論文ID: CJ-22-0561
論文ID: CJ-22-0561
Background: Because of revisions to insurance reimbursement costs, medical fees have changed for investigations and percutaneous coronary intervention (PCI) treatment of chronic coronary syndrome (CCS). In this retrospective study, we investigated these changes and their effects on mortality and cardiovascular events.
Methods and Results: We included 1,483 patients who underwent elective PCI for CCS between April 2010 and September 2019. The primary outcomes were changes in PCI procedure fees and all included hospitalization fees due to the biennial revisions of reimbursement costs across 5 time periods (~2 years each). Secondary outcomes were rates of survival and freedom from major adverse cerebral and cardiovascular events (MACCE) in each time period. Patient characteristics were generally unchanged over the study period; however, treatment procedures changed significantly, with changes in the approach site (from transfemoral to transradial access; P<0.0001) and final device (from bare-metal stents to drug-eluting stents; P<0.0001), and an increase in the use of imaging modalities (P<0.0001). Medical fee parameters (primary outcomes) decreased significantly from 2010 to 2019 (P<0.001): PCI procedure fees decreased by 25%, whereas all included hospitalization fees decreased by 20%. There were no significant differences in survival or freedom-from-MACCE rates between periods.
Conclusions: Because of revisions to reimbursement prices, there were rapid and significant decreases in PCI procedure and hospitalization fees for CCS. These changes had no effect on mortality or cardiovascular events.
Since 1977, percutaneous coronary intervention (PCI) has expanded rapidly in Japan due to its diagnostic techniques, treatment methods, and the large number of cath labs that have been established across the whole country.1,2
Between 2013 and 2017, approximately 200,000 PCI procedures were performed in Japan annually.3 PCI is an effective treatment for acute coronary syndromes (ACS), improving life expectancy.4 The approval of drug-eluting stents (DES) in 2004 greatly improved patency results.5 In 2020, 260,000 PCIs were performed in Japan. In addition to ACS, PCI is being used more frequently for the treatment of chronic coronary syndromes, such as stable angina pectoris and asymptomatic myocardial ischemia.3 Despite the more frequent use of PCI for the treatment of chronic coronary syndromes, the efficacy of this therapy is often questioned in large-scale clinical studies, including randomized control trials (RCTs) and sham studies.6–8
Insurance reimbursement in Japan is revised approximately every 2 years, primarily with regard to device prices and technical fees. The trend for reimbursement for PCI has been a continual decrease. It has not been verified how revisions to insurance reimbursement change the revenue and costs of hospitals in terms of actual clinical practice change, and whether there is a correlation between changes in insurance reimbursement and event rates. In this study, we retrospectively examined how medical costs have changed for chronic coronary syndrome treated with elective PCI.
This study was a single-center, retrospective observational study. We analyzed 1,483 consecutive patients with chronic coronary syndrome who underwent elective PCI at Kishiwada Tokushukai Hospital between April 2010 and the end of September 2019. The exclusion criteria were elective PCI for ACS non-culprit lesion, additional PCI during the same admission, other treatment-associated PCI, and cases of PCI failure (Figure 1). ACS includes ST-elevation myocardial infarction, non-ST-elevation myocardial infarction, and unstable angina pectoris (UAP). PCI failure was defined as guidewire failure or insufficiency of stent or balloon dilation requiring additional treatment, such as bypass surgery. There was only a small number of cases of PCI failure in this cohort, and costs are likely to vary due to the use of fewer stents and balloons. These cases were excluded to accurately represent the purpose of this study.

Schema of this study. Of the original 3,801 patients, 1,483 patients who met the inclusion criteria were included in the study, which was divided into 5 time periods (A–E). ACS, acute coronary syndrome; NSTEMI, non-ST segment elevation myocardial infarction; PCI, percutaneous coronary intervention; STEMI, ST-elevation myocardial infarction; TAVR, transcatheter aortic valve replacement; UAP, unstable angina pectoris.
All patient and procedure information and medical fees were extracted from electronic medical records. The study site used the Diagnosis Procedure Combination (DPC) system from the beginning of the study, and there were no major payment system changes during the study period.
The study was conducted in accordance with the Declaration of Helsinki and was approved by the Ethics Committee of Kishiwada Tokushukai Hospital (No. 20-04; date of approval, February 10, 2020). Because this study only analyzed existing medical records, it was considered exempt from the need for informed consent, in accordance with the Ethical Guidelines for Medical and Health Research Involving Human Subjects in Japan. Instead, relevant information regarding the study was open to the public and opportunities for refusal were ensured.
PCI Procedure and Follow-upRevascularization premedications, such as aspirin, clopidogrel, and oral anticoagulant agents, were administered according to hospital policy and at the physician’s discretion. Dual antiplatelet therapy was recommended as per guidelines after the procedure. PCI procedures were adapted to the method and operator’s choice at the time, and in consideration of the patient’s background. All procedures were performed under local anesthesia. Bare-metal stents (BMS) or DES were primarily used for treatment, but bare balloon angioplasty or drug-coated balloons (DCB) were used in some cases. The use of imaging devices (intravascular ultrasound [IVUS], optical coherence tomography, or optical frequency domain imaging) was left to the discretion of operators, based on their usual clinical practice and not a routine use. The diagnosis of asymptomatic myocardial ischemia was made using preoperative myocardial scintigrams or fractional flow reserve (FFR) whenever possible.9 Patients with an FFR <0.80 in all vessels with indicated stenoses were treated by PCI.
Follow-up evaluations of clinical symptom were scheduled at 1 month, 12±2 months, and 24±2 months after the procedure. Thereafter, follow-up evaluations were set to occur every other year. Follow-up evaluations assessed all-cause mortality, target lesion revascularization (TLR), acute myocardial infarction (AMI), and stroke. In this study, routine follow-up angiography was not performed for asymptomatic patients with no abnormal examination findings.
Study OutcomesThe primary measure outcomes were PCI procedure fees and all included hospitalization fees, categorized into 7 time periods (Periods A–E) according to when changes in insurance reimbursement costs occurred in Japan: Period A, April 1, 2010 to March 31, 2012; Period B, April 1, 2012 to March 31, 2014; Period C, April 1, 2014 to March 31, 2016; Period D, April 1, 2016 to March 31, 2018; Period E, April 1, 2018 to September 2019. Device prices and technical fees for each period are presented in Table 1. The October 2019 revision was made as a special exception due to the consumption tax increase normally revised every 2 years. PCI procedure fees included all reimbursable device costs, including sheaths, guidewires, balloons, stents, and imaging devices, as well as PCI technical fees. Hospitalization costs were defined as all costs incurred for all hospitalizations (including meals) and including PCI procedure fees.
| April 2004– | April 2006– | April 2008– | April 2010– | April 2012– | April 2014– | April 2016– | April 2018– | October 2019– | April 2020– | April 2022– | |
|---|---|---|---|---|---|---|---|---|---|---|---|
| Period | A | B | C | D | E | ||||||
| Technical fees (¥) | |||||||||||
| PCI for AMI | 228,000 | 220,000 | 220,000 | 220,000 | 220,000 | 320,000 | 320,000 | 320,000 | 320,000 | 360,000 | 360,000 |
| Stent implantation | 229,000 | 220,000 | 220,000 | 220,000 | 243,800 | 343,800 | 343,800 | 343,800 | 343,800 | 343,800 | 343,800 |
| PCI for UAP | 228,000 | 220,000 | 220,000 | 220,000 | 220,000 | 220,000 | 220,000 | 220,000 | 220,000 | 220,000 | 220,000 |
| Stent implantation | 229,000 | 220,000 | 220,000 | 220,000 | 243,800 | 243,800 | 243,800 | 243,800 | 243,800 | 243,800 | 220,000 |
| PCI for non-ACS | 228,000 | 220,000 | 220,000 | 220,000 | 220,000 | 193,000 | 193,000 | 193,000 | 193,000 | 193,000 | 193,000 |
| Stent implantation | 229,000 | 220,000 | 220,000 | 220,000 | 243,800 | 216,800 | 216,800 | 216,800 | 216,800 | 216,800 | 193,000 |
| Device fees (¥) | |||||||||||
| DES | 421,000 | 409,000 | 378,000 | 345,000 | 295,000 | 261,000 | 226,000 | 193,000 | 173,000 | 161,000 | 136,000 |
| BMS | 301,000 | 279,000 | 258,000 | 230,000 | 221,000 | 169,000 | 162,000 | 125,000 | 119,000 | 113,000 | 97,000 |
| DCB | – | – | – | – | – | 178,000 | 170,000 | 170,000 | 173,000 | 173,000 | 173,000 |
| PTCA balloon | 192,000 | 151,000 | 127,000 | 100,000 | 79,100 | 67,300 | 59,200 | 45,400 | 38,900 | 35,500 | 32,000 |
| Guiding catheter | 34,400 | 30,300 | 26,400 | 22,500 | 19,000 | 16,600 | 14,600 | 12,400 | 11,300 | 10,700 | 9,320 |
| Guidewire | 28,700 | 26,100 | 24,600 | 22,000 | 19,100 | 17,100 | 15,400 | 13,300 | 12,500 | 11,900 | 10,900 |
| IVUS | 161,000 | 151,000 | 141,000 | 128,000 | 115,000 | 109,000 | 100,000 | 89,500 | 84,200 | 80,300 | 72,500 |
| OCT/OFDI | – | – | 151,000 | 148,000 | 146,000 | 145,000 | 143,000 | 141,000 | 140,000 | 139,000 | 136,000 |
| Atherectomy catheter | 319,000 | 304,000 | 243,000 | 234,000 | 223,000 | 218,000 | 215,000 | 211,000 | 210,000 | 210,000 | 207,000 |
Periods A–E represent the time covered in the present study. Fees before and after Periods A–E are included for reference purposes. ACS, acute coronary syndrome; AMI, acute myocardial infarction; BMS, bare-metal stent; DCB, drug-coated balloon; DES, drug-eluting stent; IVUS, intravascular ultrasound; OCT, optical coherence tomography; OFDI, optical frequency domain imaging; PCI, percutaneous coronary intervention; PTCA, percutaneous transluminal coronary angioplasty; UAP, unstable angina.
Secondary outcomes were changes in all-cause mortality and the rate of freedom from major adverse cerebral and cardiovascular events (MACCE) in each period.10 MACCE was defined as a composite of all-cause death, non-fatal stroke, non-fatal myocardial infarction, and TLR.
The influence of imaging devices was also evaluated, the use of which rapidly became more common during that period. Data were also collected for PCI procedure-related complications, which were defined as vascular perforation, stent thrombosis, aortic dissection, cardiac tamponade, puncture site bleeding/hematoma requiring blood transfusion, and stroke/embolism.
Statistical AnalysesContinuous data were assessed for normal distribution using the Kolmogorov-Smirnov test. Normally distributed variables are presented as the mean±SD. Non-normally distributed variables are presented as median values with the interquartile range. The Kruskal-Wallis test was used for comparisons of non-parametric values among 3 or more groups. Kaplan-Meier estimates were used to summarize the time-to-outcome according to imaging device use and non-parametric log-rank tests were used to compare the time-to-outcome curves. P<0.05 was considered statistically significant; 95% confidence intervals (CIs) are reported as appropriate. All statistical analyses were performed using R version 4.1.1 (R Development Core Team, Vienna, Austria).
In all, 1,483 cases were evaluated in the primary analysis. The baseline characteristics of the study population are summarized in Table 2. The most common coexisting conditions were hypertension (84.6%), diabetes (44.3%), dyslipidemia (69.2%), and chronic renal failure (10.2%), including regular dialysis (8.1%). Aspirin, P2Y12 inhibitors, and oral anticoagulant agents were used by 98.3%, 96.2%, and 7.4% patients, respectively. Approximately 73.8% of patients were symptomatic, and the rest (26.2%) had asymptomatic ischemia. Preoperative coronary computed tomography angiography was obtained in 35.4% of patients and myocardial stress scintigrams were performed in 17.7% of patients.
| Patient characteristics | |
| Male sex | 1,023 (69.0) |
| Age (years) | 69.7±9.7 |
| Body mass index (kg/m2) | 23.9±3.5 |
| Current smoker | 393 (26.5) |
| Hypertension | 1,255 (84.6) |
| Dyslipidemia | 1,026 (69.2) |
| Diabetes | 657 (44.3) |
| CRF (eGFR <30 mL/min/1.73 m2) | 151 (10.2) |
| On dialysis | 120 (8.1) |
| Past history of stroke | 167 (11.2) |
| Postoperative CABG | 141 (9.5) |
| Atrial fibrillation | 91 (6.1) |
| EF by echocardiogram (%) | 58.8±11 |
| Aspirin use | 1,458 (98.3) |
| P2Y12 inhibitor use | 1,428 (96.2) |
| Anticoagulant use | 110 (7.4) |
| Statin use | 907 (61.1) |
| Chronic coronary syndrome | |
| Effort angina pectoris | 1,094 (73.8) |
| Silent myocardial ischemia | 389 (26.2) |
| Preoperative diagnosis | |
| Coronary CTA | 525 (35.4) |
| Stress myocardial scintigraphy | 262 (17.7) |
| Exercise stress test | 132 (8.9) |
| Catheter-based angiographyA | 182 (12.2) |
| Perioperative features | |
| Access site | |
| TFI | 399 (27.0) |
| Transbrachial intervention | 29 (2.0) |
| TRI | 1,055 (71.1) |
| Target lesion | |
| Left main trunk | 49 (3.3) |
| Left anterior descending coronary artery | 654 (44.1) |
| Right coronary artery | 519 (35.0) |
| Left circumflex coronary artery | 283 (19.1) |
| Bypass graft | 55 (3.7) |
| Chronic total occlusion | 131 (8.8) |
| Final device | |
| BMS | 248 (16.7) |
| DES | 1,202 (81.0) |
| Drug-coated stent | 19 (1.3) |
| Bare balloon angioplasty | 14 (0.9) |
| No. stents implanted | 1.3±0.6 |
| Imaging modality | |
| IVUS | 587 (40.0) |
| OCT/OFDI | 46 (3.1) |
| Fractional flow reserve | 25 (1.7) |
| Rotational atherectomy device | 31 (2.1) |
| Procedure-related complications | 11 (0.7) |
| Medical fees (¥) | |
| PCI procedure | 762,150 [648,540–964,475] |
| All included hospitalization costs | 861,070 [743,305–1,072,280] |
| LOS (days) | 2.4±1.6 |
Categorical variables are presented as n (%). Normally distributed continuous variables are presented as the mean±SD; non-normally distributed data are presented as the median [interquartile range]. ADifferent from PCI hospitalization. CABG, coronary artery bypass graft; CRF, chronic renal failure; CTA, computed tomography angiography; EF, ejection fraction; eGFR, estimated glomerular filtration rate; LOS, length of hospital stay; TFI, transfemoral intervention; TRI, transradial intervention. Other abbreviations as in Table 1.
Perioperative features are also presented in Table 2. The transfemoral artery was the treatment access site of choice for 27.0% of procedures, the transbrachial artery for 2.0%, and the transradial artery for 71.1%. No treatment was accessed from the distal radial artery during the study period.
Target lesions were the left anterior descending coronary artery, right coronary artery, left circumflex coronary artery, and bypass grafts in 44.1%, 35.0%, 19.1%, and 3.7% of patients, respectively. Imaging modalities were used in 44% of patients. The final devices were DES in 81% of patients, BMS in 16.7%, DCB in 1.3%, and bare balloon angioplasty in the remaining 0.9%. Major procedure-related complications were low, at 0.7%, with an overall hospital stay of 2.4 days.
The number of patients, patient and lesion characteristics, and treatment background by period are presented in Table 3. The patient characteristics were generally unchanged across periods; however, treatment procedures changed significantly during the study, with the main approach site changing from transfemoral intervention to transradial intervention (P<0.001) and the main final device changing from BMS to DES (P<0.001). The use of imaging modalities also increased during the study period.
| Period | P value | |||||
|---|---|---|---|---|---|---|
| A | B | C | D | E | ||
| No. patients enrolled | 301 | 319 | 321 | 357 | 185 | – |
| Age (years) | 69.2±9.3 | 68.8±8.7 | 70.2±9.6 | 69.7±10.0 | 70.8±10.2 | 0.13 |
| Diabetes (%) | 115 (38.2) | 149 (46.7) | 146 (45.5) | 158 (44.3) | 89 (48.1) | 0.16 |
| Dyslipidemia (%) | 208 (69.1) | 220 (69.0) | 215 (67.0) | 264 (73.9) | 119 (64.3) | 0.16 |
| Renal failure (%) | 13 (11) | 28 (8.8) | 38 (11.8) | 36 (10.1) | 16 (8.7) | 0.67 |
| TFI (%) | 120 (39.9) | 97 (30.4) | 79 (24.6) | 73 (20.5) | 30 (16.2) | <0.001 |
| TRI (%) | 172 (57.1) | 215 (67.4) | 237 (73.8) | 279 (78.1) | 152 (82.2) | |
| BMS (%) | 173 (57.5) | 65 (20.4) | 9 (2.8) | 1 (0.3) | 0 | <0.001 |
| DES (%) | 123 (40.9) | 253 (79.3) | 302 (94.1) | 349 (97.8) | 175 (94.6) | |
| Imaging modalities (%) | 128 (45.5) | 92 (28.8) | 115 (28.8) | 189 (52.9) | 122 (66.0) | <0.001 |
| Procedure-related complications | 2 (0.6) | 0 (0) | 2 (0.62) | 3 (0.84) | 4 (2.2) | 0.084 |
| LOS (days) | 2.36±1.6 | 2.29±1.9 | 2.38±1.9 | 2.36±1.6 | 2.42±2.2 | 0.43 |
Categorical variables are presented as n (%). Normally distributed continuous variables are presented as the mean±SD. P<0.05 was considered statistically significant. Abbreviations as in Tables 1,2.
The primary outcomes of median PCI procedure fees and all included hospitalization fees for the entire cohort were ¥762,150 (IQR ¥648,540–964,475) and ¥861,070 (IQR ¥743,305–1,072,280), respectively. Figure 2 shows the trends in both medical cost parameters according to period (A–E). These data were not normally distributed (Kolmogorov-Smirnov test), so a non-parametric test (Kruskal-Wallis test) was performed. In addition, some patients with extreme costs were identified by the Smirnov-Grubbs test and excluded as outliers. Both medical cost parameters decreased significantly over the 10-year study period, as shown in Figure 2: PCI procedure fees decreased by 25% and all included hospitalization fees decreased by 20%.
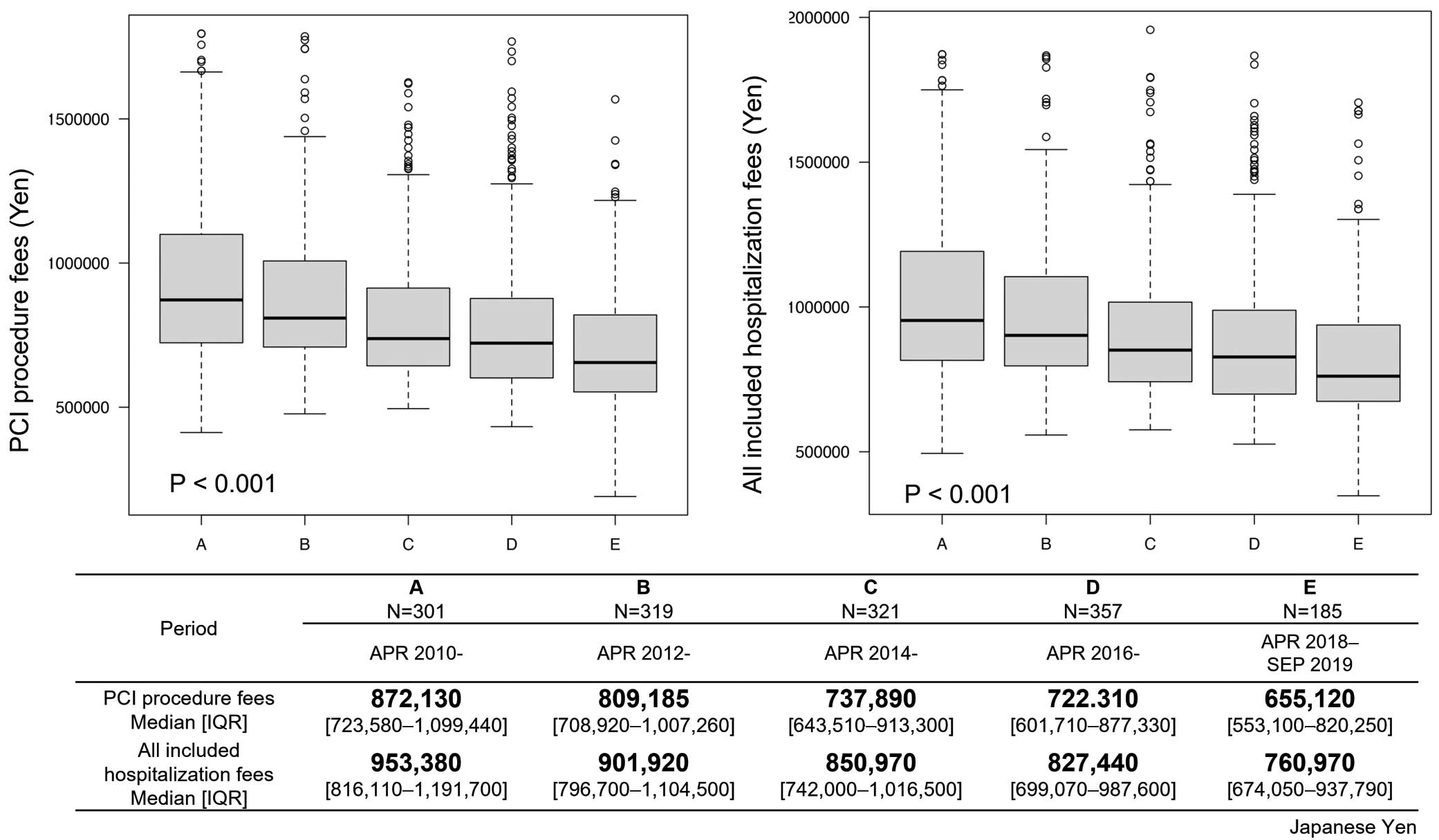
Medical costs for percutaneous coronary intervention (PCI) procedures (Left) and all included hospitalization fees (Right) in Periods A–E. The boxes show the interquartile range (IQR), with the median value indicated by the horizontal line; whiskers show the range.
Survival and MACCE rates (secondary outcomes) are shown in Figure 3 for each period. There were no significant differences in the occurrence of events in each period, despite a decrease in medical costs.
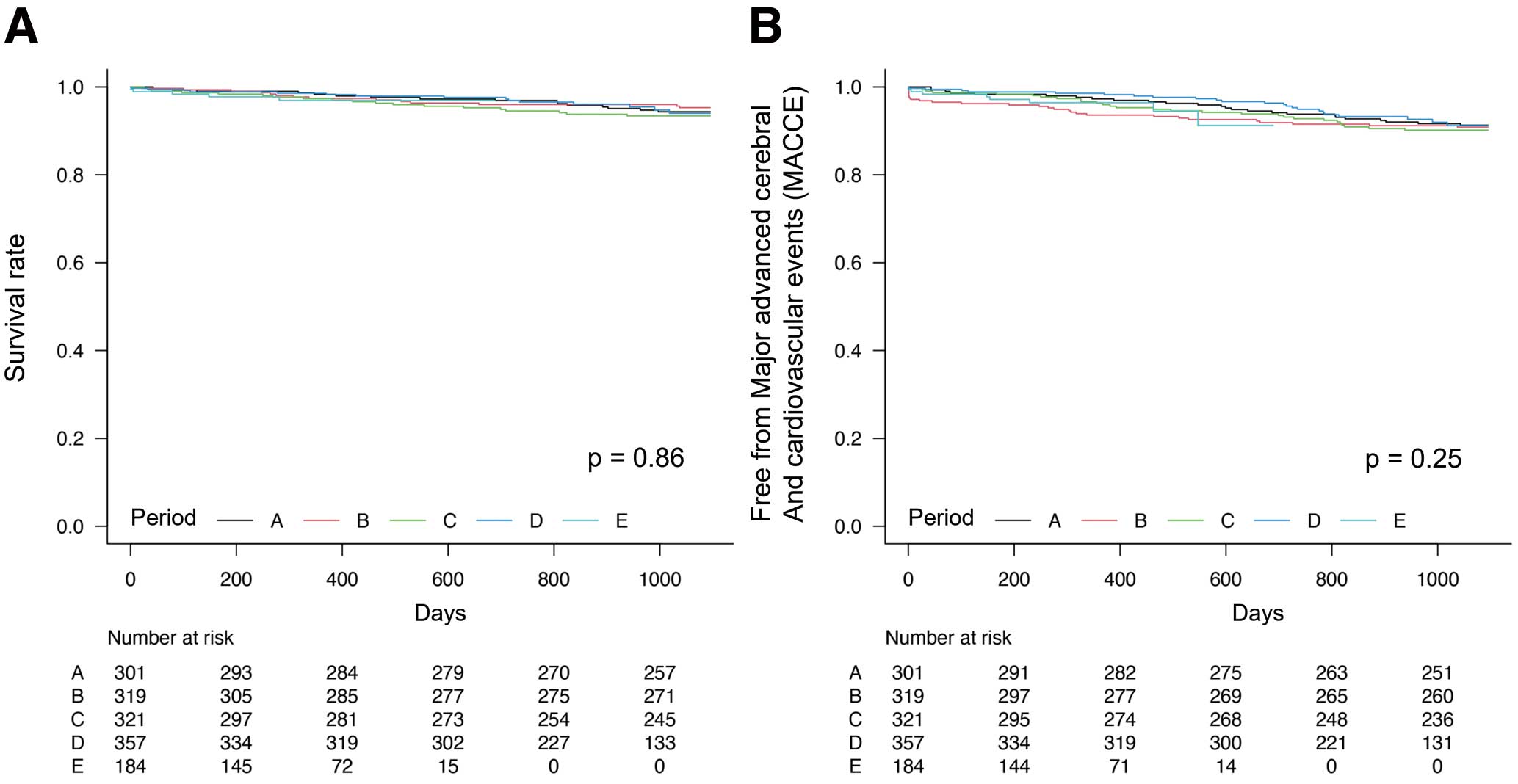
Kaplan-Meier estimates of (A) the survival rate and (B) freedom from major adverse cardiac and cerebrovascular events (MACCE) in Periods A–E.
The rate of use of imaging modalities was different in each period, which may have affected the results. Figure 4A shows an analysis that excludes imaging modality cost from the PCI procedure fee. A more marked decrease in PCI procedure fees was also observed in this analysis. However, the difference from the total cost was smaller than the imaging modality cost at all-time points, suggesting that conditional imaging device use may have reduced the use of other devices, such as stents and balloons. As shown in Figure 4B, there was no difference in the rates of all-cause mortality and MACCE avoidance between IVUS use and non-use.
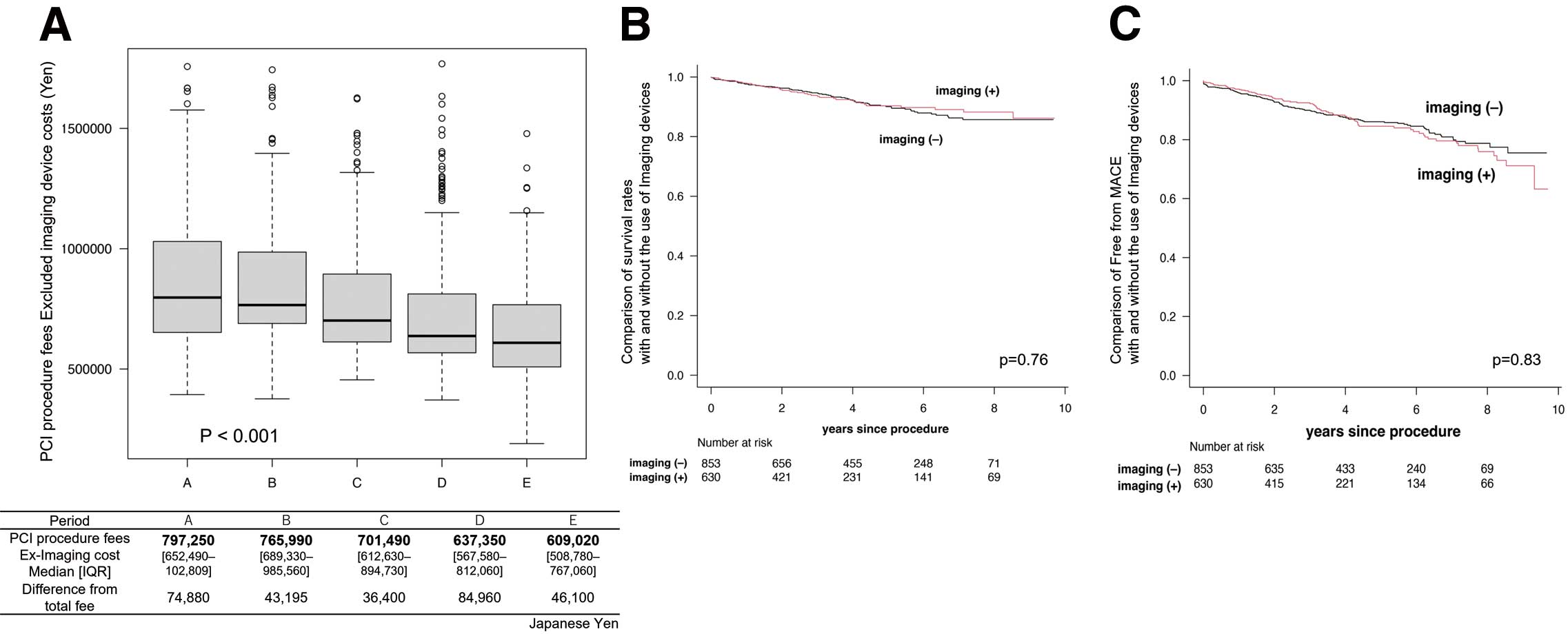
Analyses excluding imaging modality costs. (A) Percutaneous coronary intervention (PCI) procedure fees excluding imaging modality costs. The boxes show the interquartile range (IQR), with the median value indicated by the horizontal line; whiskers show the range. (B,C) Kaplan-Meier estimates of survival rate (B) and freedom from major adverse cardiac and cerebrovascular events (MACCE; C) with or without imaging modalities.
In the present study we retrospectively evaluated changes in medical fees for chronic coronary artery disease patients who underwent standby PCI. The primary outcome, PCI procedure fees and all hospitalization fees, decreased significantly over the study period (2010–2019), down to approximately three-quarters and four-fifths, respectively (Figure 2).
The PCI cost in Japan is primarily determined by the technical fee associated with the procedure and the fee for the device used for catheterization. Pricing has been changed by biennial reimbursement revisions and, as indicated in Table 1, has continued to decrease compared with the beginning of the PCI era.
Reimbursement revision after this study has resulted in further reductions in technical fees for chronic coronary syndrome and PCI device fees. The current medical fee would be even lower.
Specifically, technical fees were differentiated between ACS and non-ACS in the 2014 revision: increasing by over 50% compared with 2004, primarily for AMI, UAP fees remaining flat, and chronic coronary syndrome fees decreasing by 15%. The decrease in device fees was even more marked, with reimbursed DES falling from ¥421,000 in 2004 to ¥136,000.
Comprehensive medical care is already available in other countries. In the US, drug-eluting coronary stent treatment with angioplasty costs US$20,602 with major complication or comorbidity (MCC) or US$12,356 without MCC all-inclusive for inpatient care, or US$10,258 for day treatment. Device cost is set by individual manufacturers, who select devices through negotiations with hospitals and earn revenue from the marginal profit with comprehensive care. There is no difference in costs between ACS and non-ACS, and the costs of IVUS or atherectomy devices are calculated additionally in some situations. Comparing the results of the present study with current US costs,11 the fees in Japan are considerably cheaper.
There are some possible reasons for the recent price declines in contrast to general increase in prices in Japan. One is the urgent need to curb the overall rise in medical costs due to an aging society.12,13 Another is that the superiority of PCI over optimal medical therapy in chronic coronary syndrome has not been proven,14,15 compared with reasonable ACS evidence and guidelines.16 The status of these trials remains unclear,6–8 although many RCTs have been conducted to evaluate the efficacy of revascularization in patients with chronic coronary syndrome. There is no solid evidence to support the proposition that revascularization clearly improves outcomes in chronic coronary syndrome despite it still being widely used worldwide.3,17
It is known that approximately one-third of all PCI procedures in Japan are performed on asymptomatic patients. Furthermore, it has long been known that more than 70% of PCI procedures performed in Japan are elective, which is much higher than the 30% of procedures performed in the US and other countries.3,18
Appropriate PCI has been advocated overseas based on recent evidence, and the use of elective PCI for the chronic coronary syndrome is rapidly decreasing.15,16,19,20 In the present cohort, reported 1,931 of 3,801 patients (51%) had ACS, so the data from this patient cohort are relatively valid. Compared with other countries, there are more inappropriate cases of elective PCI than emergency PCI in Japan.21,22
It is undeniable that inappropriate cases were included in this cohort, also considering that asymptomatic myocardial ischemia accounted for 26% of all cases and myocardial scintigrams for 18%. The cost of PCI remains the same, whether the procedure is appropriate or inappropriate.
Minimally invasive tests such as preoperative scintigrams must be used in combination with more appropriate PCI procedures.23 Hospitalization and catheterization devices are necessary and the medical cost of such testing remains a future challenge, although FFR testing in the cath lab is proving efficacious.9 It is still unclear whether the number of PCI procedures could be reduced.
The techniques and devices used also changed significantly over the study period. DES is now primarily used and transradial intervention has become more common. This is considered an advance in safer and more effective treatment. It is likely that PCI procedures have become somewhat standardized in Japan. However, the procedure and device fees continue to decrease, which are the compensation for this standardization.
There were no significant changes in the secondary outcomes (all-cause mortality and MACCE-free rate) over the study period. Safety and efficacy were maintained, despite statistically significant decreases in PCI and hospitalization costs.
A subanalysis taking into account the use of imaging modalities showed no difference in major event rates whether imaging modalities were used or not.24 However, the use of imaging modalities may not be necessary in all cases in clinical practice, and their conditional use may be important.
PCI in this study was a relatively simple procedure, with 3.3% left main trunk lesions and a mean number of 1.3 stents used and a small percentage. Japan is considering a shift from the current device volume-based system to comprehensive care in the future. This would make it more difficult for many cath labs to perform elective PCI and may result in a decrease in case numbers. Profitability may suffer, especially for complex and chronic total occlusion lesions that require many devices.25 There will be a need for centralized cath labs that perform many emergency PCIs, but operator education and the introduction of new devices in Japan may become problematic if this situation arises.
The present study examined the costs associated with PCI, a topic that has not been discussed much in the past from the perspective of a real-world clinical situation. Continued discussion is needed to ensure that appropriate medical care continues in this area in the future.
Study LimitationsThere are some limitations to this study. First, it was a single-center, retrospective study and the procedures were not standardized because of the long study period (10 years). In particular, a small bias in device selection and whether imaging devices are used must be considered. Second, only the more stable waitlisted patients were enrolled in the study, which may have introduced a bias in case selection to minimize variability.
Catheterization and hospitalization fees for chronic coronary syndrome decreased significantly due to revised reimbursement prices. This change in costs had no effect on mortality or the rate of cardiovascular events.
Special thanks go to Shinji Shiotani, Akihiro Higashimori (Higashimori Clinic), Keisuke Fukuda (Tane General Hospital), Yu Morishita (Osaka University), Osami Kawarada (Kawarada Cardio Foot Vascular Clinic), and Shinji Nakata (Hino Clinic), as well as those who performed PCI procedures during this period and all the catheterization room and ward staff.
The authors have received no financial support for the research, authorship, and publication of this article.
The authors have no conflicts of interest to disclose.
This study was approved by the Ethics Committee of Kishiwada Tokushukai Hospital (No. 20-04; date of approval, February 10, 2020).