論文ID: CJ-22-0577
論文ID: CJ-22-0577
Background: In patients with acute myocardial infarction (AMI), elevated natriuretic peptide (NP) concentrations are reportedly associated with worse clinical outcomes. This study evaluated the prognostic value of NP concentrations and in-hospital heart failure (HF) events after AMI.
Methods and Results: The present bicenter registry included 600 patients with AMI undergoing percutaneous coronary intervention. HF was evaluated at 3 different time points after AMI: on admission, during hospitalization, and at the short-term follow-up at 1 month. When HF was present at each time point, 1 point was assigned to the “HF time points” (HFTP) risk scoring system; possible total scores on this system ranged from 0 to 3. The primary endpoint was a composite of all-cause death and HF rehospitalization after discharge. Among the 600 patients who survived to discharge, the primary outcome occurred in 69 (11.5%) during a mean follow-up period of 488 days. HF on admission, during hospitalization, and at the short-term follow-up were all significantly associated with subsequent clinical outcomes. Higher scores on the HFTP scoring system were related to an increased risk of the primary endpoint. Multivariable analysis indicated scores of 2 and 3 were independently associated with outcome events in a stepwise manner.
Conclusions: Among patients with AMI, HF evaluation at different time points was useful in stratifying risks of mortality and HF rehospitalization after discharge.
Ischemic heart disease, such as acute myocardial infarction (AMI), is a major underlying pathogenic factor in heart failure (HF), accounting for approximately 50% of cases in the current era,1,2 and HF event is a strong predictor of worse outcomes among patients with AMI.3,4 B-type natriuretic peptide (BNP) and N-terminal pro-BNP (NT-proBNP) are natriuretic peptides (NPs) secreted by the cardiac ventricles in response to end-diastolic pressure and volume,5 and are widely used as diagnostic biomarkers for HF in clinical practice.6 Elevated NP concentrations are associated with an increased risk of death and HF in patients with AMI,5,7 and thus recent guidelines recommend measurement of NPs to gain prognostic information in patients with AMI.8 However, the trajectory in BNP and NT-proBNP concentrations varies widely among patients, and the appropriate timing for NPs evaluation after AMI remains unclear.9 For example, early studies demonstrated that the BNP concentration at the time of initial presentation of patients with ST-segment elevation myocardial infarction (STEMI) predicted mortality,10,11 whereas other reports indicated that NP concentrations during short-term follow-up after AMI were more predictive than those at baseline.12,13 In addition, acute HF often develops during hospitalization for AMI,14 but its prognostic value in combination with NP concentrations is uncertain.3,15 Thus, the aim of the present study was to evaluate the effects of elevated NP concentrations on admission and at the short-term follow-up, and in-hospital HF events, individually and in combination, on clinical outcomes after discharge in patients with AMI.
This was a retrospective, observational, bicenter study.16–21 Between January 2012 and March 2020, 1,102 AMI patients underwent primary percutaneous coronary intervention (PCI) at 2 tertiary referral centers, Chiba University Hospital and the affiliated Eastern Chiba Medical Center. AMI, including STEMI and non-STEMI, was defined according to the fourth universal definition of MI.22 Primary PCI was performed per local standard practice with the predominant use of radial access, intracoronary imaging, and new-generation drug-eluting stents.23–27 Major exclusion criteria included in-hospital-onset AMI, late (>48 h) presentation, in-hospital death, missing data on NP concentrations at admission or at the short-term follow-up, and no follow-up information after hospital discharge (Figure 1). Thus, 600 patients were included in the present study. All participants provided written informed consent for the PCI procedure; informed consent for this study was obtained in the form of an opt-out option.

Study flow. MI, myocardial infarction; PCI, percutaneous coronary intervention.
This study was performed in accordance with the Declaration of Helsinki and was approved by the ethics committees of Chiba University Hospital and Eastern Chiba Medical Center.
HF AssessmentAt Chiba University Hospital and Eastern Chiba Medical Center, patients with AMI undergoing PCI are usually followed-up at the outpatient clinic at 1 month after discharge and routinely undergo NP measurements during hospitalization and at a follow-up visit. BNP was measured at Chiba University Hospital, whereas NT-proBNP was measured performed at Eastern Chiba Medical Center. All patients included in the present analysis underwent NP (either BNP or NT-proBNP) measurements at admission and at the short-term follow-up. Short-term follow-up NP concentrations were obtained immediately before discharge or at the 1-month outpatient visit. When NP concentrations before discharge and at the 1-month follow-up were both available, the NP concentration measured at the time closest to 30 days after the onset of AMI was used as the short-term follow-up value. According to the guidelines,28 patients were considered to have HF when NP concentrations on admission and at the short-term follow-up were ≥200 pg/mL BNP or ≥900 pg/mL NT-proBNP (Figure 2).

Time points of heart failure (HF) assessment and calculation of the “HF time points” score. BNP, B-type natriuretic peptide; IQR, interquartile range; IV, intravenous; LOS, length of hospital stay; NT-proBNP, N-terminal pro-BNP.
In addition, we evaluated HF during hospitalization. In-hospital HF was defined as the use of intravenous diuretics (e.g., furosemide) or vasopressors/inotropes (e.g., norepinephrine, dobutamine, and dopamine).16,29 The use of intravenous vasodilators such as nitrates and nicorandil did not fulfill the definition of in-hospital HF. One point was assigned to the presence of HF at each time point (i.e., on admission, during hospitalization, and at the short-term follow-up) to create the “HF time points” (HFTP) risk scoring system, which ranged from 0 to 3 (Figure 2).
Endpoints and Statistical AnalysisFollow-up data were obtained from medical records at Chiba University Hospital and Eastern Chiba Medical Center. The primary outcome of the present study was a composite of all-cause death and HF rehospitalization after discharge. Further analysis was performed among patients with scores of 1 and 2 on the HFTP risk scoring system to evaluate the impact of HF at different time points. In addition, the relationship between treatment strategies (i.e., intravenous diuretics and vasopressors/inotropes) and clinical outcomes was investigated among patients with in-hospital HF.
Statistical analyses were conducted using EZR (Saitama Medical Center, Jichi Medical University, Saitama, Japan), which is a graphical user interface for R (R Foundation for Statistical Computing, Vienna, Austria). Data are expressed as the mean±SD, median with interquartile range (IQR), or as frequencies with percentages. Continuous variables were compared using Student’s t-test or the Mann-Whitney U test depending on the normality of distribution. Categorical variables were assessed with Fisher’s exact test. The time to the primary outcome was estimated using the Kaplan-Meier method, and the log-rank test was used for between-group comparisons. A Cox proportional-hazards model was used to estimate adjusted hazard ratios with 95% confidence intervals for the primary outcomes. Univariable analyses were performed to identify factors associated with the primary outcomes. Associated factors with P<0.01 on univariable analyses were included into a multivariable model with age and sex (regardless of P values on univariable analyses). Two-sided P<0.05 was considered statistically significant.
In all, 600 AMI patients who underwent primary PCI and survived to discharge were included in the present analysis. The median (IQR) length of hospital stay, duration of short-term follow-up for NP measurements, and follow-up period for the primary outcome was 9 (6–14), 32 (18–40), and 488 (343–934) days, respectively. During the follow-up period, 69 (11.5%) patients experienced the primary outcome (death or HF rehospitalization after discharge). No patients experienced either of the primary outcome events before the short-term follow-up.
Baseline characteristics are presented in Table 1. Compared with patients who did not experience the primary outcome events, those who did were older and had a lower body mass index, a higher prevalence of comorbidities, and impaired left ventricular ejection fraction (Table 1). The rates of high NP concentrations (i.e., BNP ≥200 pg/mL or NT-proBNP ≥900 pg/mL) on admission (53.6% vs. 22.1%; P<0.001) and at the short-term follow-up (69.6% vs. 31.1%; P<0.001) were significantly higher in patients with that without the primary outcome events. Similarly, in-hospital HF was more frequently observed in patients experiencing all-cause death and HF rehospitalization after discharge (53.6% vs. 20.2%; P<0.001; Table 1). The HFTP score (Figure 2) assessment revealed 291 (48.5%), 153 (25.5%), 110 (18.3%), and 46 (7.7%) patients with a score of 0, 1, 2, and 3, respectively. Baseline characteristics and clinical outcomes were then compared among these 4 groups (Tables 2,3).
| Variable | All (n=600) | Adverse eventA | P value | |
|---|---|---|---|---|
| No (n=531) | Yes (n=69) | |||
| Age (years) | 66.8±11.9 | 66.1±12.0 | 72.2±9.7 | <0.001 |
| Male sex | 464 (77.3) | 411 (77.4) | 53 (76.8) | 0.88 |
| BMI (kg/m2) | 24.4±3.6 | 24.6±3.6 | 23.1±3.7 | 0.002 |
| Hypertension | 412 (68.7) | 354 (66.6) | 58 (84.1) | 0.003 |
| Diabetes | 208 (34.7) | 182 (34.3) | 26 (37.7) | 0.59 |
| Dyslipidemia | 394 (65.7) | 356 (67.0) | 38 (55.1) | 0.06 |
| Current smoker | 209 (34.8) | 191 (36.0) | 18 (26.1) | 0.11 |
| Previous MI | 39 (6.5) | 29 (5.4) | 10 (14.5) | 0.009 |
| Previous PCI | 55 (9.2) | 43 (8.1) | 12 (17.4) | 0.02 |
| Previous HF | 11 (1.8) | 8 (1.5) | 3 (4.3) | 0.12 |
| eGFR (mL/min/1.73 m2) | 65.6±23.6 | 67.4±22.6 | 51.5±26.5 | <0.001 |
| Hemoglobin (g/dL) | 13.9±2.1 | 14.2±2.0 | 12.2±2.3 | <0.001 |
| High NP on admission | 154 (25.7) | 117 (22.1) | 37 (53.6) | <0.001 |
| High NP at follow-up | 213 (35.5) | 165 (31.1) | 48 (69.6) | <0.001 |
| Peak CK (U/L) | 1,569 [524–3,518] | 1,564 [515–3,380] | 1,890 [747–4,908] | 0.16 |
| LVEF (%) | 47.7±12.2 | 48.4±12.0 | 42.5±12.5 | <0.001 |
| Cardiogenic shock | 67 (11.2) | 52 (9.8) | 15 (21.7) | 0.007 |
| Triple vessel disease | 127 (21.2) | 107 (20.2) | 20 (29.0) | 0.12 |
| Type of MI | 0.33 | |||
| STEMI | 421 (70.2) | 376 (70.8) | 45 (65.2) | |
| NSTEMI | 179 (29.8) | 155 (29.2) | 24 (34.8) | |
| Medication at discharge | ||||
| Aspirin | 564 (94.0) | 501 (94.4) | 63 (91.3) | 0.29 |
| P2Y12 inhibitor | 579 (96.5) | 515 (97.0) | 64 (92.8) | 0.07 |
| Statin | 562 (93.7) | 502 (94.5) | 60 (87.0) | 0.03 |
| ACEI or ARB | 600 (100) | 531 (100) | 69 (100) | 1.00 |
| β-blocker | 471 (78.5) | 416 (78.3) | 55 (79.7) | 0.88 |
| MRA | 111 (18.5) | 89 (16.8) | 22 (31.9) | 0.005 |
| Diuretic | 134 (22.3) | 96 (18.1) | 38 (55.1) | <0.001 |
| SGLT2 inhibitor | 20 (3.3) | 18 (3.4) | 2 (2.9) | 1.00 |
| In-hospital HFB | 144 (24.0) | 107 (20.2) | 37 (53.6) | <0.001 |
Unless indicated otherwise, data are given as the mean±SD, median [interquartile range], or n (%). AAdverse event indicates the primary outcome, a composite of all-cause death and heart failure (HF) rehospitalization after discharge. BIn-hospital HF was defined as the use of intravenous vasopressors, inotropes, and diuretics during the index hospitalization for acute myocardial infarction (MI). ACEI, angiotensin-converting enzyme inhibitor; ARB, angiotensin II receptor blocker; BMI, body mass index; CK, creatine kinase; eGFR, estimated glomerular filtration rate; LVEF, left ventricular ejection fraction; MRA, mineralocorticoid receptor antagonist; NP, natriuretic peptide; NSTEMI, non ST-segment elevation myocardial infarction; PCI, percutaneous coronary intervention; SGLT2, sodium-glucose cotransporter 2; STEMI, ST-segment elevation myocardial infarction.
| Variable | HFTP score | P value | |||
|---|---|---|---|---|---|
| 0 (n=291) | 1 (n=153) | 2 (n=110) | 3 (n=46) | ||
| Age (years) | 63.2±11.6 | 68.3±11.6 | 71.1±10.6 | 73.8±11.3 | <0.001 |
| Male sex | 239 (82.1) | 111 (72.5) | 80 (72.7) | 34 (73.9) | 0.051 |
| BMI (kg/m2) | 24.9±3.8 | 24.2±3.5 | 23.8±3.5 | 23.3±3.0 | 0.006 |
| Hypertension | 184 (63.2) | 114 (74.5) | 78 (70.1) | 36 (78.3) | 0.04 |
| Diabetes | 98 (33.7) | 48 (31.4) | 34 (30.9) | 19 (41.3) | 0.43 |
| Dyslipidemia | 200 (68.7) | 107 (69.9) | 63 (57.3) | 24 (52.2) | 0.02 |
| Current smoker | 123 (42.2) | 45 (29.4) | 32 (29.1) | 9 (19.6) | 0.002 |
| Previous MI | 22 (7.6) | 5 (3.3) | 8 (7.3) | 4 (8.7) | 0.25 |
| Previous PCI | 27 (9.3) | 10 (6.5) | 12 (10.9) | 6 (13.0) | 0.43 |
| Previous HF | 1 (0.3) | 2 (1.3) | 6 (5.5) | 2 (4.3) | 0.002 |
| eGFR (mL/min/1.73 m2) | 73.4±20.8 | 63.7±22.5 | 54.4±25.4 | 48.8±19.2 | <0.001 |
| Hemoglobin (g/dL) | 14.4±1.9 | 14.1±2.1 | 13.2±2.1 | 12.1±2.4 | <0.001 |
| High NP on admission | 0 (0) | 39 (25.5) | 70 (63.6) | 46 (100) | <0.001 |
| High NP at follow-up | 0 (0) | 75 (49.0) | 92 (83.6) | 46 (100) | <0.001 |
| Peak CK (U/L) | 1,045 [284–2,156] | 2,557 [1,187–4,430] | 2,497 [778–5,607] | 2,429 [2,021–5,038] | <0.001 |
| LVEF (%) | 53.3±10.0 | 44.8±11.2 | 41.5±11.3 | 36.5±11.9 | <0.001 |
| Cardiogenic shock | 8 (2.7) | 23 (15.0) | 26 (23.6) | 10 (21.7) | <0.001 |
| Triple vessel disease | 52 (17.9) | 26 (17.0) | 30 (27.3) | 19 (41.3) | 0.001 |
| Type of MI | <0.001 | ||||
| STEMI | 173 (59.5) | 126 (82.4) | 86 (78.2) | 36 (78.3) | |
| NSTEMI | 118 (40.5) | 27 (17.6) | 24 (21.8) | 10 (21.7) | |
| Medication at discharge | |||||
| Aspirin | 285 (97.9) | 142 (92.8) | 98 (89.1) | 39 (84.8) | <0.001 |
| P2Y12 inhibitor | 286 (98.3) | 150 (98.0) | 99 (90.0) | 44 (95.7) | 0.001 |
| Statin | 277 (95.2) | 146 (95.4) | 99 (90.0) | 40 (87) | 0.050 |
| ACEI or ARB | 291 (100) | 153 (100) | 110 (100) | 46 (100) | 1.00 |
| β-blocker | 211 (72.5) | 127 (83.0) | 96 (87.3) | 37 (80.4) | 0.004 |
| MRA | 19 (6.5) | 27 (17.6) | 41 (37.3) | 24 (52.2) | 0.005 |
| Diuretic | 12 (4.1) | 34 (22.2) | 55 (50.0) | 33 (71.7) | <0.001 |
| SGLT2 inhibitor | 13 (4.5) | 1 (0.7) | 4 (3.6) | 2 (4.3) | 0.12 |
| In-hospital HFA | 0 (0) | 40 (26.1) | 58 (52.7) | 46 (100) | <0.001 |
Unless indicated otherwise, data are given as the mean±SD, median [interquartile range], or n (%). AIn-hospital HF was defined as the use of intravenous vasopressors, inotropes, and diuretics during the index hospitalization for acute MI. HFTP, heart failure time points. Other abbreviations as in Table 1.
| Variable | All (n=600) | HFTP score | P value | |||
|---|---|---|---|---|---|---|
| 0 (n=291) | 1 (n=153) | 2 (n=110) | 3 (n=46) | |||
| Primary endpoint | 69 (11.5) | 15 (5.2) | 7 (4.6) | 26 (23.6) | 21 (45.7) | <0.001 |
| All-cause death | 40 (6.7) | 13 (4.5) | 3 (2.0) | 13 (11.8) | 11 (23.9) | <0.001 |
| HF rehospitalization | 35 (5.8) | 2 (0.7) | 4 (2.6) | 15 (13.9) | 14 (32.6) | <0.001 |
Unless indicated otherwise, data are given as n (%). HFTP, heart failure time points.
Kaplan-Meier analysis revealed that higher HFTP scores were associated with an increased risk of all-cause death and HF rehospitalization after discharge (Figure 3). In patients with HFTP scores of 1 and 2, there was no significant difference in the primary outcome among the 3 groups with HF at different time points (i.e., HF on admission, during hospitalization, or at the short-term follow-up; Supplementary Figures 1,2). With respect to in-hospital HF, patients treated with both intravenous diuretics and vasopressors/inotropes had the worst clinical outcomes, followed by those treated with either treatment strategy and no in-hospital HF (Supplementary Figure 3). Multivariable analysis showed that HFTP scores of 2 and 3, as well as older age and a lower hemoglobin level, were independently associated with the primary endpoint in a stepwise manner (Table 4).
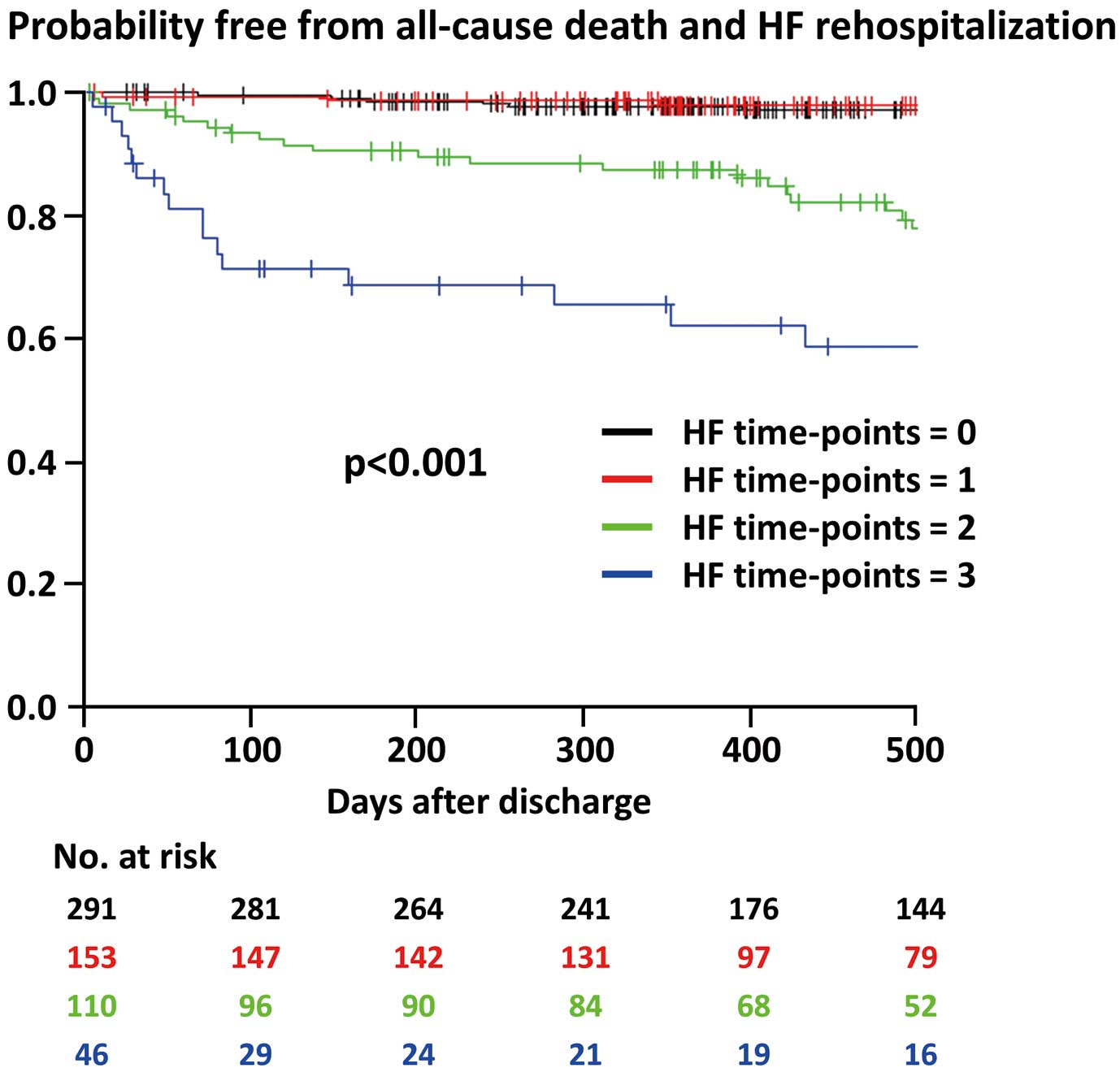
Probability of being free from all-cause death and heart failure (HF) rehospitalization events after discharge according to the HF time points score.
| Variable | Univariable | Multivariable | ||
|---|---|---|---|---|
| HR (95% CI) | P value | HR (95% CI) | P value | |
| Age | 1.06 (1.04–1.09) | <0.001 | 1.03 (1.00–1.06) | 0.048 |
| Male sex | 0.91 (0.52–1.59) | 0.73 | 1.59 (0.88–2.86) | 0.13 |
| BMI | 0.91 (0.85–0.98) | 0.008 | 0.95 (0.88–1.03) | 0.25 |
| Hypertension | 2.51 (1.32–4.80) | 0.005 | 1.73 (0.86–3.50) | 0.13 |
| eGFR | 0.97 (0.96–0.98) | <0.001 | 0.99 (0.98–1.00) | 0.13 |
| Hemoglobin | 0.72 (0.65–0.79) | <0.001 | 0.85 (0.75–0.97) | 0.01 |
| LVEF | 0.96 (0.95–0.98) | <0.001 | 0.99 (0.97–1.01) | 0.43 |
| Cardiogenic shock | 2.20 (1.24–3.90) | 0.007 | 1.34 (0.68–2.61) | 0.39 |
| HFTP score | ||||
| 0 | Reference | Reference | ||
| 1 | 0.90 (0.37–2.2) | 0.82 | 0.64 (0.25–1.64) | 0.35 |
| 2 | 4.96 (2.62–9.38) | <0.001 | 2.61 (1.24–5.47) | 0.01 |
| 3 | 11.71 (6.00–22.85) | <0.001 | 4.17 (1.72–10.11) | 0.002 |
CI, confidence interval; HFTP, heart failure time points; HR, hazard ratio. Other abbreviations as in Table 1.
In the present bicenter registry, HF events were frequently observed on admission, during hospitalization, and at the short-term follow-up (median 32 days after the index myocardial infarction [MI]) in patients with AMI who underwent PCI and survived to discharge (25.7%, 24.0%, and 35.5%, respectively); all of these events were associated with an increased risk of subsequent clinical events, including all-cause death and HF rehospitalization. When the presence or absence of HF events at the 3 time points was compounded, patients with HFTP scores of 2 and 3 had a higher risk of death and HF rehospitalization after discharge, whereas those with a score of 1 did not, regardless of the timing of HF (i.e., on admission, during hospitalization, or at the short-term follow-up).
NP Concentrations in AMIA large future increase in the prevalence of cardiovascular diseases such as MI and HF is currently projected across the world, including Japan and the US.30,31 By 2060, in the US, it is estimated that the prevalence of MI and HF will increase by 16.9% and 33.4%, respectively, compared with 2025 levels (i.e., 4.9% of the population [16.0 million people] and 4.0% of the population [or 12.9 million people], respectively).31 Because AMI is a major etiology of HF,1,2 identifying AMI patients who are at a high risk of developing future HF is clinically relevant in the current “heart failure pandemic” era.30,32 In this context, recent guidelines recommended (Class IIa) measuring plasma BNP or NT-proBNP concentrations to gain prognostic information in patients with non-STEMI;8 however, the appropriate timing for evaluation of NP concentrations in AMI patients remains unclear. A single-center, observational study (n=1,034) showed that NT-proBNP between ≥150 and 600 pg/mL at initial presentation of STEMI patients was associated with higher mortality during the median follow-up period of 901 days in a stepwise manner.11 Further, previous studies have indicated the prognostic impact of NP concentrations at the short-term follow-up after AMI, such as those measured at a median of 3 days,33 a median of 6 days,13 3–4 weeks,7 and a median of 56 days.12 In a previous study, the plasma BNP concentration measured 3–4 weeks after the onset of AMI was an independent predictor of cardiac death, with the best-cut off value of 180 pg/mL, which is in line with the results of the present study (i.e., BNP ≥200 pg/mL or NT-proBNP ≥900 pg/mL).7 Among patients with the HFTP score of 2, the lack of HF at the short-term follow-up was apparently associated with better outcomes, although the difference compared with patients with HF at the short-term follow-up was not significant.
In addition to individual NP assessment on admission and at the short-term follow-up, a combination NP evaluation has been investigated to stratify future cardiovascular risks after AMI. A single-center study by Lee et al (n=442) reported that when patients were divided into 4 groups according to initial and follow-up median BNP concentrations, patient with high BNP levels at both the initial and follow-up measurements (i.e., high-high group) had worst clinical outcomes after AMI, followed by the low-high and low-low groups, suggesting that prognosis was good for patients with elevated BNP concentrations at the initial admission but subsequent reductions in BNP.12 The multicenter TRIUMPH registry (n=803) reported similar results,34 although the “low–high” group was excluded in that study because of the small sample size. Thus, whether elevated NP concentrations at admission and the short-term follow-up are independently prognostic after AMI remains uncertain.
In the present study that elevated BNP or NT-proBNP concentrations at admission and at a median of 32 days were both associated with an increased risk of clinical events, but patients with a high NP concentration at either time point were not at increased risk if no in-hospital HF events occurred (Supplementary Figure 1). Therefore, serial assessment of NP may be useful to determine future cardiovascular risks after AMI, especially when levels are persistently elevated. Given the fact that, in the present study, hazard ratios were increased in a stepwise manner in the multivariable model (Table 4), the presence of HF at 2 and 3 time points was predictive for worse clinical outcomes after AMI.
In-Hospital HF After AMIAlthough the incidence has declined over time owing to advances in early reperfusion therapy and medical treatment, in-hospital HF developing after AMI is still a major complication, affecting up to 30% of patients;14,35 this is in line with our results (i.e., 24.0%). The presence of HF during hospitalization for AMI was intuitively associated with short- and long-term morality.14,35 In addition, it was reported that HF developing >3 days after AMI was more predictive for cardiovascular events than early-onset HF (≤3 days).3 Nevertheless, the prognostic value of in-hospital HF in combination with initial and follow-up NP concentrations has been poorly investigated. In the present study, in-hospital HF itself was not predictive for subsequent mortality and HF readmission risks unless accompanied by elevated NP concentrations either on admission or at short-term follow-up or both (i.e., HFTP score of 1). Thus, we believe that combined evaluation of HF after AMI using the novel scoring system (HFTP) may be useful in daily practice. Interestingly and of note, the intensity of HF treatment, represented by the use of intravenous diuretics and/or vasopressors/inotropes, was significantly associated clinical outcomes after AMI (Supplementary Figure 3). Therefore, further risk stratification would be possible with more factors associated with clinical outcomes, especially using artificial intelligence technology.36
Study LimitationsThe present study has some limitations. The present study was a retrospective study with a moderate sample size, and the number of patients excluded was relatively large. Because of the retrospective and exploratory nature of the present study, no sample size calculation was performed. Future studies are needed to externally confirm the diagnostic ability of the HFTP scoring system. Despite recent advances in HF treatment, including sodium–glucose cotransporter 2 inhibitors, the number of patients receiving such treatment was limited. In the present study, we used measures of both BNP and NT-proBNP because of institutional availability. Thus, NP levels were used as being dichotomous not as being continuous (i.e., BNP ≥200 pg/mL or NT-proBNP ≥900 pg/mL). NP was routinely measured on admission and at the short-term follow-up, whereas NP measurements during hospitalization for AMI varied widely among individual cases in our institutions, preventing HF evaluation by NP concentrations during hospitalization. The timing of the short-term follow-up NP measurement was not uniform in the present study, although none of the participants experienced the primary outcome events before NP measurement at the short-term follow-up. Further investigations are warranted to improve clinical outcomes in patients with AMI complicated by HF.37–39
Among patients with AMI who underwent PCI and survived to discharge, the evaluation of HF on admission, during hospitalization, and at the short-term follow-up was useful in stratifying risks of mortality and HF rehospitalization after discharge when HF was present at 2 or 3 time points.
This work was supported by grants from Chiba Foundation for Health Promotion & Disease Prevention.
Y.K. is a member of Circulation Journal’s Editorial Team. The remaining authors have no conflicts of interest to disclose.
This study was approved by the Ethics Committee at Chiba University Graduate School of Medicine (Approval No. 3,933) and Eastern Chiba Medical Center (Approval No. 131).
The deidentified participant data will not be shared.
Please find supplementary file(s);
https://doi.org/10.1253/circj.CJ-22-0577