論文ID: CJ-22-0109
論文ID: CJ-22-0109
Background: The fibrosis-4 (FIB-4) index is used to evaluate liver disease patients. It can also be used to evaluate the prognosis for heart disease patients; however, its ability to determine the prognosis of severe isolated tricuspid regurgitation (TR) patients is unclear. This study aimed to clarify the association between FIB-4 index scores and the cardiovascular prognosis for severe isolated TR patients.
Methods and Results: This was a dual-center, retrospective study. From 2011 to 2019, 111 consecutive outpatients with severe isolated TR (mean age, 68.6 years; 53.2% male) were evaluated. Major adverse cardiovascular events (MACEs) were defined as the composite of cardiovascular death, hospitalization for heart failure, myocardial infarction, and stroke. The association between FIB-4 index scores and echocardiography was also evaluated. During a median follow up of 3.0 years, 24 patients were lost to follow up and 40 MACEs occurred. Baseline FIB-4 index scores for patients with MACEs were significantly higher than those for patients without MACEs. A multivariate analysis revealed that FIB-4 index scores are significantly associated with MACEs (hazard ratio, 1.89; 95% confidence interval, 1.01–3.54; P=0.046). A linear regression analysis indicated that FIB-4 index scores were correlated with echocardiographic parameters, including the left atrial volume index and left ventricular end-diastolic diameter.
Conclusions: The FIB-4 index score may be a useful predictor of MACEs for patients with severe isolated TR.
Tricuspid regurgitation (TR) is associated with a poor prognosis for patients with heart failure (HF), regardless of left ventricular ejection fraction (LVEF) or pulmonary artery pressure.1 Concomitant surgical intervention for severe TR during left-side valve surgery is recommended because severe TR complicated with left-side valvular diseases is a prognostic marker of future cardiovascular events.2 Surgical intervention is not performed for severe isolated TR without significant left-side valvular disease because of the high operative mortality rate.3,4 However, recently, there has been renewed interest in early surgery for severe isolated TR before the onset of organ damage.2,5 Therefore, estimation of the risk of systemic organ damage is important in the management of severe isolated TR.
Having severe TR and liver disorders is associated with poor postoperative clinical outcomes.6,7 Liver stiffness measured using transient elastography, which may reflect hepatic congestion, can predict the prognosis for patients with HF.8–13 However, transient elastography requires special equipment; therefore, a simple index that can evaluate liver fibrosis is more convenient. The fibrosis-4 (FIB-4) index is a simple and useful test that can assess liver fibrosis in patients with liver diseases and is recommended as a clinically useful tool for screening or determining the risk of non-alcoholic fatty liver disease.14–17 Recently, the FIB-4 index has been used to determine the prognosis for patients with HF.18–20 At Iwakuni Clinical Center, it was determined that the FIB-4 index score reflects right ventricular dysfunction and a poor prognosis for patients with HF and preserved ejection fraction.17 However, the association between the FIB-4 index score and the prognosis for patients with severe isolated TR remains unclear.
During this study, we evaluated whether the FIB-4 index score could predict major adverse cardiovascular events (MACEs) for patients with severe isolated TR. We also aimed to clarify the association between the FIB-4 index score and cardiovascular prognosis for patients with severe isolated TR based on MACEs.
This was a dual-center, retrospective study. From January 2011 to December 2019, 304 outpatients with severe TR were diagnosed at the Iwakuni Clinical Center and the Okayama University Hospital. Isolated TR was defined as TR without significant left-side valvular heart disease or severe pulmonic stenosis or insufficiency based on a previous study.3 Significant left-side valvular dysfunction was defined as moderate or severe insufficiency or stenosis of the mitral or aortic valves. Figure 1 shows the flow diagram of the study design. After excluding patients according to the exclusion criteria, we identified 188 outpatients with severe isolated TR. Additionally, we excluded patients with a history of valvular surgery (surgical aortic valve replacement, n=13; mitral valve replacement, n=10; mitral valve plasty, n=6; open mitral commissurotomy, n=2; double valve replacement, n=6; Rastelli-type repair with a valved conduit, n=1), decompensated HF that required hospitalization on the same day as echocardiography (n=21), and congenitally corrected transposition of great arteries such as the tricuspid valve and systemic atrioventricular valve (n=1). Patients with chronic liver disease (hepatitis B, n=2; hepatitis C, n=12; alcoholic liver disease, n=2; history of hospitalization because of liver disorder of unknown cause, n=1) were also excluded. Chronic liver diseases were diagnosed by a hepatologist based on the blood examination results, pre-existing liver disease, and/or history of treatment. Finally, 111 patients with isolated severe TR were evaluated.

Flow diagram of patient selection. Among 304 outpatients with severe TR, we identified 188 patients with isolated TR. From these, those with history of valvular surgery, decompensated HF that required hospitalization on the day of echocardiography, ccTGA, and chronic liver disease were excluded. A total of 111 patients were finally evaluated. ccTGA, congenitally corrected transposition of great arteries; HF, heart failure; TR, tricuspid regurgitation.
This investigation conformed to the principles outlined in the Declaration of Helsinki and was approved by the institutional review boards of Iwakuni Clinical Center (0264) and Okayama University Graduate School of Medicine (2206-020). The requirement for informed consent was waived because of the low-risk nature of the study and because consent could not be directly obtained from all enrolled patients.
FIB-4 IndexResults of the blood examinations performed on the same day as echocardiography were used. The FIB-4 index score was calculated as follows: FIB-4 index score = age (years) × aspartate aminotransferase (AST) (U/L) / [alanine aminotransferase (ALT) (U/L)1/2 × platelet count (109/L)].14,17 We also calculated the non-alcoholic fatty liver disease fibrosis score (NFS), which is recommended for the assessment of advanced liver disorders and is similar to the FIB-4 index score, as follows: NFS = −1.675 + 0.037 × age (years) + 0.094 × body mass index (kg/m2) + 1.13 × impaired fasting glucose/diabetes (yes=1; no=0) + 0.99 × AST/ALT ratio − 0.013 × platelet count (109/L) − 0.66 × serum albumin (g/dL).21 Routine laboratory tests and measurements of N-terminal pro-B-type natriuretic peptide (NT-pro-BNP) and B-type natriuretic peptide (BNP) were performed using an automated analyzer at the Iwakuni Clinical Center and Okayama University Hospital. The estimated glomerular filtration rates were calculated as follows: estimated glomerular filtration rate = 194 × creatinine−1.094 × age−0.287 for men and 194 × creatinine−1.094 × age−0.287 × 0.739 for women.
Echocardiography AssessmentsEchocardiographic assessments of cardiac function were performed by experienced technicians using an iE33 (Philips Japan, Ltd., Tokyo, Japan) and Aplio echo machine (Canon Medical Systems, Otawara, Japan). Two-dimensional and Doppler measurements were performed and analyzed by using standard views and techniques. The severity of TR was assessed according to multiparametric integrative approaches based on the accepted criteria. We divided patients into 3 grade groups: mild, moderate, and severe. Severe patients had a color flow jet area >10 cm2, vena contracta width ≥0.7 cm, proximal isovelocity surface area >0.9 cm2, systolic flow reversal in the hepatic vein, effective regurgitant orifice area ≥0.40 cm2, and regurgitant volume ≥45 mL.22 When echocardiography showed discordant findings that made it difficult to determine the TR severity, the evaluation was based on clinical findings or other modalities according to the established guidelines.22 We measured the left atrial volume index (LAVI), LV end-diastolic diameter (LVDd), and LV end-systolic diameter (LVDs) using general methods. The LVEF was measured by using the modified Simpson technique with B-mode presentation in the apical 2-chamber and 4-chamber views. We also measured the peak early diastolic velocities (E) of LV inflow, deceleration time (DcT), and early diastolic myocardial velocities (e’). The ratio of E to e’ (E/e’) was calculated. Using an apical approach, we measured the tricuspid annular plane systolic excursion (TAPSE) and tricuspid lateral annular systolic velocity (S’) to assess right ventricular function. TAPSE was measured as the length of the systolic excursion of the right ventricle (RV) annulus along its longitudinal plane using M-mode presentation in an RV-focused apical 4-chamber view. S’ was measured as the velocity of the tricuspid lateral annular plane using Doppler tissue imaging in an RV-focused apical 4-chamber view. Each echocardiographic parameter was measured based on the American Society of Echocardiography guidelines.23
Endpoints and Follow upThe incidence of MACEs was investigated by retrospective review of the medical records or telephone interviews when laboratory data were blinded. MACEs were defined as a composite of the following clinical outcomes: cardiac death; hospitalization for HF; non-fatal non-ST elevation/ST elevation myocardial infarction; and ischemic/non-ischemic stroke. Cardiac death was defined as death caused by any of the following: acute coronary syndrome; HF; arrhythmic death; and unclear causes of death for which a cardiac origin could not be excluded.24
Statistical AnalysisCategorical variables are presented as numbers (%) and were compared using the chi-squared test or Fisher’s exact test as appropriate. Continuous variables that were normally distributed are presented as means±standard deviation and were compared by using the Student’s t-test. Continuous variables that were not normally distributed are presented as medians with interquartile ranges (IQRs) and were compared using the Mann-Whitney U-test. Data normality was evaluated by using the Shapiro-Wilk test. To assess MACEs, a receiver-operating characteristic (ROC) curve analysis was performed for the FIB-4 index score. The optimal cut-off value was defined as the point maximizing the Youden index (=max [sensitivity + specificity − 1]). We classified patients into 2 groups according to the optimal cut-off values of the FIB-4 index. Cumulative event-free rates of the clinical endpoints during follow up were compared between groups by using Kaplan-Meier curves, post-hoc comparisons, and log-rank tests. The effects of variables on MACEs were evaluated by using the Cox proportional hazard analysis. The results are reported as hazard ratios (HRs) and 95% confidence intervals (CIs). Factors that had statistically significant associations with clinical endpoints in the univariate analysis were included in the multivariate Cox proportional hazard analysis. Atrial fibrillation, hemoglobin, serum albumin, and estimated glomerular filtration rate were included in the analysis of the FIB-4 index score and MACEs; however, age and platelet count were not included because they are components of the calculation for the FIB-4 index score. Age, platelet count and serum albumin were not included in the multivariate analysis of NFS and MACEs because these are components of the calculation for NFS. We also evaluated the prognostic value of the FIB-4 index score as categorized variables divided by the optimal cut-off values of both the univariate and multivariate analyses and adjusted for the Cox regression analysis model. The association between echocardiographic parameters and the FIB-4 index score was also investigated by using linear regression analyses. Continuous variables that were not normally distributed were applied to the regression analysis after natural logarithmic transformation. Statistical significance was set at P<0.05. These analyses were performed by using SPSS statistical software (version 25; IBM Corp., Armonk, NY, USA).
Table 1 shows the clinical characteristics of the patients. The median follow-up period was 3.0 years. Twenty-four patients were lost to follow up. The median follow-up period until loss to follow up was 3.7 years. The FIB-4 index score and NFS were significantly higher for patients with MACEs than for those without MACEs (P<0.001 and P=0.004, respectively). BNP levels (n=53) and NT-proBNP levels (n=45) for patients with MACEs were significantly higher than those for patients without MACEs (BNP: 340.9 [236.1–527.0] pg/mL and 169.4 [48.0–260.7] pg/mL, P=0.001; NT-proBNP: 1940.0 [1,204.5–3,225.5] pg/mL and 1392.0 [740.0–1,960.0] pg/mL, P=0.083).
| Variables | Missing, N | All patients (N=111) |
CV events | P value | |
|---|---|---|---|---|---|
| Present (N=40) | Absent (N=71) | ||||
| Age, years | 0 | 68.6±17.0 | 75.3±15.2 | 64.9±16.9 | 0.002 |
| Male | 0 | 59 (53.2) | 25 (62.5) | 34 (47.9) | 0.139 |
| Body mass index, kg/m2 | 1 | 20.6±2.8 | 20.4±2.2 | 20.8±3.1 | 0.551 |
| NYHA class, n (%) | 0 | <0.001 | |||
| I/II/III/IV | 31/48/29/3 | 4/16/19/1 | 27/32/10/2 | ||
| SBP, mmHg | 29 | 123.9±23.8 | 121.6±24.6 | 125.6±23.4 | 0.459 |
| Heart rate, beats/min | 1 | 72.6±14.1 | 74.2±14.5 | 71.6±14.0 | 0.374 |
| Hypertension | 0 | 35 (31.5) | 11 (27.5) | 24 (49.7) | 0.493 |
| Diabetes mellitus | 2 | 20 (18.3) | 7 (17.5) | 13 (18.3) | 0.889 |
| Atrial fibrillation | 0 | 69 (62.2) | 30 (75.0) | 39 (54.9) | 0.036 |
| Implantation of CIEDs | 1 | 34 (30.9) | 18 (45.0) | 16 (22.9) | 0.016 |
| Chronic kidney disease | 0 | 47 (42.3) | 9 (22.5) | 38 (53.5) | 0.001 |
| History of heart failure | 0 | 36 (32.4) | 22 (55.0) | 14 (19.7) | <0.001 |
| COPD | 2 | 7 (6.4) | 5 (12.5) | 2 (2.9) | 0.098 |
| Ischemic heart disease | 0 | 13 (11.7) | 5 (12.5) | 8 (11.3) | 1.000 |
| Medications on admission | |||||
| β-blockers | 0 | 56 (50.5) | 20 (50.0) | 36 (50.7) | 0.943 |
| ACEIs/ARBs | 0 | 34 (30.6) | 16 (40.0) | 18 (25.4) | 0.108 |
| MRA | 0 | 47 (42.3) | 21 (52.5) | 26 (36.6) | 0.104 |
| Digoxin | 1 | 12 (10.9) | 7 (17.9) | 5 (7.0) | 0.110 |
| Nitrates | 0 | 9 (8.1) | 5 (12.5) | 4 (5.6) | 0.279 |
| Statin | 0 | 20 (18.0) | 3 (7.5) | 17 (23.9) | 0.030 |
| Laboratory data | |||||
| Hemoglobin, g/dL | 0 | 12.4±1.9 | 11.9±2.0 | 12.7±1.8 | 0.043 |
| Platelet count, 109/L | 0 | 175.9±61.8 | 155.7±55.2 | 187.2±62.8 | 0.009 |
| AST, IU/L | 0 | 27 (21.5–33) | 29 (22–34) | 26 (21–32) | 0.178 |
| ALT, IU/L | 0 | 17 (13–24) | 17 (13–23) | 16 (12.5–24) | 0.638 |
| Serum albumin, g/dL | 15 | 4.0 (3.8–4.4) | 3.9 (3.7–4.3) | 4.2 (3.8–4.4) | 0.015 |
| FIB-4 index | 0 | 2.63 (1.71–3.86) | 3.63 (2.57–4.45) | 2.24 (1.58–3.10) | <0.001 |
| NFS | 15 | −0.3±1.7 | 0.3±1.5 | −0.7±1.7 | 0.004 |
| Total bilirubin, g/dL | 7 | 0.8 (0.6–1.2) | 0.8 (0.6–1.2) | 0.8 (0.6–1.0) | 0.247 |
| Total cholesterol, mg/dL | 40 | 169.5±40.2 | 155.5±31.2 | 175.4±42.4 | 0.056 |
| Cholinesterase, U/L | 49 | 224.5 (174–299) | 187.5 (164.5–220) | 246.5 (199–318) | 0.003 |
| BUN, mEq/L | 0 | 19.2 (15.7–23.4) | 22.0 (18.6–32.6) | 17.4 (14.2–20.7) | <0.001 |
| Serum creatinine, mg/dL | 0 | 0.9 (0.7–1.1) | 1.1 (0.9–1.5) | 0.9 (0.7–1.0) | <0.001 |
| eGFR, mL/min/1.73 m2 | 0 | 57.1±20.1 | 48.2±19.1 | 62.2±20.0 | <0.001 |
Data are presented as n (%), mean±standard deviation, or median (25th–75th percentile). ACEIs, angiotensin-converting enzyme inhibitors; ALT, alanine aminotransferase; ARBs, angiotensin II receptor blockers; AST, aspartate aminotransferase; BUN, blood urea nitrogen; CIEDs, cardiovascular implantable electronic devices; COPD, chronic obstructive pulmonary disease; CV, cardiovascular; eGFR, estimated glomerular filtration rate; FIB-4 index, fibrosis-4 index; MRA, mineralocorticoid receptor antagonist; NFS, non-alcoholic fatty liver disease fibrosis score; NYHA, New York Heart Association; SBP, systolic blood pressure.
Primary TR was observed in 25 patients (endocarditis, n=1; pacing lead, n=8; prolapse, n=4; iatrogenic perforation of the tricuspid valve n=1; Ebstein anomaly, n=10; repaired tetralogy of Fallot, n=1). Secondary TR was observed in 86 patients (left heart disease, n=4; right ventricular volume overload, n=2; right ventricular cardiomyopathy, n=5; pulmonary arterial hypertension, n=3; chronic lung disease, n=5; pulmonary thromboembolism, n=4; left-to-right shunt, n=14; atrial fibrillation, n=48; compression by pectus excavatum, n=1). Table 2 presents the echocardiographic findings of this study. No significant differences in the echocardiographic parameters, except for TAPSE and maximum inferior vena cava diameter, were observed.
| Variables | All patients (N=111) |
CV events | P value | |
|---|---|---|---|---|
| Present (N=40) | Absent (N=71) | |||
| LAVI, mL/m2 | 50.0 (34.0–66.0) | 46.0 (33.0–66.0) | 51.0 (38.5–65.0) | 0.625 |
| LVDd, mm | 42.3±6.6 | 42.6±6.3 | 42.1±6.8 | 0.712 |
| LVDs, mm | 28.0 (24.0–32.5) | 29.0 (24.0–34.0) | 27.0 (24.5–31.0) | 0.442 |
| LVEF, % | 62.0 (55.0–69.0) | 60.5 (50.0–68.0) | 64.0 (57.0–69.0) | 0.115 |
| DcT, ms | 187 (149–222) | 177 (143–215) | 187 (151–224) | 0.474 |
| E/e’ | 10.4 (8.4–13.0) | 10.1 (8.2–13.2) | 10.5 (8.4–12.4) | 0.695 |
| TAPSE, mm | 18.6±6.5 | 16.3±5.5 | 20.2±6.7 | 0.008 |
| S’, cm/s | 11.0±3.8 | 10.3±3.9 | 11.5±3.6 | 0.200 |
| TRPG, mmHg | 30.0 (25.0–40.0) | 31.0 (25.0–38.5) | 30.0 (25.0–40.0) | 0.702 |
| Maximum IVC diameter, mm | 20.0 (16.0–23.0) | 21.5 (18.5–24.0) | 19.0 (13.5–22.0) | 0.002 |
| IVC respiratory change rate, % | 44.8±18.7 | 40.6±18.5 | 47.1±18.5 | 0.077 |
| Estimated systolic PAP, mmHg | 40.0 (31.0–51.0) | 41.0 (34.5–51.0) | 39.5 (31.0–51.0) | 0.730 |
Data are presented as n (%), mean±standard deviation, or median (25th–75th percentile). CV, cardiovascular; DcT, deceleration time; E/e’, early diastolic filling velocity/early diastolic velocity of the mitral annulus; IVC, inferior vena cava; LAVI, left atrial volume index; LVDd, left ventricular end-diastolic diameter; LVDs, left ventricular end-systolic diameter; LVEF, left ventricular ejection fraction; PAP, pulmonary arterial pressure; TAPSE, tricuspid annular plane systolic excursion; TR, tricuspid regurgitation; TRPG, tricuspid regurgitation pressure gradient; S’, tricuspid lateral annular systolic velocity.
During the follow-up periods, 40 MACEs occurred (cardiovascular death, n=6; hospitalization for HF, n=29; myocardial infarction, n=1; stroke, n=4). Tricuspid valve surgery was performed for 7 patients. Two patients underwent tricuspid valve surgery after hospitalization for HF. One patient had cardiovascular death after tricuspid valve surgery. One patient had hospitalization for HF after tricuspid valve surgery.
Relationship Between FIB-4 Index Scores and MACEsThe ROC curve analysis showed that the C-statistic of the FIB-4 index score for MACE prediction was 0.713 (95% CI, 0.611–0.815; P<0.001), with a sensitivity of 62.5% and a specificity of 80.3%, and that the value maximizing the Youden index was 3.22. The C-statistic of NFS for MACE prediction was 0.684 (95% CI, 0.573–0.794; P=0.003). Figure 2 shows the Kaplan-Meier analyses of patients stratified according to the optimal cut-off value for the FIB-4 index score. Patients with higher FIB-4 index scores had a significantly poor cardiovascular prognosis compared with patients with lower FIB-4 index scores (P<0.001). As shown in Table 3, the Cox regression analysis showed that the MACE incidence was significantly associated with age, atrial fibrillation, hemoglobin, platelet count, serum albumin, FIB-4 index score, FIB-4 index score without multiplication by age, NFS, and estimated glomerular filtration rate. As shown in Table 4, the FIB-4 index scores as logarithmic-transformed values were also significantly associated with the MACE incidence in the multivariate Cox regression analysis (model 1; P=0.046). However, the NFS was not significantly associated with the MACE incidence in the multivariate Cox regression analysis (HR, 1.248; 95% CI, 0.978–1.594; P=0.075). Additionally, the FIB-4 index scores as categorical variables divided by the optimal cut-off value were significantly associated with the MACE incidence in multivariate analysis adjusted for the model (HR, 5.069; 95% CI, 2.119–12.121; P<0.001).
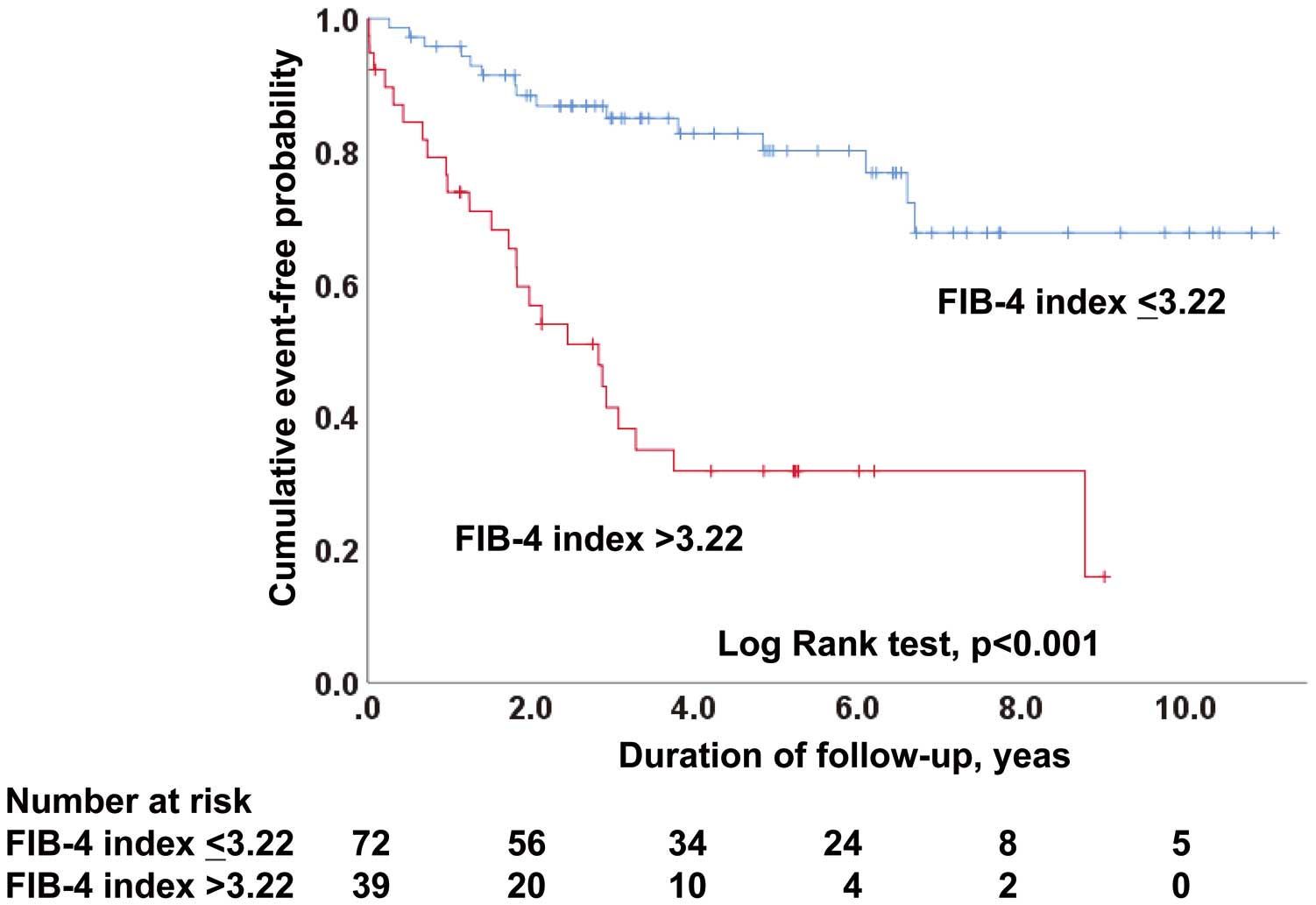
Kaplan-Meier analysis of the event-free rate of major cardiovascular events in the groups divided by the optimal cut-off value of the FIB-4 index. FIB-4 index, fibrosis-4 index.
| Variable | HR | 95% CI | P value |
|---|---|---|---|
| Age, year | 1.049 | 1.020–1.079 | 0.001 |
| Male | 1.861 | 0.978–3.544 | 0.059 |
| Body mass index, kg/m2 | 0.988 | 0.883–1.105 | 0.829 |
| Atrial fibrillation | 2.286 | 1.114–4.690 | 0.024 |
| Hemoglobin, g/dL | 0.848 | 0.721–0.998 | 0.048 |
| Platelet count, 109/L | 0.993 | 0.987–0.998 | 0.006 |
| AST*, point | 2.055 | 0.884–4.778 | 0.094 |
| ALT*, point | 1.295 | 0.715–2.347 | 0.394 |
| Serum albumin*, point | 0.038 | 0.006–0.223 | <0.001 |
| FIB-4 index*, point | 2.311 | 1.438–3.715 | 0.001 |
| FIB-4 index without age*, point | 2.216 | 1.246–3.943 | 0.007 |
| NFS, point | 1.381 | 1.120–1.702 | 0.003 |
| Total bilirubin*, point | 1.641 | 0.826–3.262 | 0.157 |
| eGFR, 1 mL/min/1.73 m2 | 0.970 | 0.954–0.985 | <0.001 |
CI, confidence interval; HR, hazard ratio. Other abbreviations as in Table 1. *Values are logarithm-transformed.
| Variable | HR | 95% CI | P value |
|---|---|---|---|
| FIB-4 index, point* (model 1) | 1.892 | 1.011–3.540 | 0.046 |
| Age, year (model 2) | 1.027 | 0.992–1.064 | 0.132 |
| FIB-4 index without age, point* (model 3) | 1.936 | 0.956–3.923 | 0.067 |
Each multivariate model was adjusted for atrial fibrillation, hemoglobin, serum albumin, and eGFR. CI, confidence interval; HR, hazard ratio. Other abbreviations as in Table 1. *Values are logarithm-transformed.
Because the FIB-4 index score includes age, further analyses were performed to evaluate the influence of age on the association between FIB-4 index scores and MACEs. In the multivariate Cox regression models (Table 4), age (model 2; P=0.132) and FIB-4 index score without multiplication by age (model 3; P=0.067) were not associated with MACE incidence. Next, patients were divided into 2 groups based on a median age of 72 years. MACEs were documented for 27 in patients aged ≥72 years and for 13 patients aged <72 years. For patients aged ≥72 years, the univariate Cox regression analysis showed that FIB-4 index scores as logarithmic-transformed continuous values (HR, 1.929; 95% CI, 1.004–3.705; P=0.049) and as categorical variables divided by the optimal cut-off value (HR, 3.707; 95% CI, 1.492–9.211; P=0.005) were significantly associated with MACE incidence. For patients aged <72 years, FIB-4 index scores as categorical variables divided by the optimal cut-off value (HR, 6.299; 95% CI, 1.771–22.401; P=0.004), but not logarithmic-transformed continuous values (HR, 2.295; 95% CI, 0.738–7.131; P=0.151), were significantly associated with MACE incidence.
Finally, the association between primary TR and MACEs was analyzed. MACEs were documented in 4 patients with primary TR (16%). In the univariate Cox regression analysis, FIB-4 index scores as categorical variables divided by the optimal cut-off value (HR, 24.191; 95% CI, 2.081–281.217; P=0.011), but not logarithmic-transformed continuous values (HR, 14.074; 95% CI, 0.618–320.671; P=0.097), were significantly associated with the MACE incidence in patients with primary TR.
Association Between FIB-4 Index Scores and Echocardiographic ParametersThe FIB-4 index score was significantly associated with age (P<0.001), platelet count (P<0.001), AST (P<0.001), total bilirubin (P=0.001), and NFS (P<0.001). Table 5 shows the liner regression analysis of echocardiographic parameters. The FIB-4 index score was significantly related to the LAVI (P<0.001), LVDd (P=0.006), LVDs (P=0.001), DcT (P=0.004), E/e’ (P=0.002), and maximum inferior vena cava diameter (P<0.001).
| FIB-4 index* | β | P value |
|---|---|---|
| Color flow jet area of TR | 0.302 | 0.223 |
| Vena contracta width of TR | −0.250 | 0.949 |
| PISA of TR* | −0.127 | 0.593 |
| Systolic flow reversal in the hepatic vein | 0.287 | 0.053 |
| EROA of TR* | 0.021 | 0.927 |
| Regurgitant volume of TR* | −0.025 | 0.944 |
| LAVI* | 0.366 | <0.001 |
| LVDd | 0.261 | 0.006 |
| LVDs* | 0.311 | 0.001 |
| LVEF* | −0.160 | 0.093 |
| DcT* | −0.276 | 0.004 |
| E/e’* | 0.290 | 0.002 |
| TAPSE | −0.143 | 0.209 |
| S’ | −0.016 | 0.890 |
| TRPG* | −0.029 | 0.765 |
| Maximum IVC diameter* | 0.351 | <0.001 |
| IVC respiratory change rate | −0.163 | 0.087 |
| Estimated systolic PAP* | −0.041 | 0.670 |
EROA, effective regurgitant orifice area; FIB-4 index, fibrosis-4 index; PISA, proximal isovelocity surface area. Other abbreviations as in Table 2. *Values are logarithm-transformed.
This study investigated the effect of the FIB-4 index score on MACEs of patients with severe isolated TR. A higher FIB-4 index score was significantly associated with a higher MACE incidence. The FIB-4 index score was also significantly associated with the LAVI, LVDd, LVDs, DcT, E/e’, and maximum inferior vena cava diameter. To the best of our knowledge, this is the first study to evaluate whether the FIB-4 index score reflects a poor cardiovascular prognosis for patients with severe isolated TR.
FIB-4 Index Score and MACE Incidence in Patients With Severe Isolated TRA high FIB-4 index score was reportedly associated with worse outcomes for patients hospitalized for acute decompensated HF.18–21 During this study, we evaluated patients with severe isolated TR and found that a high FIB-4 index score was associated with an increased MACE incidence. The FIB-4 index score may be a predictor of cardiovascular events for patients with severe isolated TR.
For patients with liver disease, the accuracy of the FIB-4 index score for the estimation of liver conditions among older adults may be reduced because the FIB-4 index score includes age, and the index was mainly developed based on studies involving young adults.14,15,25 Previous studies have suggested that the cut-off FIB-4 index score for the diagnosis of liver fibrosis for patients with non-alcoholic fatty liver disease should increase with age.26,27 During this study, the value of the FIB-4 index score might have been affected by age because the mean age of this study population was older than that of populations involved in previous studies of liver disease. However, most previous studies that have investigated the relationship between the FIB-4 index score and cardiovascular disease prognosis included older adults, similar to this study, and reported that the FIB-4 index score was a significantly useful prognostic marker for patients with cardiovascular disease.18–21,28 Therefore, the FIB-4 index score may be used as a prognostic marker for older adults with cardiovascular disease, including those with severe isolated TR.
During this study, the NFS was not significantly associated with MACEs in the multivariate Cox analysis; however, the FIB-4 index score was significantly associated when a similar statistical approach was used. The NFS was developed to differentiate between patients with and without prognostically significant non-alcoholic fatty liver disease.29 Furthermore, the NFS includes more variables than the FIB-4 index score. Therefore, the NFS may be a more specific score for diagnosing non-alcoholic fatty liver disease. In contrast, although the FIB-4 index was developed to predict hepatic fibrosis in patients with human immunodeficiency virus (HIV) and hepatitis C virus coinfections using a simple formula, the FIB-4 index score is associated with liver fibrosis in patients with other liver diseases.14,17,25 Hence, the FIB-4 index score might be more versatile than the NFS and more suitable as a prognostic marker of cardiovascular diseases.
Correlation Between the FIB-4 Index Score and Left Heart-Derived Echo ParametersDuring this study, the FIB-4 index score was associated with left heart-derived echo parameters, excluding LVEF. Previously, the FIB-4 index score was reported to be associated with right atrial pressure and inferior vena cava diameter.18 This study also revealed a significant association between the FIB-4 index score and maximum inferior vena cava diameter, suggesting that the FIB-4 index score is correlated with cardiac preloads. This may be why the FIB-4 index score is associated with the LAVI, LVDd, and LVDs; these parameters can be influenced by cardiac preloads. Furthermore, 48 (43.2%) patients with secondary TR because of atrial fibrillation were included in this study. The FIB-4 index score may reflect the degree of hemodynamic disorders caused by atrial fibrillation in these patients. The FIB-4 index score may be significantly associated with LAD and LAVI, which are influenced by atrial fibrillation.
Liver stiffness measured by transient elastography reportedly increased with an increase in E/e’.11 We demonstrated that a high FIB-4 index score was also associated with a high E/e’. The American Society of Echocardiography recommends using the E/e’ to assess LV diastolic dysfunction because it is correlated with the LV filling pressure.30,31 Therefore, the FIB-4 index score may be considered a biomarker of LV diastolic dysfunction in patients with TR. The FIB-4 index score was also significantly inversely associated with DcT in this study. DcT was previously reported to be inversely associated with LV diastolic dysfunction in patients with atrial fibrillation and depressed LVEF.32,33 This finding may also support the association between the FIB-4 index score and LV diastolic dysfunction, although the LVEF of patients in this study was preserved.
Previously, we reported that the FIB-4 index was associated with right ventricular dysfunction in patients with HF and preserved ejection fraction.21 In another study, liver stiffness assessed using transient elastography was also reported to be associated with TAPSE rather than E/e’ in acute decompensated HF patients.10,11 However, during this study, the FIB-4 index was not significantly associated with TAPSE or S’ in patients with severe isolated TR. Therefore, the meaning of the FIB-4 index score for patients with HF may vary depending on the etiology of HF. This requires further investigation.
Study LimitationsThis study had several limitations. First, the study had a relatively small sample size. Second, we classified TR into only 3 grades according to the standard American Society of Echocardiography grading scheme.22 When severe TR is further classified as severe, massive, and torrential, the therapeutic effect on severe TR becomes clearer.34 Third, the C-statistic of the FIB-4 index score for MACE prediction was 0.713, which suggests that the FIB-4 index score alone does not have high accuracy for predicting prognosis. Further studies are necessary to determine the optimal prognostic biomarker panel, including the FIB-4 index score. Fourth, age, which may be a determinant of poor outcomes, was included in the calculation of the FIB-4 index score. Although we evaluated the impact of age on the association between FIB-4 index scores and MACEs in this study, it was difficult to clarify the effect of age on the prognostic value of the FIB-4 index score using such a stratification analysis because of the small sample size. Fifth, patients may have started medications or other treatments after the baseline examination, which may have affected TR and underestimated the risk. Finally, although the FIB-4 index score was associated with a worse prognosis for patients with severe isolated TR, the association between the FIB-4 index score and liver fibrosis was not evaluated during this study. Only a few previous studies have reported the relationship between the FIB-4 index score and liver conditions of patients with cardiovascular disease.14,16 Further studies including liver biopsy or transient elastography performed for patients with cardiovascular disease are required.
In conclusion, the FIB-4 index score is associated with a high risk of MACEs for patients with severe isolated TR. In the clinical setting, the FIB-4 index score may be useful for assessing the risk of future cardiovascular events, thus allowing the implementation of strategies aimed at avoiding cardiovascular events.
None.
This work was not supported by any specific grant from funding agencies in the public, commercial, or not-for-profit sectors.
H.I. is an Editorial Board member of Circulation Journal.
This investigation was approved by the institutional review board of Iwakuni Clinical Center (0264) and Okayama University Graduate School of Medicine (2206-020).
The deidentified participant data will be shared on a request basis. Please directly contact the corresponding author to request data sharing. Data on patient and procedural characteristics, and long-term outcomes will be shared. The study protocol will also be available. The data will be available for 2 years after the publication of the manuscript. The data will be provided in PDF files and sent by E-mail.