 (20.0%) |
| PE only |
6 (60.0%) |
| DVT only |
2 (20.0%) |
| Severity of PE (n=8) |
| Massive |
0/8 (0%) |
| Submassive |
0/8 (0%) |
| Low-risk |
8/8 (100%) |
| Location of DVT (n=4) |
| Proximal DVT |
2/4 (50.0%) |
| Distal DVT |
2/4 (50.0%) |
The severity of PE was classified according to the clinical severity: (1) massive PE: shock and/or hypotension (systolic blood pressure <90 mmHg or a pressure drop ≥40 mmHg for >15 min if not caused by a new-onset arrhythmia, hypovolemia or sepsis); (2) submassive PE: hemodynamically stable with right ventricular dysfunction; and (3) low-risk PE: hemodynamically stable without right ventricular dysfunction. Proximal DVT was defined as venous thrombosis located in the popliteal, femoral, or iliac vein. Distal DVT was defined as venous thrombosis located in calf veins, including the peroneal, posterior tibial, anterior tibial, and soleus muscle veins below the knee without proximal DVT. DVT, deep vein thrombosis; ICU, intensive care unit; PE, pulmonary embolism; VTE, venous thromboembolism.
Discussion
The main findings of the current study were as follows. (1) The number of patients who were evaluated with contrast-enhanced CT examination during hospitalization in real-world clinical practice was considerably small. (2) There were no VTE patients among mild COVID-19 patients, whereas the incidence of VTE seemed to be relatively high among severe COVID-19 patients. (3) COVID-19 patients with VTE showed a higher body weight and BMI, and a higher proportion had a severe status for COVID-19 compared with those without. (4) There was no significant difference in the mortality risk between patients with and without VTE, and all PE events were low-risk in the population with high suspicion of PE.
A number of previous studies suggested that hospitalized patients with COVID-19 could have a high risk for developing VTE, with a higher incidence associated with routine screening by imaging and inclusion of critically ill patients including ICU patients,19,20
Notably, the current study found only a small number of patients were evaluated with contrast-enhanced CT examination in Japanese real-world clinical practice, which suggests some reluctance to conduct imaging examinations due to the risk of infection to healthcare providers. The current study also showed that there were no cases of VTE among mild COVID-19 patients who did not require oxygen. Although previous studies reported relatively high incidences, even in non-ICU hospitalized patients,19,20
our results suggested that patients with the least severity for COVID-19, such as patients who do not require oxygen, were at a lower risk of VTE among non-ICU hospitalized patients in Japan. However, the current study showed that the incidence of VTE was considerably high among severe COVID-19 patients who might be suspected of VTE and evaluated with contrast-enhanced CT examination, which seems consistent with the quite high incidence of VTE in previous studies of ICU patients with routine screening by imaging.19,20
Considering the especially high risk of VTE in severe COVID-19 patients, clinicians might wish conduct an appropriate imaging examination of these patients when they are suspected of VTE during the course of COVID-19 treatment to avoid under-diagnosis of VTE.
Although all hospitalized patients have a risk for developing VTE, the risk could differ widely depending on each patient. A previous study reported that VTE patients with COVID-19 had a high median BMI (30.2 kg/m2).6
Another study also reported a high mean body weight (87 kg).7
Consistent with those previous reports, the current study showed COVID-19 patients with VTE had a higher body weight and BMI compared with those without VTE. Obesity is a well-known risk factor for the development of VTE,21
and must be considered as an especially important risk factor for the development of VTE in COVID-19 patients. The current study also revealed that among COVID-19 patients with VTE a higher proportion had severe status for COVID-19 compared with those without. Most of the patients with VTE received anticoagulants as thromboprophylaxis. In line with the current study, the previous studies also showed a higher incidence of VTE in ICU patients despite thromboprophylaxis by anticoagulants,5,7
which suggests that patients with severe disease are at especially high risk of VTE despite anticoagulant drugs.
In addition to developing VTE, the severity of VTE, including PE, might be clinically relevant in terms of the clinical impact of VTE on prognosis. The previous studies reported a high prevalence of incidental thrombosis in small and mid-sized pulmonary arteries despite thromboprophylaxis.22,23
In particular, studies using a screening strategy with imaging examinations for critically ill patients showed a strikingly high prevalence of VTE, including minor and asymptomatic VTE, although it remains controversial whether VTE was the cause of death or only a concurrent event.12
Interestingly, the current study showed that all PE events were low-risk, and there was no significant difference in the mortality risk between patients with and without VTE. It is unknown whether these results could be due to the high proportion of thromboprophylaxis by anticoagulants or a relatively low risk of severe PE in COVID-19 patients. On the other hand, COVID-19 patients are also reported to be at a high risk for bleeding,20
and bleeding events sometimes can be critical, in which case anticoagulation therapy might be potentially harmful. Patients with COVID-19 have been reported to be at a relatively high risk for bleeding events with anticoagulation therapy.24
In addition, there is concern that anticoagulation might be associated with a higher risk of bleeding, more particularly in Asians than in Caucasians.25,26
Thus, it is important to assess the balance between thrombotic and bleeding risks in individual patients, including ethnic differences, when clinicians consider thromboprophylaxis by anticoagulants.27,28
Currently, based on the concept of a high risk for VTE in patients with COVID-19, the primary prevention of VTE in COVID-19 by anticoagulants has been drawing attention. In fact, some studies report that use of anticoagulants was associated with reduced mortality in patients hospitalized with COVID-19,29,30
and the effect was more remarkable in patients with severe condition. These results suggest a potential benefit of anticoagulants for better prognosis and prevention of VTE in COVID-19, especially those with severe condition such as ICU patients. Although several consensus statements have recommended thromboprophylaxis by anticoagulants in all patients hospitalized due to COVID-19,31–33
the optimal strategies for the prevention of VTE in COVID-19, including good candidates, intensity, and duration of anticoagulation therapy, as well as ethnic differences still remain unknown, and need to be revealed through further studies.
Study Limitations
First, the current study was based on observational cohort data and the decisions regarding the management strategies, including imaging examination, were at the discretion of the attending physicians. Therefore, we could not deny the potential for selection bias. Second, the absolute number of patients was relatively small, although it was derived from a multicenter cohort study in Japan. Third, demographics and practice patterns as well as the clinical outcomes in patients at the participating centers may be different from those at other centers. Thus, it should be interpreted with caution whether the current results can be extrapolated to patients at all institutions in Japan. Fourth, we could not completely deny the possibility of concomitant VTE on admission because patients were not evaluated with screening contrast-enhanced CT examination at that time.
Conclusions
Among a relatively small number of patients undergoing contrast-enhanced CT examination in Japanese real-world clinical practice, there were no cases of VTE among mild COVID-19 patients, but the incidence of VTE seemed to be relatively high among severe COVID-19 patients, although all PE events were low-risk PE without a significant impact on mortality risk.
Acknowledgments
We appreciate the support and collaboration of the Japanese Society of Phlebology and the Japanese Society of Pulmonary Embolism Research throughout the current study. We are indebted to Ms. Emi Kuroki from the Japanese Society of Phlebology for technical support.
Data Availability
The data, analytic methods, and study materials will not be made available to other researchers for the purposes of reproducing the results or replicating the procedure. However, if the relevant review board or ethics committee approve the data sharing and all investigators give their consent, the deidentified participant data will be shared on a request basis through the principal investigator. The study protocol and statistical analysis plan will also be available. The data will be shared as Excel files via E-mail during the proposed publication period.
Conflicts of Interest
T.K. has received honorariums from Nippon Covidien Ltd for lectures. All other authors report that they have no relationships relevant to the contents of this paper to disclose.
Funding
This research did not receive any specific grant from funding agencies in the public, commercial, or not-for-profit sectors.
Disclosures
T.K. has received honorariums from Nippon Covidien Ltd for lectures. All other authors report that they have no relationships relevant to the contents of this paper to disclose. This research did not receive any specific grant from funding agencies in the public, commercial, or not-for-profit sectors.
IRB Information
The relevant review boards or ethics committees in participating centers approved the research protocol. The ethics committee of the primary institution was the Ethics Committee of Kuwana City Medical Center (approval no. 2020-171).
References
- 1.
Guan WJ, Ni ZY, Hu Y, Liang WH, Ou CQ, He JX, et al. Clinical characteristics of coronavirus disease 2019 in China. N Engl J Med 2020; 382: 1708–1720.
- 2.
Huang C, Wang Y, Li X, Ren L, Zhao J, Hu Y, et al. Clinical features of patients infected with 2019 novel coronavirus in Wuhan, China. Lancet 2020; 395: 497–506.
- 3.
Zhang Y, Xiao M, Zhang S, Xia P, Cao W, Jiang W, et al. Coagulopathy and antiphospholipid antibodies in patients with Covid-19. N Engl J Med 2020; 382: e38.
- 4.
Tang N, Li D, Wang X, Sun Z. Abnormal coagulation parameters are associated with poor prognosis in patients with novel coronavirus pneumonia. J Thromb Haemost 2020; 18: 844–847.
- 5.
Cui S, Chen S, Li X, Liu S, Wang F. Prevalence of venous thromboembolism in patients with severe novel coronavirus pneumonia. J Thromb Haemost 2020; 18: 1421–1424.
- 6.
Llitjos JF, Leclerc M, Chochois C, Monsallier JM, Ramakers M, Auvray M, et al. High incidence of venous thromboembolic events in anticoagulated severe COVID-19 patients. J Thromb Haemost 2020; 18: 1743–1746.
- 7.
Klok FA, Kruip M, van der Meer NJM, Arbous MS, Gommers D, Kant KM, et al. Confirmation of the high cumulative incidence of thrombotic complications in critically ill ICU patients with COVID-19: An updated analysis. Thromb Res 2020; 191: 148–150.
- 8.
Poissy J, Goutay J, Caplan M, Parmentier E, Duburcq T, Lassalle F, et al. Pulmonary embolism in patients with COVID-19: Awareness of an increased prevalence. Circulation 2020; 142: 184–186.
- 9.
Zhang L, Feng X, Zhang D, Jiang C, Mei H, Wang J, et al. Deep vein thrombosis in hospitalized patients with COVID-19 in Wuhan, China: Prevalence, risk factors, and outcome. Circulation 2020; 142: 114–128.
- 10.
Cohen SL, Gianos E, Barish MA, Chatterjee S, Kohn N, Lesser M, et al. Prevalence and predictors of venous thromboembolism or mortality in hospitalized COVID-19 patients. Thromb Haemost, doi:10.1055/a-1366-9656.
- 11.
Li JY, Wang HF, Yin P, Li D, Wang DL, Peng P, et al. Clinical characteristics and risk factors for symptomatic venous thromboembolism in hospitalized COVID-19 patients: A multicenter retrospective study. J Thromb Haemost, doi:10.1111/jth.15261.
- 12.
Bikdeli B, Madhavan MV, Jimenez D, Chuich T, Dreyfus I, Driggin E, et al. COVID-19 and thrombotic or thromboembolic disease: Implications for prevention, antithrombotic therapy, and follow-up [JACC State-of-the-Art Review]. J Am Coll Cardiol 2020; 75: 2950–2973.
- 13.
Yamashita Y, Yamada N, Mo M. The primary prevention of venous thromboembolism in patients with COVID-19 in Japan: Current status and future perspective. Ann Vasc Dis 2021; 14: 1–4.
- 14.
Yamashita Y, Hara N, Obana M, Ikeda S, Furuichi M, Ishiguro S, et al. Clinical features of venous thromboembolism in patients with coronavirus disease 2019 (COVID-19) in Japan: A case series study. Circ J 2021; 85: 309–313.
- 15.
Schulman S, Kearon C, Subcommittee on Control of Anticoagulation of the Scientific and Standardization Committee of the International Society on Thrombosis and Haemostasis. Definition of major bleeding in clinical investigations of antihemostatic medicinal products in non-surgical patients. J Thromb Haemost 2005; 3: 692–694.
- 16.
Raskob GE, van Es N, Verhamme P, Carrier M, Di Nisio M, Garcia D, et al. Edoxaban for the treatment of cancer-associated venous thromboembolism. N Engl J Med 2018; 378: 615–624.
- 17.
Barbar S, Noventa F, Rossetto V, Ferrari A, Brandolin B, Perlati M, et al. A risk assessment model for the identification of hospitalized medical patients at risk for venous thromboembolism: The Padua Prediction Score. J Thromb Haemost 2010; 8: 2450–2457.
- 18.
Jaff MR, McMurtry MS, Archer SL, Cushman M, Goldenberg N, Goldhaber SZ, et al. Management of massive and submassive pulmonary embolism, iliofemoral deep vein thrombosis, and chronic thromboembolic pulmonary hypertension: A Scientific Statement from the American Heart Association. Circulation 2011; 123: 1788–1830.
- 19.
Nopp S, Moik F, Jilma B, Pabinger I, Ay C. Risk of venous thromboembolism in patients with COVID-19: A systematic review and meta-analysis. Res Pract Thromb Haemost 2020; 4: 1179–1191.
- 20.
Jiménez D, García-Sanchez A, Rali P, Muriel A, Bikdeli B, Ruiz-Artacho P, et al. Incidence of VTE and bleeding among hospitalized patients with coronavirus disease 2019: A systematic review and meta-analysis. Chest 2021; 159: 1182–1196.
- 21.
Klovaite J, Benn M, Nordestgaard BG. Obesity as a causal risk factor for deep venous thrombosis: A Mendelian randomization study. J Intern Med 2015; 277: 573–584.
- 22.
Danzi GB, Loffi M, Galeazzi G, Gherbesi E. Acute pulmonary embolism and COVID-19 pneumonia: A random association? Eur Heart J 2020; 41: 1858.
- 23.
Lax SF, Skok K, Zechner P, Kessler HH, Kaufmann N, Koelblinger C, et al. Pulmonary arterial thrombosis in COVID-19 with fatal outcome: Results from a prospective, single-center, clinicopathologic case series. Ann Intern Med 2020; 173: 350–361.
- 24.
Al-Samkari H, Gupta S, Leaf RK, Wang W, Rosovsky RP, Brenner SK, et al. Thrombosis, bleeding, and the observational effect of early therapeutic anticoagulation on survival in critically ill patients with COVID-19. Ann Intern Med, doi:10.7326/M20-6739.
- 25.
Chiang CE, Wang KL, Lip GY. Stroke prevention in atrial fibrillation: An Asian perspective. Thromb Haemost 2014; 111: 789–797.
- 26.
Oldgren J, Healey JS, Ezekowitz M, Commerford P, Avezum A, Pais P, et al. Variations in cause and management of atrial fibrillation in a prospective registry of 15,400 emergency department patients in 46 countries: The RE-LY Atrial Fibrillation Registry. Circulation 2014; 129: 1568–1576.
- 27.
Iba T, Connors JM, Spyropoulos AC, Wada H, Levy JH. Ethnic differences in thromboprophylaxis for COVID-19 patients: Should they be considered? Int J Hematol 2021; 113: 330–336.
- 28.
Yamashita Y, Morimoto T, Amano H, Takase T, Hiramori S, Kim K, et al. Anticoagulation therapy for venous thromboembolism in the real world: From the COMMAND VTE registry. Circ J 2018; 82: 1262–1270.
- 29.
Tang N, Bai H, Chen X, Gong J, Li D, Sun Z. Anticoagulant treatment is associated with decreased mortality in severe coronavirus disease 2019 patients with coagulopathy. J Thromb Haemost 2020; 18: 1094–1099.
- 30.
Paranjpe I, Fuster V, Lala A, Russak AJ, Glicksberg BS, Levin MA, et al. Association of treatment dose anticoagulation with in-hospital survival among hospitalized patients with COVID-19. J Am Coll Cardiol 2020; 76: 122–124.
- 31.
Spyropoulos AC, Levy JH, Ageno W, Connors JM, Hunt BJ, Iba T, et al. Scientific and Standardization Committee communication: Clinical guidance on the diagnosis, prevention, and treatment of venous thromboembolism in hospitalized patients with COVID-19. J Thromb Haemost 2020; 18: 1859–1865.
- 32.
Zhai Z, Li C, Chen Y, Gerotziafas G, Zhang Z, Wan J, et al. Prevention and treatment of venous thromboembolism associated with coronavirus disease 2019 infection: A Consensus Statement before Guidelines. Thromb Haemost 2020; 120: 937–948.
- 33.
Moores LK, Tritschler T, Brosnahan S, Carrier M, Collen JF, Doerschug K, et al. Prevention, diagnosis, and treatment of VTE in patients with coronavirus disease 2019: CHEST Guideline and Expert Panel Report. Chest 2020; 158: 1143–1163.