論文ID: CJ-22-0257
論文ID: CJ-22-0257
Background: Although regenerative cell therapy is expected to be an alternative treatment for peripheral artery disease (PAD), many regenerative cell therapies have failed to show sufficient efficacy in clinical trials. Most preclinical studies have used acute ischemia models, despite PAD being a chronic disease. In addition, aging and atherosclerosis decrease the quality of a patient’s stem cells. Therefore, using a non-acute ischemic preclinical model and stem cells with high regenerative potency are important for the development of effective regenerative therapy. In this study, we assessed the tissue regenerative potential of umbilical cord-derived mesenchymal stromal cells (UCMSCs), which could potentially be an ideal cell source, in a rat model of established ischemia.
Methods and Results: The regenerative capacity of UCMSCs was analyzed in terms of angiogenesis and muscle regeneration. In vitro analysis showed that UCMSCs secrete high amounts of cytokines associated with angiogenesis and muscle regeneration. In vivo experiments in a rat non-acute ischemia model showed significant improvement in blood perfusion after intravenous injection of UCMSCs compared with injection of culture medium or saline. Histological analysis revealed UCMSCs injection enhanced angiogenesis, with an increased number of von Willebrand factor-positive microcapillaries, and improved muscle regeneration.
Conclusions: These results suggest that intravenous administration of UCMSCs may be useful for treating patients with PAD.
The prevalence of peripheral artery disease (PAD) is increasing worldwide. Chronic limb-threatening ischemia (CLTI), the most severe form of PAD, is a devastating disease associated with poor functional and survival prognosis.1,2 The current treatment for CLTI is primarily revascularization. Recently, regenerative therapy has drawn attention as an alternative treatment option, and various studies have been performed to demonstrate the efficacy of regenerative therapy for PAD.3–7 However, current guidelines do not recommend regenerative therapy, including cell and gene therapies, for PAD because of insufficient evidence of efficacy.2,8 Efficacy has not been satisfactory in previous studies using various types of cell therapies, due, in part, to the low regenerative capacity of autologous cells, such as bone marrow-derived stem cells.9–11 During the aging process, the regenerative potency and number of autologous cells gradually decrease; atherosclerosis further worsens the quality of autologous cells.10–12 In this context, allogenic cells with high regenerative capacity would be beneficial for achieving a satisfactory angiogenic effect, although immune reactions are a matter of concern. Mesenchymal stromal cells (MSCs) are well known for their immunomodulatory properties,13 and allogeneic MSCs can be safely administered without causing a significant immune response.9,14 In this context, MSCs derived from the umbilical cord (UCMSCs) are of significant benefit. UCMSCs, which are derived from fetal tissues, have high proliferative potency.9,15 In addition, the properties of MSCs change according to the culture conditions. Therefore, ideal culture conditions will help produce MSCs with high proliferative capacity and potency to secrete various cytokines and enhance tissue regeneration.
Another reason for the insufficient efficacy of cell-based therapies in clinical trials, despite their promising regenerative effect in preclinical studies, could be the inappropriate preclinical animal models used, which is the most widely used model for acute hind limb ischemia. The efficacy of the source cells is usually assessed by administering them immediately after the induction of ischemia.16–20 However, considering the pathophysiology of PAD and CLTI as chronic diseases, an acute model would be inappropriate for assessing the regenerative capacity of cell administration because an acute ischemia model, with concomitant acute inflammation, is known to be advantageous for angiogenesis.21,22 In fact, a preclinical study comparing acute and established non-acute ischemia models showed that the administration of cells in the established ischemia model failed to increase angiogenesis 3 days after induction of ischemia, despite significant angiogenesis detected in the acute model,21 similar to the unsatisfactory clinical results reported for various cell therapies in PAD. Therefore, to assess the applicability of regenerative cell therapy in clinical settings, preclinical studies using established ischemia or a more chronic ischemia model are necessary.
The aim of the present study was to assess the tissue regenerative potential of intravenously administered UCMSCs using a rat model of established ischemia.
Umbilical cords were obtained from 2 infants delivered at 40 weeks gestation without complications. Written consent was obtained from all parents. This study was approved by the ethics committees at the Sun Field Clinic and Osaka University, and was conducted in accordance with the approved guidelines.
Preparation of UCMSCsHuman umbilical cords (weight 2–3 g) were collected, as noted above. The umbilical cords were enzymatically dissociated with collagenase AF Type A (Worthington Biochemical, Worthington, OH, USA) at 37℃ for 180 min and diluted with 10 times the volume of phosphate-buffered saline (PBS). The cell suspension was then filtered using a 100-μm Falcon cell strainer (Corning, Corning, NY, USA). The resulting cells derived from the umbilical cords were cultured at 37℃ (5% CO2) in PRIME-XV MSC Expansion XSFM medium (FUJIFILM Irvine Scientific, Santa Ana, CA, USA) until the primary cultures reached confluence. The confluent cells were dissociated using TrypLE Select (Gibco, Waltham, MA, USA) and seeded in fresh T150 flasks (Corning). The cells were cultured until they reached confluence and were then cryopreserved in STEM CELL BANKER (Nippon Zenyaku Kogyo, Fukushima, Japan; Figure 1A).
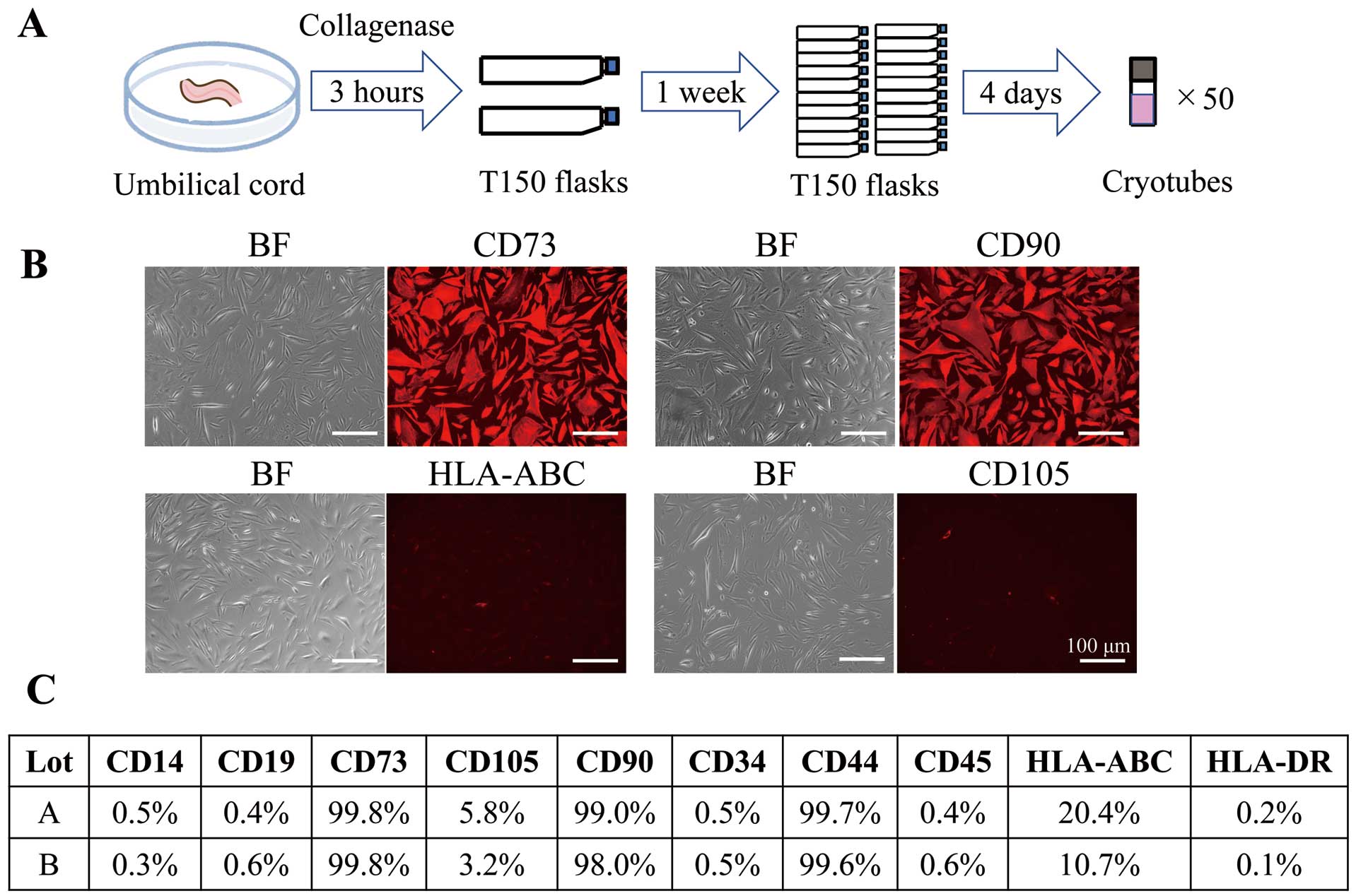
Establishment and characterization of umbilical cord-derived mesenchymal cells (UCMSCs). (A) Protocol for establishing UCMSCs. (B) Representative images of UCMSCs stained with phycoerythrin-conjugated antibodies against CD73, CD90, human leukocyte antigen (HLA)-ABC, and CD105. Scale bars, 100 μm. BF, bright field. (C) Evaluation of cell surface markers using 2 different strains of UCMSCs (lots A and B). Values show the percentage of cells positive for each surface marker.
UCMSCs were dissociated with 0.25% trypsin-EDTA for 10 min, washed with PBS, and suspended at a density of 1×106 cells/mL in flow cytometry buffer containing 1 × PBS, 2 mM EDTA, and 10% Block Ace (Dainippon Pharmaceutical, Osaka, Japan). The cells were incubated with phycoerythrin (PE)-conjugated mouse primary antibodies against CD14, CD19, CD34, CD44, CD45, CD73, CD90, CD105, human leukocyte antigen (HLA)-ABC, or HLA-DR (BD Biosciences, San Jose, CA, USA) for 45 min at 37℃, washed with PBS, and filtered using a 70-μm cell strainer (BD Biosciences). PE-conjugated mouse IgG1, IgG2a, or IgF2b isotype (BD Biosciences) was used as a negative control for each primary antibody. Flow cytometry analysis was performed using an SA3800 flow cytometer and the corresponding analysis software (Sony, Tokyo, Japan). Immunostaining was performed with the same antibodies. The antibody reaction was allowed to proceed in culture medium for 60 min at 37℃, after which samples were washed with PBS and photographed using a BZ-X810 fluorescence microscope (Keyence, Osaka, Japan).
Evaluation of Cell Culture Efficacy on Flasks With or Without Laminin CoatingTo establish a mass culture system for actual clinical use, we compared the cell proliferation efficacy of UCMSCs using flasks coated with or without iMatrix-511 laminin (Nippi, Tokyo, Japan). Cryopreserved UCMSCs were thawed from master cell banks and seeded at a density of 1,000 cells/cm2 in T512 peel-off mass culture vessels (Sumitomo Bakelite, Tokyo, Japan) using serum-free medium with or without an iMatrix-511 laminin coating. Differences in culture efficacy were evaluated across treatments.
Evaluation of the Proliferation Capacity of UCMSCsWe evaluated the proliferative capacity of UCMSCs compared with 2 other types of MSCs: adipose tissue-derived MSCs (ADMSCs; ATCC, Manassas, VA, USA) and bone marrow-derived MSCs (BMMSCs; PuREC, Shimane, Japan). Briefly, cryopreserved MSCs were thawed and cultured in a T512 peel-off mass culture vessel coated with iMatrix-511 laminin. PRIME-XV MSC expansion XSFM medium was used as the culture medium. After the MSCs reached confluence, they were dissociated with TrypLE, selected, collected, and passaged. The cultured MSCs were then used in in vitro experiments to evaluate differences in the cell proliferation rate among the 3 types of MSCs.
ELISA Analysis of Cytokine ProductionAfter the MSC cultures had reached confluence in each flask, they were washed twice with PBS. Culture supernatants were collected after 2 days of medium exchange with Dulbecco’s modified Eagle’s medium (DMEM)/F12 (Sigma-Aldrich, St Louis, MO, USA). Concentrations of hepatocyte growth factor (HGF), monocyte chemoattractant protein-1 (MCP-1), and transforming growth factor (TGF)-β1) in the culture supernatant were measured by ELISA, using commercially available kits (R&D Systems, Minneapolis, MN, USA).
Animals and Ethical ConsiderationsThis study used 12-week-old male Sprague-Dawley (SD) rats and 4-week-old male C57BL/6J mice. All animals were purchased from CLEA Japan (Osaka, Japan). All animal experiments were performed in accordance with the Guide for the Care and Use of Laboratory Animals (National Institute of Health Publication No. 85-23, revised 1996). The experimental protocols were approved by the Animal Experimentation Committee of Osaka University.
Induction of Hind Limb Ischemia and Intravenous Administration of UCMSCsTwelve-week-old SD rats were anesthetized by intraperitoneal administration of a combination of anesthetics (medetomidine/midazolam/butorphanol: 0.3/4/5) at a dose of 5 mL/kg. Hind limb ischemia was induced in the left hind limb by exposing and ligating the external and internal iliac arteries and veins, the saphenous artery and vein at a level just proximal to the ankle, all branches, and then resecting all the vessels between the ligations (Figure 2A). Blood perfusion was measured 3 days after the induction of hind limb ischemia induction using laser Doppler perfusion imaging (LDPI). As an inclusion criterion for treatment intervention, we set an LDPI relative value of ≤60%. We previously described the importance of setting the cut-off value for enrollment to assess the therapeutic efficacy of regenerative therapy.22–24 Only rats with LDPI relative values ≤60% in the non-ischemic limb were classified as having established ischemia and were included in this study. To evaluate the actual efficacy of cell treatment, rats with prominent autoregenerative capacity were excluded from the study. The rats included in the study were randomly assigned to 3 groups and injected with either UCMSCs, culture medium (DMEM/F12), or saline. Cells were injected intravenously into the tail vein using a 27-gauge needle 4 times: on Day 0 (3 days after induction of hind limb ischemia) and on Day 1, 2, and 6 (Figure 2B). The number of cells administered per injection was 1.25×106, with 5.0×106 cells administered in total. After the cryopreserved UCMSCs had been thawed, DMEM/F12 culture medium was used to suspend the UCMSCs. This is because DMEM/F12 was better at maintaining cell viability than saline, primarily due to the various growth factors present in DMEM/F12 (data not shown). Therefore, we included a culture medium (DMEM/F12)-injected group in this experiment to evaluate the treatment capacity of the culture medium due to the growth factors it contains.

In vivo experiments to evaluate the regenerative efficacy of umbilical cord-derived mesenchymal cells (UCMSCs). (A) A severe hind limb ischemia model was created by ligating arteries and veins, as indicated in the diagram, including the external iliac (EIA), internal iliac (IIA), popliteal (PA), the distal portion of the saphenous (SA) at the level of the ankle, and the proximal caudal femoral (PCFA) arteries and veins. The arteries and veins were resected between the ligations. (B) Timeline of the in vivo administration protocol. LDPI, laser Doppler perfusion imaging.
Blood perfusion was evaluated using a Moor LDI 2.0 system (Moor Instruments, Devon, UK). LDPI measurements were performed before the induction of hind limb ischemia, immediately after the induction of ischemia, just before initial cell administration, and then every 7 days until 28 days after initial cell administration (Figure 2B). The relative perfusion value was calculated by dividing the perfusion value of the ischemic limb by that of the contralateral healthy non-ischemic limb.
Histological AnalysisThe adductor muscles of the ischemic limb were harvested 28 days after initial cell administration. The harvested muscles were fixed in formalin, embedded in paraffin, and then cut into 2-μm slices using a microtome. To evaluate angiogenesis after injection, immunohistochemical staining with a rabbit anti-rat von Willebrand factor (vWF) polyclonal antibody (EMD Millipore, Burlington, MA, USA) was performed. To evaluate muscle regeneration, sections were stained with hematoxylin and eosin (HE) and the cross-sectional area of the muscle fibers was measured. Images were examined using optical microscopy (Keyence). Five fields were randomly selected per sample to count the number of capillaries and measure the area of the muscle fibers.
Analysis of the Therapeutic Efficacy of UCMSCs for Muscle Regeneration In VitroSatellite cells, which are muscle progenitor cells, were isolated from 4-week-old C57BL/6J mice via magnetic-activated cell sorting and cultured, as described previously.22 The isolated and cultured satellite cells were resuspended in 12-well plates (IWAKI, Shizuoka, Japan) at a density of 1.0×104 cells/well) and incubated for 48 h at 37℃ under hypoxic (5% O2) and normoxic conditions. The resuspended satellite cells were cocultured with or without the same number of UCMSCs using Costar Transwell cell culture inserts (Corning), and the effect of UCMSCs on the proliferation of satellite cells was assessed (Figure 3A). The number of satellite cells was counted in 5 randomly selected fields per sample at a magnification of ×20 using an optical microscope (Keyence). The proliferation rate of satellite cells was calculated by dividing the number of satellite cells after 48 h of coculture by the initial number of satellite cells.
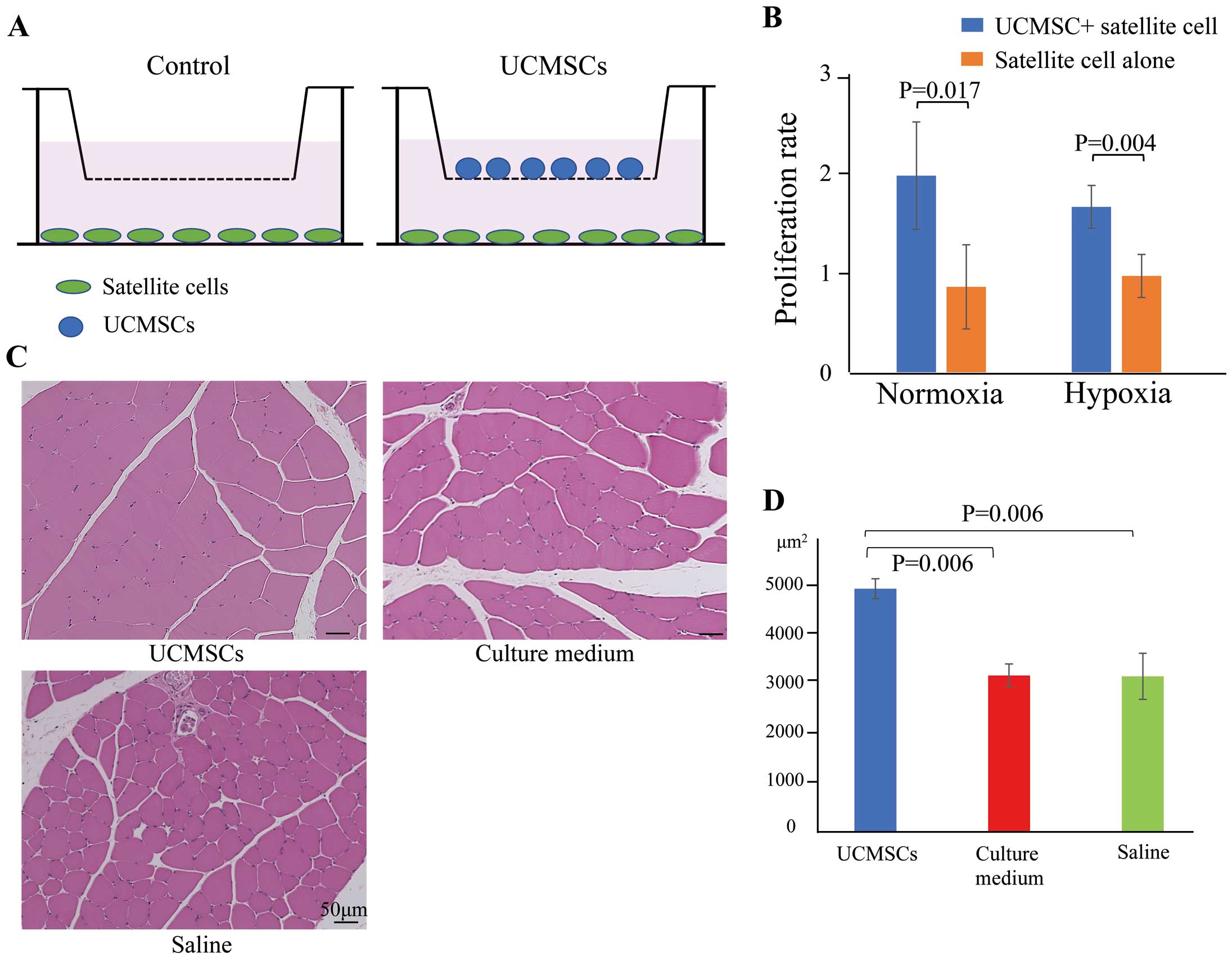
Muscle regeneration induced by umbilical cord-derived mesenchymal cells (UCMSC) administration. (A) UCMSCs were cocultured with satellite cells using a Transwell assay under normoxic or hypoxic conditions for 48 h. (B) Proliferation rate of satellite cells cocultured with or without UCMSCs under normoxic and hypoxic conditions. Data are the mean±SEM. (C) Representative images of hematoxylin-eosin-stained ischemic muscles following the administration of UCMSCs, culture medium, or saline. Scale bars, 50 μm. (D) Mean (±SEM) muscle fiber area in the UCMSCs, culture medium, and saline groups.
LDPI values, capillary density, and the area of muscle fibers were compared among 3 groups using analysis of variance followed by Tukey’s post hoc test. Comparisons between 2 groups were made using Student’s t-test. Unless otherwise noted, all values are reported as the mean±SEM. The threshold for significance was set at 2-tailed P<0.05. JMP version 13.1 (SAS Institute, Cary, NC, USA) was used for statistical analyses.
UCMSCs seeded in flasks were stained with PE-tagged antibodies for 10 days after culture (Figure 1B). The evaluation of UCMSC surface markers was performed using cells derived from 2 different strains. The cultured UCMSCs expressed representative surface markers of MSCs, including CD73, CD90, and CD44, but were negative for hematopoietic cell, macrophage, and endothelial cell markers, such as CD14, CD19, CD34, CD45, and HLA-DR.25 Although UCMSCs expressed surface markers representative of MSC, the expression levels of CD105 and HLA-ABC were low (Figure 1C).
Effect of Laminin on UCMSC ProliferationWe tested the effect of laminin on UCMSC proliferation by using laminin-coated and uncoated flasks. After seeding flasks with 5×105 cells, the number of UCMSCs had increased significantly in laminin-coated flasks on Days 5 and 10 after dissemination (Figure 4A). In laminin-coated flasks, UCMSCs became confluent (approximately 3.2×107 cells/flask) within 10 days of culture, whereas 15 days were required for UCMSCs cultured in uncoated flasks to reach confluence (2.7×105 cells/flask), demonstrating the capacity of laminin to promote the growth of UCMSCs.
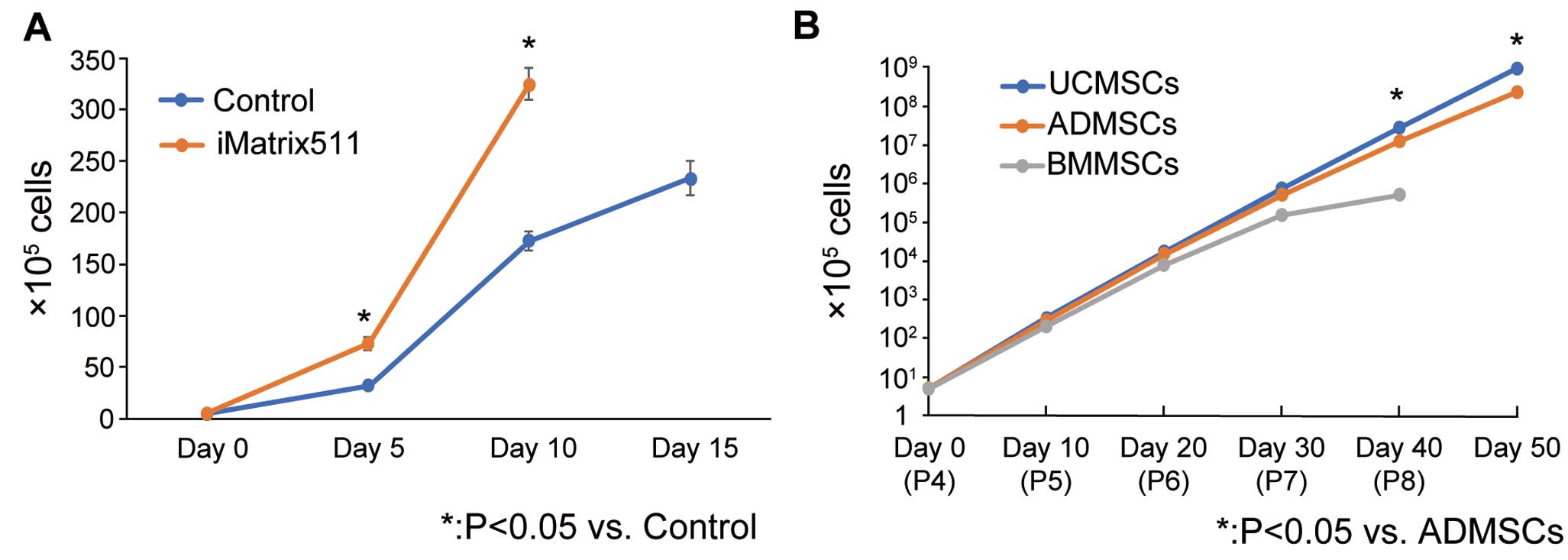
Proliferative capacity of umbilical cord-derived mesenchymal cells (UCMSCs). (A) Differences in the proliferation of UCMSCs cultured in iMatrix-511 laminin-coated flasks (iMatrix511) and uncoated flasks (control) at Days 0, 5, 10, and 15 after seeding 5×105 cells per flask (n=3 each). Data are the mean±SEM. *P<0.05 compared with control (Student’s t-test). (B) Proliferation rates of 3 types of mesenchymal cells (MSCs), namely UCMSCs, adipose-tissue-derived MSCs (ADMSCs), and bone marrow-derived MSCs (BMMSCs), cultured on laminin-coated flasks. Starting from passage (P) 4, defined as Day 0, passages were performed every 10 days, and the total number of cells was plotted on Days 0, 10 (P5), 20 (P6), 30 (P7), 40 (P8), and 50 (P9; n=3 for each). BMMSCs stopped proliferating at P8, and the cultures were terminated. Data are the mean±SEM. *P<0.05 compared with ADMSCs (Student’s t-test).
We compared the proliferative capacity of UCMSCs with that of ADMSCs and BMMSCs cultured in laminin-coated flasks. Starting from the passage 4, passages were repeated every 10 days until Day 50, corresponding to passage 9. The total number of cells was then plotted to compare the proliferative capacity of each type of MSC type (Figure 4B). At passage 8, BMMSCs stopped proliferating, whereas UCMSCs and ADMSCs continued to proliferate. Therefore, the culture of BMMSCs was terminated at Day 40, and a comparison of UCMSCs and ADMSCs was performed, excluding BMMSCs. The proliferative rate of UCMSCs was significantly higher than that of ADMSCs (P<0.05) at passage 8 and later, indicating a better proliferative capacity of UCMSCs.
UCMSC Cytokine ProductionThe ability of 3 types of MSCs (UCMSCs, ADMSCs, and BMMSCs) to secrete cytokines was evaluated using ELISA (Figure 5). UCMSCs secreted significantly higher concentrations of HGF, MCP-1, and TGF-β1 than ADMSCs and BMMSCs (HGF: P=0.002 vs. ADMSCs, P<0.001 vs. BMMSCs; MCP-1: P=0.03 vs. ADMSCs, P=0.03 vs. BMMSCs: TGF-β1: P<0.001 vs. ADMSCs, P<0.001 vs. BMMSCs).
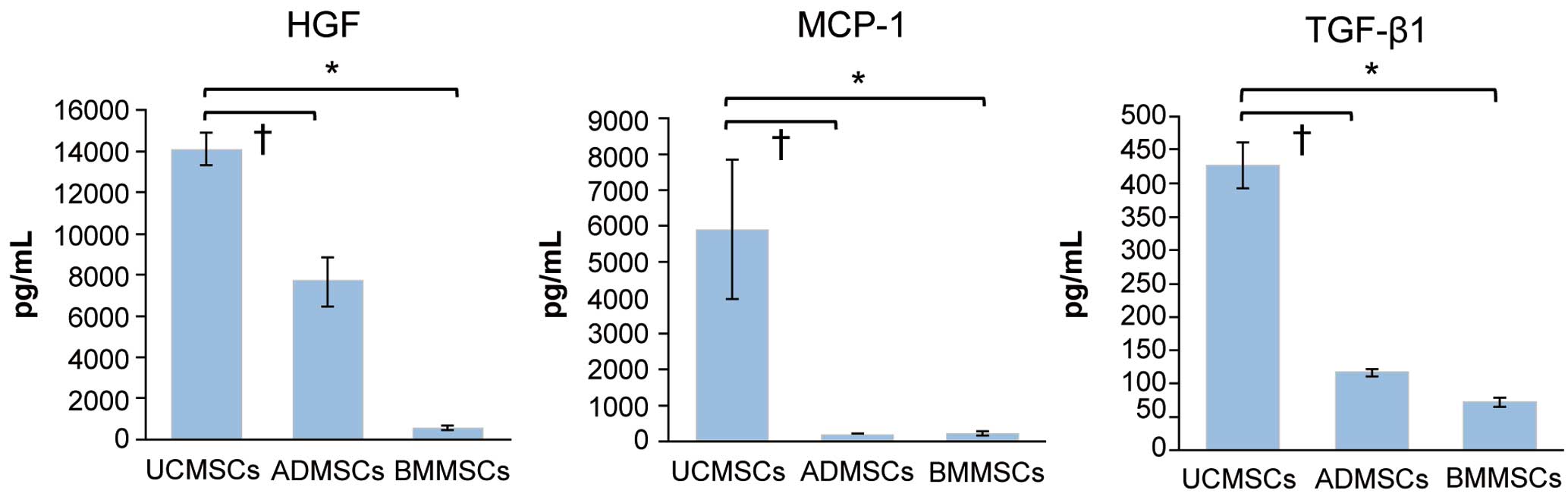
Differences in cytokine production among umbilical cord-derived mesenchymal cells (UCMSCs; n=6), adipose-tissue-derived mesenchymal cells (ADMSCs; n=6), and bone marrow-derived mesenchymal cells (BMMSCs; n=4), evaluated using ELISA. Data are the mean±SEM. *P<0.05 (Tukey’s post hoc test). HGF, hepatocyte growth factor; MCP-1, monocyte chemoattractant protein-1; TGF-β1, transforming growth factor-β1.
The time course of LDPI values and representative images of blood perfusion are shown in Figure 6A and 6B, respectively. LDPI values were significantly increased in the UCMSCs-injected group than in the culture medium- and saline-injected groups 28 days after initial cell administration (76.5±6.6% vs. 54.0±3.9% and 53.1±5.0%, respectively). There was no significant difference in LDPI values between the culture medium and saline groups, indicating that the culture medium has no treatment capacity even if it contains various growth factors.
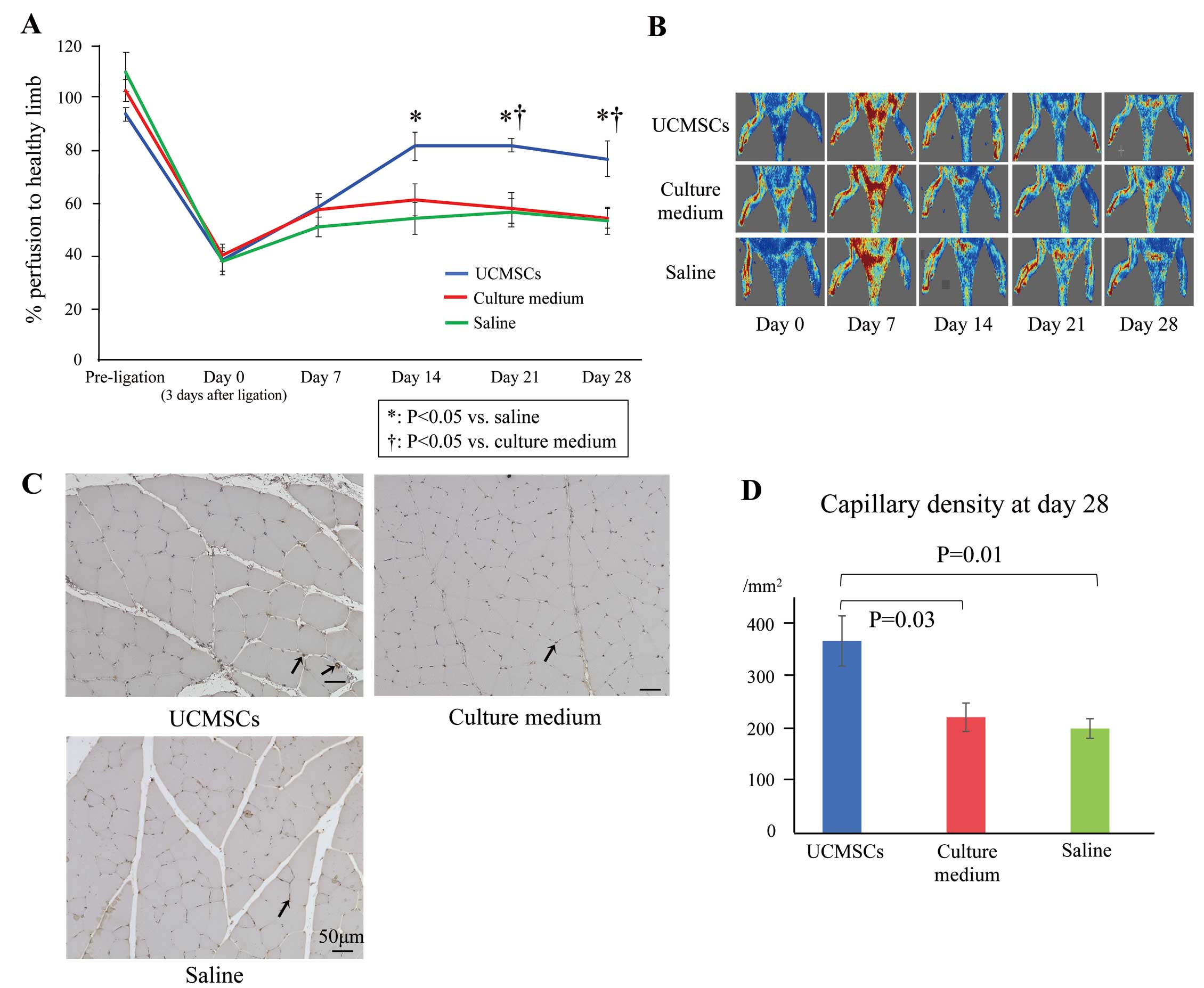
Time course of changes in blood perfusion and angiogenesis. (A) Changes in hind limb perfusion before ligation and on Days 0 (3 days after the induction of ischemia and the day of cell administration), 7, 14, 21, and 28 in rats intravenously administered umbilical cord-derived mesenchymal cells (UCMSCs; n=5), culture medium (n=7), or saline (n=8). Data are the mean±SEM. *P<0.05 compared with the saline group; †P<0.05 compared with the culture medium group (Tukey’s post hoc test). (B) Representative images of laser Doppler perfusion imaging (LDPI) performed on Days 0, 7, 14, 21, and 28. (C) Representative immunohistochemistry images of the microvasculature in ischemic muscles 28 days after initial administration. Sections were stained with an anti-von Willebrand factor (vWF) antibody (brown). Arrows indicate vWF-positive capillaries. Scale bars, 50 μm. (D) Density of vWF-positive capillaries 28 days after initial administration. Data are the mean±SEM (n=5 in each group).
Consistent with the results of LDPI, histological analysis showed significantly augmented angiogenesis, evaluated as the density of vWF-positive capillaries (/mm2), in the UCMSCs group compared with culture medium and saline groups on Day 28 (366.4±47.8 vs. 221.4±26.9 and 199.9±18.7/mm2, respectively [P=0.03 and P=0.01, respectively]; Figure 6C,D).
Muscle Regenerative Capacity of UCMSCs In VitroThe ability of UCMSCs to promote muscle proliferation was evaluated by coculturing them with satellite cells, which are muscle progenitor cells (Figure 3A). The number of satellite cells was significantly increased upon coculture with UCMSCs under both normoxic and hypoxic conditions (P=0.017 and P=0.004, respectively), indicating the capacity of UCMSCs to induce muscle proliferation in vitro (Figure 3B).
UCMSCs-Induced Augmented Muscle Regeneration In VivoHistological analysis revealed differences in muscle regeneration among the UCMSCs-, culture medium-, and saline-injected groups on Day 28 after cell administration (Figure 3C,D). Compared with the UCSMCs group, the size of the muscle fibers was not uniform in the culture medium and saline groups, with a mixture of small and large fibers, indicating insufficient muscle regeneration.26 Measuring the cross-sectional area of the muscle fibers showed that although the mean areas were equivalent between the culture medium and saline groups (3,130.7±230.7 and 3,114.9±460.8 μm2/fiber, respectively; P=0.99), the fibers in the UCMSCs group were more mature and larger in size (4,865.3±198.5 μm2/fiber; P=0.006 vs. culture medium, P=0.006 vs. saline). These results suggest that muscle regeneration was augmented by the administration of UCMSCs in vivo.
In the present study, the UCMSCs harvested and cultured using our method showed high regenerative capacity in a non-acute, established hind limb ischemia model, especially with regard to angiogenesis and muscle regeneration in vitro and in vivo.
In this study we used a non-acute, established ischemia model to appropriately test the regenerative capacity of UCMSCs for actual patients with PAD who are refractory to various regenerative therapies.8 Hence, at least a treatment-refractory preclinical model should be used to confirm the concept of target treatment in preclinical studies. We included rats with limited perfusion recovery because rodents are well known for their high autoregenerative capacity.27 In pilot experiments that induced severe hind limb ischemia without any subsequent intervention, approximately 20–30% of rats recovered blood perfusion >80% of the LDPI value at 4 weeks after the induction of ischemia (data not shown). This result would make appropriate preclinical tests difficult without the selection of individual treatment-refractory rats based on setting a cut-off value, as in the present and previous studies.22–24
Historically, various MSCs from different sources have been used for regenerative therapy.28–30 The present study suggests that the umbilical cord would be an ideal cell source of MSCs, considering their higher proliferative capacity compared with MSCs derived from adipose tissue and bone marrow. In addition to the tissue or organ source, culture conditions are also important because they change the characteristics of MSCs, including proliferative capacity and the expression of surface markers.31 In the present study, in addition to the proliferative capacity, a similar cytokine secretion profile and surface marker expression was maintained during passaging (data not shown). With regard to surface markers, although the UCMSCs in the present study expressed most of the markers representative of MSCs, including CD33, CD73, and CD90, they expressed CD105 and HLA-ABC only at low levels, probably because of the serum-free culture conditions used in this study.25,32 The low HLA-ABC levels would be advantageous for regenerative effects because HLA-ABC is immunogenic; hence, low HLA-ABC levels may help evade immune rejection.33
Advantages of cell therapy include the potential therapeutic effect caused by the secretion of multiple cytokines and the various cell interactions among administered cells and host cells.9,34,35 These could have various positive effects on tissue regeneration through the induction of angiogenesis, muscle regeneration, and regulation of inflammation.22 In vitro experiments showed that UCMSCs secrete large amounts of HGF, which is important for angiogenesis and muscle regeneration.26,34,36,37
Consistent with the in vivo results, injection of UCMSCs in the present study resulted in augmented angiogenesis and muscle regeneration. In patients with PAD, muscle regeneration is an important target of treatment, in addition to angiogenesis. Patients with PAD have both ischemic symptoms and muscle wasting.38 Deteriorated muscle function and quality negatively affect walking ability, which is closely related to survival prognosis in these patients.39,40 Therefore, to alter the prognosis of patients with PAD, it is necessary to provide them with a treatment that improves their functional status and walking ability. UCMSCs secrete various cytokines with inflammation-modulatory properties, including MCP-1 and TGF-β.41 Although we did not scrutinize the effect of UCMSCs on inflammation in the present study, considering the importance of controlling inflammation to promote healthy tissue regeneration,22,39,40,41 these cytokines could potentially contribute to augmented tissue regeneration.
In the present study, UCMSCs were delivered intravenously. Although the intramuscular route is the preferred route of administration, the optimal method for delivering these cells remains unclear. Potential routes of administration include intramuscular, subcutaneous, intra-arterial, and intravenous, but each route has its limitations.42 Focal administration would not show regenerative capacity in large ischemic areas. Intramuscular injection would lead to an immediate loss of administered cells due to the ischemic and inflammatory microenvironment, although direct administration of cells into the ischemic area is possible.43 Intra-arterial administration would enable administration into several ischemic arterial areas, but the fate of the administered cells remains unclear, and a potential risk of vascular occlusion has been reported. The major concern regarding systemic intravenous administration is the potential loss of cells before they reach target sites, either by the cells being washed out or trapped mainly in the lungs and partially in the liver and spleen;44,45 administered MSCs have been reported to disappear within hours to days.30 In this context, the homing of MSCs to the sites of injury would not be a major contributor to tissue regeneration, although in specific disease conditions, transiently augmented homing of MSCs has been reported.28,30 Considering the therapeutic effect of intravenously administered MSCs detected in the present and previous studies, the homing of MSCs to the site of injury is not necessarily a prerequisite. Potential therapeutic mechanisms could be attributed to the interaction with various immune cells, leading to the modification of inflammation.30 Various chemokines secreted by UCMSCs, including MCP-1, attract monocytes and macrophages, subsequently leading to the phagocytosis of administered MSCs. Monocytes and macrophages, which phagocytose MSCs, regulate inflammation and contribute to tissue regeneration.30,46 In addition, the paracrine effect of the administered MSCs, exosomes, and macrovesicles, which transmit various signaling pathways, may have a therapeutic effect.30 Nonetheless, the mechanism of tissue regeneration following intravenous infusion of MSCs is still not fully understood, and further investigations into this mechanism are needed.
Intravenous administration of UCMSCs cultured using our method showed significantly augmented angiogenesis and muscle regeneration in a rat model of established ischemia, suggesting the clinical possibility of using this approach in treating patients with PAD.
The UCMSCs used in this study were supplied by Cell Exosome Therapeutics (Tokyo, Japan).
This work was supported by Cell Exosome Therapeutics (Tokyo, Japan).
N.A. and Y.S. are members of Circulation Journal’s Editorial Board.
This study was approved by the Osaka University Clinical Research Review Committee (Reference no. 20262).