論文ID: CJ-21-0278
論文ID: CJ-21-0278
Background: Because it is unclear whether lower urinary tract symptoms (LUTS) are associated with cardiovascular disease (CVD) in the Japanese population, we explored the association in general Japanese men aged 55–75 years.
Methods and Results: The cross-sectional study included male participants who had both national health checkup data and the International Prostate Symptom Score (IPSS) in the same calendar year between 2009 and 2017. LUTS severity was evaluated by IPSS. A robust Poisson regression model was used to assess the association between LUTS severity and the composite CVD outcome [coronary artery disease (CAD), stroke, or atrial fibrillation (AF)] and each component of the composite outcome. Prevalence ratio (PR) was adjusted for conventional cardiovascular risk factors. Of 16,781 male participants (mean age, 67±5 years), mild LUTS were observed in 9,243 (55.1%); moderate, 6,445 (38.4%); and severe, 1,093 (6.5%). Compared with the mild LUTS group, moderate LUTS [PR 1.18, 95% confidence interval (CI) 1.10–1.25, P<0.001] and severe LUTS (PR 1.38, 95% CI 1.24–1.53, P<0.001) were significantly associated with a higher prevalence of CVD. LUTS severity was associated with higher prevalence of CAD and stroke, but not AF.
Conclusions: The severity of LUTS was associated with a higher prevalence of CVD, especially CAD and stroke, independent of conventional CVD risk factors.
Lower urinary tract symptoms (LUTS) due to benign prostatic hyperplasia (BPH) are common in older men. In fact, the incidence of moderate to severe LUTS secondary to BPH is estimated to reach up to 25%.1,2 Additionally, the prevalence of LUTS is also on the rise due to population aging. Although the underlying causes of LUTS remain unclear, some recent reports demonstrate that cardiometabolic factors such as obesity, dyslipidemia, diabetes, and hypertension are associated with LUTS development and greater prostate size.3–5 A recent meta-analysis of 15 studies showed a significant association between LUTS and major adverse cardiovascular events involving coronary artery disease (CAD) and cerebrovascular disease.6 However, most of the studies were conducted in Western countries, where the prevalence of cardiovascular disease (CVD) risk factors, particularly obesity and diabetes, are more common compared with Asian countries.7–9 In fact, previous studies suggested that both obesity and metabolic syndrome led to LUTS and CVD. Therefore, the disproportionate CVD risk between Western and Asian countries may alter the association between LUTS and CVDs, and it is important to explore this association in an Asian study population due to the lack of evidence.
Editorial p ????
Atrial fibrillation (AF) is a common and clinically important arrhythmia that leads to serious cardiovascular complications such as embolic stroke and heart failure. The prevalence of AF increases in patients with advanced age, hypertension, diabetes, and ischemic heart disease, which are also shared by those with LUTS. We found that one of the urological symptoms, erectile dysfunction (ED), was associated with incident AF using prospective cohort data from the USA.10 Other studies showed that ED was associated with LUTS and/or CVD.11,12 However, the association between LUTS and AF has not been investigated, and the only available evidence is that BPH as a major cause of LUTS was associated with increased risk for incident AF using a longitudinal health insurance database in Taiwan.13
Therefore, the present study had 2 objectives; First, to explore the association between LUTS and a composite CVD outcome including AF in the general Japanese population; Second, to examine the association of LUTS with each component of the composite outcome.
Details of the National Japanese-specific health checkups were reported previously.14–16 Briefly, the national healthcare campaign was launched by the Japanese government in 2008 to strategically focus on early screening, diagnosis, and treatment of the metabolic syndrome. All unemployed or retired residents in Japan aged 40 years or older are eligible for these health checkups, which are not mandatory, and individuals can voluntarily choose whether or not to participate. Individuals are asked to complete questionnaires regarding demographic factors, past medical history, medications, and lifestyle. The basic examination consists of anthropometric measurements, physical examinations, blood tests, chest X-ray, urine dipstick test for protein, and resting 12-lead ECG. As an extension of the health checkup, approximately half of all male participants (those with an odd-numbered age between 55 and 75 years) are invited to undergo a prostate cancer screening, including a prostate-specific antigen (PSA) test and LUTS assessment based on the International Prostate Symptom Score (IPSS) (Supplementary Table 1).17
The current study involved participants exclusively from Kanazawa City, Japan. For representativeness of the current study population, the following preliminary analysis was conducted referring to the population by age in Kanazawa City in 2014.18 Of the 260,247 residents in Kanazawa City aged ≥40 years, 48,014 (18.4%) underwent the national health checkup in 2014 after excluding those with multiple visits (≥2 visits) in the same calendar year. Of these 48,014 participants, 16,996 were male and among the male participants, 4,555 individuals underwent both prostate cancer screening and a health checkup in the calendar year of 2014. Participants who underwent prostate cancer screening were much younger (69 years vs. 76 years) and had fewer cardiovascular comorbidities (antihypertensive medication: 47.8% vs. 51.3%, CAD: 12.7% vs. 16.6%, stroke: 8.9% vs. 10.6%) compared with those who did not, although the prevalence of diabetes was much higher in those who underwent prostate cancer screening (16.7% vs. 10.0%).
We excluded individuals who underwent prostate cancer screening more than once between 2009 and 2017, those who did not undergo prostate cancer screening and a health checkup in the same calendar year, and those missing baseline data (Figure 1). The Kanazawa Medical Association collected and de-identified the data. The ethics committees of the Kanazawa Medical Association and Kanazawa University approved this study (No. 16000003 and No. 3255-1, respectively). Informed consent was exempted due to utilizing completely de-identified data. Instead, an opt-out-style announcement of the outline of the present study was posted on the Kanazawa Medical Association website (http://www.kma.jp/cyberhospital.html).

CONSORT Flow chart of study inclusion.
Baseline data were obtained between April 1, 2009, and March 31, 2017 and included self-reported age, sex, past medical history, medications, and lifestyle behaviors. Past medical history included CAD and stroke; medications (antihypertensive medication, glucose-lowering medication including insulin, and lipid-lowering medication); smoking status, and alcohol intake. Smoking status was defined as current active smoking. Current alcohol intake was defined as daily average alcohol intake and excessive alcohol intake was defined as an alcohol intake >60 g ethanol/day.18 As a part of the anthropometric measurements, height (meters), weight (kilograms), and systolic/diastolic blood pressure (mmHg) were measured. Body mass index (BMI) was calculated as weight (kg) divided by the square height (m). Baseline examinations included total serum cholesterol, high-density lipoprotein cholesterol (HDL-C), triglyceride, fasting, non-fasting blood glucose, hemoglobin-A1c (HbA1c), and serum creatinine. Serum creatinine was measured using an enzymatic method. The estimated glomerular filtration rate (eGFR) was calculated using the method of the Japanese Society of Nephrology.19 A clinical chemical analyzer automatically analyzed blood samples at local laboratories within 24 h of collection.20 Diabetes was defined as any of the following: prescribed glucose-lowering medication, fasting blood glucose ≥126 mg/dL and HbA1c ≥6.5%, or non-fasting blood glucose ≥200 mg/dL and HbA1c ≥6.5%.21 Non-HDL-C was defined as total cholesterol minus HDL-C (mg/dL). The total PSA measurement was conducted using a commercial kit (Tosoh, Tokyo, Japan) in primary medical facilities where the primary screening was carried out.22 All 12-lead ECGs were automatically coded, and experienced physicians confirmed the results. AF was confirmed by 12-lead ECG using the Minnesota code (8-3).
Severity of LUTSLUTS severity was categorized into 3 groups (mild, moderate, and severe) based on the IPSS (Supplementary Table 1).17 The IPSS is based on the answers to 7 questions regarding urinary symptoms (incomplete emptying, frequency, intermittency, urgency, weak stream, straining, and nocturia) and 1 question about quality of life. For the 7 questions, the answers are assigned points ranging from 0 to 5, with the total score ranging from 0 to 35 (asymptomatic to severely symptomatic). LUTS severity was defined based on the total IPSS: mild, 0–7: moderate, 8–19: severe, 20–35.
OutcomesThe primary outcome was defined as a composite outcome including self-reported CAD or self-reported stroke or AF confirmed by 12-lead ECG. CAD was defined based on a single question about a history of heart disease, including angina pectoris and myocardial infarction, or related treatments. Stroke was defined based on a single question regarding a history of stroke such as intracranial hemorrhage and ischemic stroke or related treatments. Ascertainment of self-reported CAD and stroke was implemented in a previous study.18 Self-reported CAD and stroke had excellent diagnostic ability (CAD: sensitivity 99.78%, specificity 99.98%, positive predictive value (PPV) 99.89%, and negative predictive value (NPV) 99.97%; Stroke: sensitivity 99.55%, specificity 100%, PPV 100%, and NPV 99.97%).
Statistical AnalysisBaseline characteristics were summarized by the 3 groups based on the severity of LUTS. Continuous variables are presented as mean±standard deviation or median (interquartile range, IQR). Categorical variables are presented as numbers (%). The distribution of the total IPSS was summarized using a histogram.
The odds ratio (OR) is frequently used as a proxy for the prevalence ratio (PR) in cross-sectional studies. We used a robust Poisson regression to measure the association between LUTS and the composite outcome because the outcome was common (>10%) and the OR is reported to overestimate the PR for common outcomes.23 To measure the association between the severity of LUTS and the composite outcome in this study, a robust Poisson regression model was used to compute unadjusted and adjusted PRs for the composite outcome compared with the mild symptom group as the referent. PRs were adjusted for the following three models: Model 1 adjusted for age; Model 2 adjusted for Model 1 covariate plus systolic blood pressure, BMI, history of diabetes, current smoking, excessive alcohol intake, antihypertensive medication, lipid-lowering medication, eGFR, and NHDL-C; Model 3 adjusted for Model 2 covariates plus total PSA. All covariates other than total PSA are components of the Framingham CVD risk score.
Subgroup Analysis The prevalence and severity of LUTS increase with aging, and a subgroup analysis by age group (≥65 years or <65 years) was added. Multiplicative interaction between age groups and the severity of LUTS was assessed. Furthermore, we also examined the effect of prostate treatment history (none or current/former) on the association between the severity of LUTS and the composite outcome.
Sensitivity Analysis The association between LUTS and the primary outcome was assessed using the IPSS as a continuous variable.
All statistical analyses were performed using statistical software (StataCorp. 2019. Stata Statistical Software: Release 16.1. StataCorp LLC, College Station, TX, USA).
Figure 1 summarizes the eligibility of the present study subjects. A total of 19,857 participants with 48,306 prostate cancer screenings between 2009 and 2017 were included. We excluded non-first prostate cancer screenings of 28,449 to include only participants with their first prostate cancer screening between 2009 and 2017. Next, participants without a simultaneous health checkup (n=2,992) and those with missing data on baseline characteristics (n=84) were also excluded, resulting in a final study population of 16,781.
Baseline characteristics are summarized in Table 1 and Figure 2. Overall, the mean age was 66.6±5.3 years, and the BMI was 23.6±3.0 kg/m2. With regard to cardiovascular comorbidities, the prevalence of diabetes was 16%, followed by CAD (12%) and stroke (8%). More than 40% of participants took antihypertensive medications, and the current smoking rate was also high (26%). Most participants did not have a history of prostate treatment (86%). Specifically, more than half of the participants accounted for mild LUTS, while severe LUTS was observed in 1,093 participants (Figure 2). Severe LUTS was more likely to be observed in older participants with >1 comorbidity. Similarly, a former or current history of prostate treatment was more frequently observed in those with moderate or severe LUTS. In contrast, the percentage of everyday alcohol intake was lower in participants with severe LUTS than moderate or mild LUTS. The median total PSA was 1.0 (IQR: 0.6–1.7) in the overall population, and a higher total PSA was associated with advanced LUTS severity (moderate or severe).
| Total (n=16,781) |
Mild LUTS (n=9,243) |
Moderate LUTS (n=6,445) |
Severe LUTS (n=1,093) |
|
|---|---|---|---|---|
| Age, years | 66.6 (5.3) | 66.1 (5.4) | 67.1 (5.1) | 67.4 (5.1) |
| SBP, mmHg | 129 (16) | 130 (16) | 129 (16) | 128 (16) |
| DBP, mmHg | 77 (11) | 78 (10) | 77 (11) | 77 (11) |
| BMI, kg/m2 | 23.6 (3.0) | 23.6 (3.0) | 23.5 (3.0) | 23.4 (3.0) |
| Diabetes, n (%) | 2,741 (16) | 1,432 (15) | 1,109 (17) | 200 (18) |
| Coronary artery disease, n (%) | 2,018 (12) | 974 (11) | 869 (13) | 175 (16) |
| Stroke, n (%) | 1,355 (8) | 632 (7) | 599 (9) | 124 (11) |
| Antihypertensive medication, n (%) | 7,313 (44) | 3,923 (42) | 2,913 (45) | 477 (44) |
| Lipid-lowering medication, n (%) | 3,434 (20) | 1,863 (20) | 1,338 (21) | 233 (21) |
| Current smoking, n (%) | 4,281 (26) | 2,356 (25) | 1,642 (25) | 283 (26) |
| Everyday alcohol intake, n (%) | 8,298 (49) | 4,691 (51) | 3,121 (48) | 486 (45) |
| Excessive alcohol intake, n (%) | 576 (3) | 304 (3) | 226 (4) | 46 (4) |
| eGFR, mL/min/1.73 m2 | 73.2 (15.7) | 73.5 (15.6) | 72.9 (15.6) | 72.5 (16.8) |
| Non-HDL cholesterol, mg/dL | 141 (34) | 142 (34) | 141 (33) | 141 (34) |
| Total PSA, ng/mL | 1.0 (0.6–1.7) | 0.9 (0.6–1.6) | 1.1 (0.6–1.9) | 1.1 (0.7–2.1) |
| Left ventricular hypertrophy, n (%) | 591 (4) | 326 (4) | 233 (4) | 32 (3) |
| Atrial fibrillation, n (%) | 373 (2) | 197 (2) | 147 (2) | 29 (3) |
| History of prostate medication, n (%) | ||||
| None | 14,437 (86) | 8,409 (91) | 5,300 (82) | 728 (67) |
| Former | 1,172 (7) | 455 (5) | 552 (9) | 165 (15) |
| Current | 1,061 (6) | 289 (3) | 575 (9) | 197 (18) |
| Missing | 111 (1) | 90 (1) | 18 (0) | 3 (0) |
Continuous variables are presented as mean (standard deviation) and median (interquartile range), and categorical variables are presented as n (%). BMI, body mass index; DBP, diastolic blood pressure; eGFR, estimated glomerular filtration rate; HDL, high-density lipoprotein; LUTS, lower urinary tract symptoms; PSA, prostate-specific antigen; SBP, systolic blood pressure.
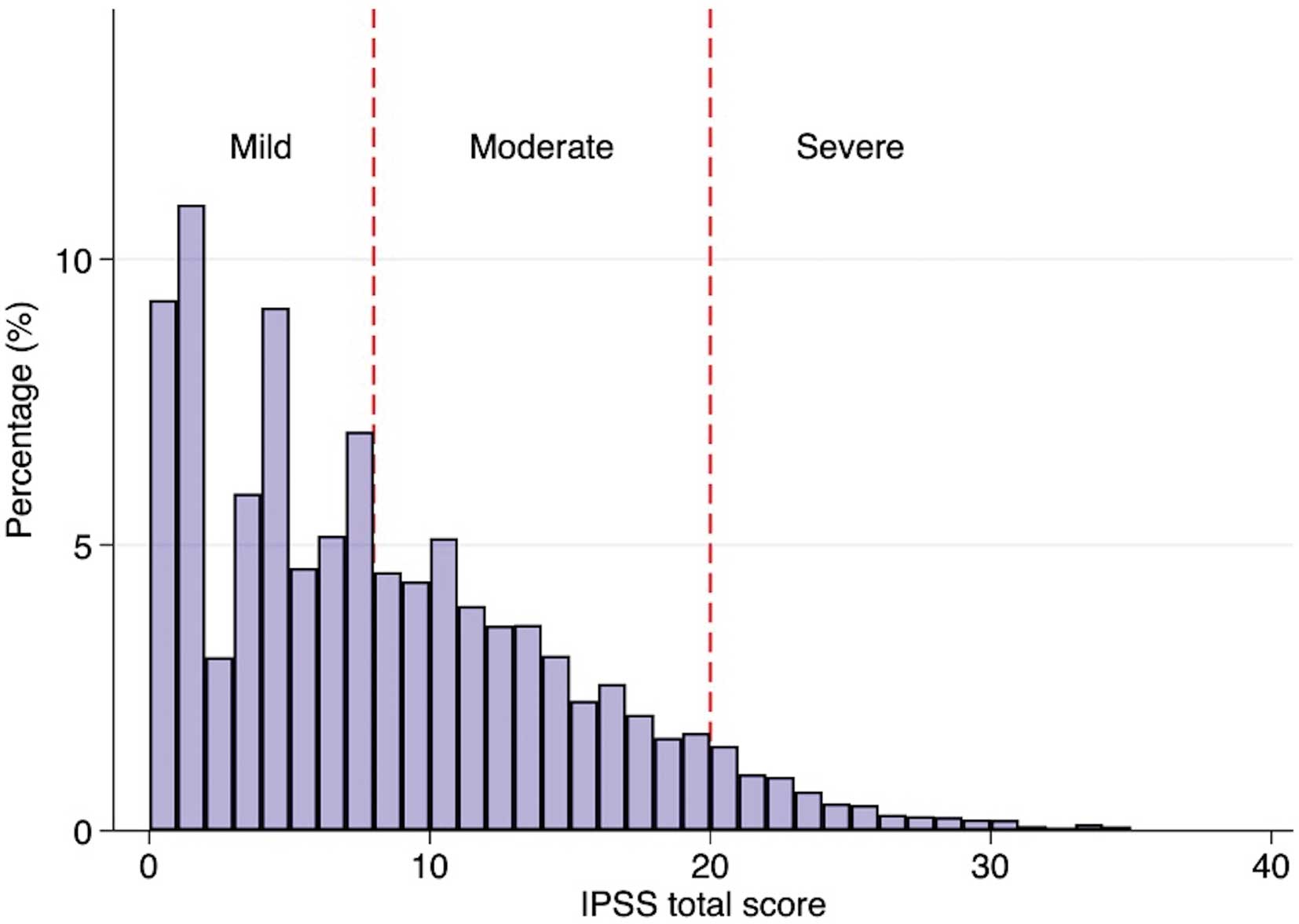
Distribution of total International Prostate Symptom Scores (IPSS). Mild symptoms were defined as total IPSS 1–7; moderate 8–19; severe 20–35.
Table 2 summarizes the association between the severity of LUTS and the composite outcome. Compared with mild LUTS as the referent, moderate or severe LUTS had significantly higher PRs across the models. Furthermore, we observed a significant dose-response association of the severity of LUTS on composite outcome (P for trend <0.0001). Given that IPSS was treated as a continuous variable, we also observed a significant association between IPSS and the composite outcome across the models (Supplementary Table 2).
| Severity of LUTS | Unadjusted | Model 1 | Model 2 | Model 3 |
|---|---|---|---|---|
| Mild | 1.0 (reference) | 1.0 (reference) | 1.0 (reference) | 1.0 (reference) |
| Moderate | 1.26 (1.18–1.34) | 1.20 (1.12–1.28) | 1.18 (1.10–1.25) | 1.18 (1.10–1.25) |
| Severe | 1.49 (1.33–1.67) | 1.41 (1.26–1.57) | 1.38 (1.24–1.54) | 1.38 (1.24–1.53) |
Model 1: PR adjusted for age. Model 2: PR further adjusted for Model 1 covariate, SBP, BMI, diabetes, current smoking, excess alcohol intake, antihypertensive medication, lipid-lowering medication, eGFR, and non-HDL cholesterol. Model 3: PR further adjusted for Model 2 covariates and PSA. PR, prevalence ratio. Other abbreviations as in Table 1.
Table 3 demonstrates the results of subgroup analysis by age groups. In moderate LUTS, the PR in the younger age group was similar to that in the older age group. In severe LUTS, the PR in the younger age group was slightly higher than that in the older age group, but no significant interaction was observed. Subgroup analysis by a history of prostate treatment is summarized in Table 4. There was no significant difference in PR between the 2 treatment groups (none vs. current/former). In contrast, in severe LUTS, the PR in participants without a history of prostate treatment was higher than those with current or former history of prostate treatment, with a significant interaction between the groups.
| Severity of LUTS |
Age category |
Prevalence ratio |
95% CI | P value | P for interaction |
|---|---|---|---|---|---|
| Moderate | <65 | 1.18 | 1.03–1.35 | 0.016 | 0.822 |
| ≥65 | 1.18 | 1.09–1.26 | <0.001 | ||
| Severe | <65 | 1.45 | 1.15–1.83 | 0.002 | 0.536 |
| ≥65 | 1.36 | 1.21–1.53 | <0.001 |
Adjusted prevalence ratio calculated using robust Poisson regression model in comparison with mild symptom as the referent. Prevalence ratio adjusted for age, SBP, BMI, diabetes, current smoking, excessive alcohol intake, antihypertensive medication, lipid-lowering medication, eGFR, and non-HDL cholesterol. CI, confidence interval. Other abbreviations as in Table 1.
| Severity of LUTS |
Prostate treatment |
Prevalence ratio |
95% CI | P value | P for interaction |
|---|---|---|---|---|---|
| Moderate | None | 1.17 | 1.10–1.26 | <0.001 | 0.962 |
| Current/former | 1.15 | 0.98–1.36 | 0.089 | ||
| Severe | None | 1.48 | 1.31–1.68 | <0.001 | 0.033 |
| Current/former | 1.14 | 0.92–1.42 | 0.240 |
History of prostate treatment was missing for 111 participants. Therefore, this analysis included 16,670 participants. The number of participants without a history of prostate treatment was 14,437, while that with former or current prostate treatment was 1,172 and 1,061, respectively. The number of participants with former or current prostate treatment was relatively small, and we evaluated these variables together. Prevalence ratio was adjusted for age, SBP, BMI, diabetes, current smoking, excessive alcohol intake, antihypertensive medication, lipid-lowering medication, eGFR, and non-HDL cholesterol. Abbreviations as in Tables 1,3.
Figure 3 is a forest plot summarizing the association of LUTS severity with each component of the composite outcome. In CAD and stroke, the severity of LUTS was significantly associated with each outcome with dose-response (P for trend <0.0001, respectively). In contrast, we observed no significant association between the severity of LUTS and AF.

Association of the severity of lower urinary tract symptoms (LUTS) with cardiovascular outcomes. A robust Poisson regression model calculated the prevalence ratio of moderate and severe LUTS for each cardiovascular outcome compared with mild LUTS. Each prevalence ratio was adjusted for age, systolic blood pressure, body mass index, history of diabetes, current smoking, antihypertensive medication, lipid-lowering medication, estimated glomerular filtration rate, and non-HDL cholesterol. AF, atrial fibrillation; CAD, coronary artery disease; CI, confidence interval.
The main finding of this study was that LUTS was independently associated with a higher prevalence of a composite cardiovascular outcome after adjusting for traditional cardiovascular risk factors. Subgroup analyses and sensitivity analyses supported the association of LUTS and the composite CVD outcome. Additionally, LUTS was significantly associated with CAD and stroke, but not AF.
We found a significant association between LUTS and CVD in a relatively lower CVD risk population to Western countries. Furthermore, even moderate LUTS was significantly associated with a higher prevalence of CVD outcomes other than AF compared with mild LUTS. These findings suggested that LUTS may be a useful clue for detecting underlying CVD rather than for addressing only urinary symptoms. Indeed, it is reported that early intervention toward cardiometabolic risk factors allows physicians to slow down the progression of both LUTS and CVD.24–27 The present findings as well as those from several previous papers highlight the importance of a holistic approach and /or cooperation of urologists and cardiologists in Japan.
The present study demonstrated a dose-dependent association between LUTS severity and the prevalence of a composite CVD outcome independently of cardiovascular risk factors. Older age and slightly higher prevalence of cardiovascular comorbidities in the advanced LUTS groups may partially explain this dose-response association. However, the differences in the baseline characteristics of the 3 groups were small, and LUTS itself can potentially exert adverse effects on CVD outcomes independently of conventional CVD risk factors. One component of the IPSS, nocturia, leads to increased sympathetic nerve activity during the night, causing loss of circadian blood pressure variability. These physiological changes in blood pressure may be considered to increase CVD risk independently of cardiovascular risk factors.28
We observed a modest but significant association between LUTS and the composite CVD outcome in the present study, but it seemed smaller compared with that in previous studies. A plausible explanation is that the present study included an older but healthier population than most of the previous studies.6 Health checkups were voluntary, not mandatory, and the sicker elderly individuals more frequently visit hospitals than undergo health checkups, whereas health-conscious and highly motivated individuals tend to take regular health checkups to maintain their health. As a result, we believe that healthier individuals were more likely to be included in the present study. The prevalence of cardiometabolic comorbidities, including obesity, was low in our study (Table 1), which may have attenuated the association between LUTS and CVD. The other possibility is that the etiology of LUTS may be different in the present study, so further study is warranted to elucidate etiological differences in the association between LUTS and the composite CVD outcome.
Pathophysiologically, LUTS or BPH is unlikely to cause CVD such as MI or stroke. On the other hand, subclinical and clinical CVD may cause LUTS via BPH secondary to ischemia or chronic inflammation, or as sequelae of stroke. Therefore, LUTS is considered a marker of underlying CVD, and CVD can be a causal factor for LUTS. In fact, recent studies demonstrated that statin therapy, and a healthy lifestyle such as low meat intake and moderate-to-vigorous physical activity could reduce the incidence of BPH and help slow down the development of LUTS from BPH in older males, suggesting a causal relationship between CVD and LUTS.25,26,29
Although a recent study found that the presence of BPH, one of the major causes of LUTS, was significantly associated with increased risk for incident AF, we did not find a significant association between them.13 One possible cause is that we did study a substantially smaller number of participants with AF at baseline compared with those with CAD or stroke. We may have underestimated the number of AF cases because of the relative insensitivity of the 12-lead ECG for detecting AF, particularly in those with asymptomatic paroxysmal AF. As a result, the present study is more likely to include high burden paroxysmal, persistent or permanent AF. Therefore, the association between LUTS and AF in this study should be read with caution. The use of long-term monitoring techniques would increase the rate of AF detection. In addition, previous studies using the same health checkup data showed that AF was infrequently observed in participants aged <70 years.16,30 Another possible explanation is that the underlying cause of AF is may be different from that of LUTS and other CVD. Dyslipidemia is an established risk factor for LUTS, CAD, and stroke, but previous mendelian randomization studies failed to show a significant association between lipid profile (low-density lipoprotein, high-density lipoprotein, and fatty acids) and incident AF.31,32
There are several strengths to this study. It had a large sample size, which allowed us to explore the association between LUTS and cardiovascular outcomes by several subgroups and sensitivity analyses. Second, most measurable confounding factors were available, which enabled us to obtain precise estimations of the association between LUTS and CVD. There are also several limitations. We included participants aged ≥55 years in Kanazawa City, and the participants may not represent the whole Japanese population. However, this is one of the biggest studies involving 16,793 participants. Additionally, lack of objective measures of prostate size or volume and data on the exact cause of LUTS may also lead to measurement biases in the present study. Furthermore, we cannot exclude potential biases due to unmeasurable confounding factors. Psychosocial factors such as depression and antidepressant intake are reportedly associated with both LUTS and CVDs.33–35 Additionally, previous studies show that sleeping disorders, such as insomnia, and socioeconomic status also can affect LUTS and CVDs.36–39 Collectively, psychosocial factors and some medications are considered as unmeasurable confounding factors in the present study. Finally, a cross-sectional study did not show a causal relationship between LUTS and CVDs.
In the present study, which included Japanese men aged between 55 and 75 years, moderate and severe LUTS were significantly associated with higher prevalence of a composite CVD outcome compared with mild LUTS. LUTS was associated with CAD and stroke, but not AF. It is conceivable that a holistic approach toward LUTS may be important for detecting underlying cardiovascular complications, especially CAD and stroke.
The authors appreciate the precious contributions of Dr. Atsushi Hashiba and other staff to the national health checkups in Kanazawa City.
None.
Y.T. was supported by the American Heart Association.
The Ethics Committee of the Kanazawa Medical Association and Kanazawa University approved this study (No. 16000003 and No. 3255-1, respectively).
Please find supplementary file(s);
http://dx.doi.org/10.1253/circj.CJ-21-0278