Abstract
Background: It remains controversial whether a cancer history increases the risk of cardiovascular (CV) events among patients with myocardial infarction (MI) who undergo revascularization.
Methods and Results: Patients who were confirmed as type 1 acute MI (AMI) by coronary angiography were retrospectively analyzed. Patients who died in hospital or those not undergoing revascularization were excluded. Patients with a cancer history were compared with those without it. A cancer history was examined in the in-hospital cancer registry. The primary outcome was a composite of cardiac death, recurrent type 1 MI, post-discharge coronary revascularization, heart failure hospitalization, and stroke. Among 551 AMI patients, 55 had a cancer history (cancer group) and 496 did not (non-cancer group). Cox proportional hazards model revealed that the risk of composite endpoint was significantly higher in the cancer group than in the non-cancer group (adjusted hazard ratio [HR]: 1.78; 95% confidence interval [CI]: 1.13–2.82). Among the cancer group, patients who were diagnosed as AMI within 6 months after the cancer diagnosis had a higher risk of the composite endpoint than those who were diagnosed as AMI 6 months or later after the cancer diagnosis (adjusted HR: 5.43; 95% CI: 1.55–19.07).
Conclusions: A cancer history increased the risk of CV events after discharge among AMI patients after revascularization.
Cancer and coronary artery disease (CAD) complicate at a high rate because they share pathophysiological mechanisms and common risk factors such as age, sex, life-style habits including smoking, and chronic inflammation.1–3 In addition, cancer therapies such as chemotherapy and radiation are associated with a higher risk of CAD.4 Previous studies have shown that patients with cancer carry a higher risk of cardiovascular (CV) events, but it remains controversial whether a history of cancer is associated with a higher risk of CV events among patients with acute myocardial infarction (AMI) who undergo revascularization including percutaneous coronary intervention (PCI) and coronary artery bypass grafting (CABG). It has been reported that a history of cancer is associated with a higher risk of cardiac death,5,6 ischemic events,7,8 heart failure hospitalization,6 and bleeding,6,7,9–11 but other studies concluded that the risk of CV events was similar between patients with and without a history of cancer.12–16 In Asia, especially, because of the lack of registries in cardio-oncology and difficulties in integrating MI data with cancer registry, none of the previous studies have evaluated the effect of a history of cancer CV events among AMI patients with detailed information on both MI and cancer.6,14 Addressing this knowledge gap is important in the field of cardio-oncology because the number of patients with a history of cancer who undergo revascularization for AMI is increasing as a result of the advancements in cancer therapy in the past quarter of the century.14,17
In this study, we investigated the effect of a cancer history on CV events among patients with AMI who underwent revascularization.
Methods
We retrospectively analyzed consecutive patients who were confirmed as type I AMI by coronary angiography between January 2009 and December 2020 in the Cardiovascular Division, National Hospital Organization Osaka National Hospital.18 We excluded patients who died in hospital or those who did not undergo revascularization. Patients with a cancer history (cancer group) were compared with those without a cancer history (non-cancer group). We defined MI according to the 4th universal definition of MI.19 The present study was approved by the Osaka National Hospital Institutional Review Board #2 (approval no. 21045). Informed consent from patients included in the study was waived because it was a retrospective cohort study.
We retrospectively collected the following data on patients: age, sex, body mass index (BMI), ST-segment elevation MI (STEMI: yes/no), history of smoking, medical history including diabetes mellitus, hypertension, hypercholesterolemia, stroke and MI, history of PCI, history of CABG, angiographic data including culprit lesion (right coronary artery [RCA]/left main trunk [LMT]/left anterior descending coronary artery [LAD]/left circumflex coronary artery [LCX]), number of diseased vessels (1/2/3), chronic total occlusion (CTO: yes/no), laboratory data including peak creatine kinase (peak CK) and peak creatine kinase myocardial band (peak CK-MB), prescription at discharge from hospital for the index AMI, including β-blocker (yes/no), angiotensin-converting enzyme (ACE) inhibitor or angiotensin II receptor blocker (ARB: yes/no), calcium-channel blocker (yes/no), statin (yes/no), aspirin (yes/no), P2Y12 inhibitor (yes/no), warfarin (yes/no), and direct oral anticoagulant (DOAC: yes/no).
A history of cancer was examined in the in-hospital cancer registry of the National Hospital Organization Osaka National Hospital between 2000 and 2020. Whether inpatient or outpatient, patients first diagnosed at Osaka National Hospital were registered in the in-hospital cancer registry. In addition, cases in which the tumor had already been diagnosed or treated at other centers and the patient subsequently visited Osaka National Hospital for diagnosis or treatment were also registered in the in-hospital cancer registry. We reviewed the medical records of all the patients included in the present study, and confirmed there was no record of cancer-related disease before the index AMI among patients in the non-cancer group. At least 1 diagnosis of cancer before the index AMI was defined as having a history of cancer. When patients had multiple cancer diagnoses before the index AMI, the data on the earliest cancer diagnosis was used for the analyses. Data on types of cancer, the clinical stage at cancer diagnosis, presence of metastasis (yes/no), treatments for cancer (surgery, chemotherapy, radiation, endoscopy, endocrine therapy: yes/no), and the date of cancer diagnosis were collected from the in-hospital cancer registry. Advanced cancer was defined as cancer with clinical stage ≥III at the time of diagnosis.20
Follow-up started at the date of discharge from the hospital for the index AMI. Patients were followed from hospital discharge, and follow-up was censored at the date when the patient was lost to follow-up, the date of death, or at 5 years from hospital discharge, whichever occurred first. The primary outcome was a composite of cardiac death, recurrent type 1 MI, post-discharge coronary revascularization, heart failure hospitalization, and stroke. The secondary outcome was all-cause death and each CV event (cardiac death, recurrent type 1 MI, post-discharge coronary revascularization, heart failure hospitalization, and stroke). Cardiac death was defined according to the previous consensus report on CV and stroke endpoints.21 Cardiac death was recorded when the primary cause of death was CV disease, and it was distinguished from non-cardiac death.21 Post-discharge coronary revascularization was defined as any CABG or PCI including target lesion revascularization (TLR), target vessel revascularization (TVR), and non-target vessel revascularization (non-TVR). For TVR and non-TVR, staged PCIs for residual lesions scheduled at the time of the index AMI were excluded.
Categorical variables are summarized as count and proportion, and compared between the cancer and the non-cancer groups using the chi-squared test. Age and BMI as continuous variables are summarized as mean and standard deviation (SD). Peak CK and peak CK-MB as continuous variables are summarized as median and interquartile range (IQR). These 4 continuous variables were compared between the cancer and non-cancer groups using Mann-Whitney U test. The Kaplan-Meier method was applied to calculate the cumulative probability of events, and the difference between groups was compared using the log-rank test. Univariate and multivariable Cox proportional hazards models were applied to elucidate the effect of a history of cancer on CV events among the study population, and hazard ratios (HR) and 95% confidence intervals (CI) were calculated. The multivariable Cox proportional hazards model was adjusted for factors associated with CV events in this population, including age, history of smoking, history of diabetes mellitus, culprit lesion: LAD (yes/no), number of diseased vessels (1/2/3), CTO (yes/no), prescription of statin at hospital discharge (yes/no), and prescription of P2Y12 antagonist at hospital discharge (yes/no). We conducted additional analyses for the cancer group to determine factors associated with their risk of CV events. Data on cancer are summarized as count and proportion. Univariate and the multivariable Cox proportional hazards models were applied to evaluate the effect of each factor, including advanced cancer (yes/no), presence of metastasis (yes/no), type of cancer (gastric cancer [yes/no], prostate cancer [yes/no], colon cancer [yes/no]), each treatment for cancer, and time from cancer diagnosis to index AMI (<6 months/≥6 months) on the primary outcome. The multivariable Cox proportional hazard model was adjusted for age, sex, and history of diabetes mellitus.
All analyses were performed using R software (version 4.1.1) and Stata/MP 16.0. P values were two-sided, and we considered P<0.05 as statistically significant.
Results
Among the 641 patients who were confirmed as type I AMI by coronary angiography between January 2009 and December 2020 in the Cardiovascular Division, National Hospital Organization Osaka National Hospital, those who died in hospital (74 patients) and those who did not undergo coronary revascularization (16 patients) were excluded, so a total of 551 consecutive patients were eligible for our analysis. The number of patients treated with PCI and CABG was 542 and 9, respectively. Table 1 describes the baseline characteristics of the cancer and non-cancer groups. The cancer group was older and more had a history of PCI, whereas the proportion of patients with STEMI was higher in the non-cancer group.
Table 1. Baseline Characteristics of the Study Population
| |
Cancer group
(n=55) |
Non-cancer group
(n=496) |
P value |
Missing,
n (%) |
| Age (years) |
76.4±7.7 |
66.1±13.3 |
<0.001 |
0 (0.0) |
| Male |
42 (76.4) |
407 (82.1) |
0.30 |
0 (0.0) |
| BMI |
23.5±3.1 |
24.2±3.7 |
0.32 |
1 (0.2) |
| STEMI |
30 (54.5) |
369 (74.4) |
<0.01 |
0 (0.0) |
| Coronary revascularization |
| PCI |
55 (100) |
487 (98.2) |
NA |
0 (0.0) |
| CABG |
0 (0) |
9 (1.8) |
|
|
| History of smoking |
39 (70.9) |
336 (67.7) |
0.63 |
0 (0.0) |
| Medical history |
| Diabetes mellitus |
25 (45.5) |
163 (32.9) |
0.06 |
0 (0.0) |
| Hypertension |
39 (70.9) |
322 (64.9) |
0.38 |
0 (0.0) |
| Hypercholesterolemia |
30 (54.5) |
256 (51.6) |
0.68 |
0 (0.0) |
| Stroke |
4 (7.3) |
26 (5.2) |
0.53 |
0 (0.0) |
| MI |
9 (16.4) |
40 (8.1) |
0.04 |
0 (0.0) |
| History of PCI |
12 (21.8) |
44 (8.9) |
<0.01 |
0 (0.0) |
| History of CABG |
0 (0.0) |
11 (2.2) |
0.26 |
0 (0.0) |
| Culprit lesion |
| RCA |
19 (34.5) |
162 (32.7) |
0.78 |
0 (0.0) |
| LMT |
2 (3.6) |
23 (4.6) |
0.74 |
0 (0.0) |
| LAD |
19 (34.5) |
246 (49.6) |
0.03 |
0 (0.0) |
| LCX |
17 (30.9) |
70 (14.1) |
<0.01 |
0 (0.0) |
| No. of diseased vessels |
|
|
0.43 |
0 (0.0) |
| 1 |
22 (40.0) |
244 (49.2) |
|
|
| 2 |
20 (36.4) |
152 (30.6) |
|
|
| 3 |
13 (23.6) |
100 (20.2) |
|
|
| CTO |
3 (5.5) |
51 (10.3) |
0.25 |
0 (0.0) |
| Laboratory data |
| Peak CK (U/L) |
758.0 (272.5–1,563.0) |
1,035.0 (357.5–2,602.0) |
0.12 |
2 (0.4) |
| Peak CK-MB (U/L) |
67.0 (17.5–159.5) |
82.0 (22.0–278.0) |
0.37 |
3 (0.5) |
| Prescription at hospital discharge |
| β-blocker |
34 (61.8) |
323 (65.1) |
0.63 |
0 (0.0) |
| ACE inhibitor or ARB |
35 (63.6) |
359 (72.4) |
0.17 |
0 (0.0) |
| Calcium-channel blocker |
16 (29.1) |
91 (18.3) |
0.06 |
0 (0.0) |
| Statin |
38 (69.1) |
387 (78.0) |
0.13 |
0 (0.0) |
| Aspirin |
53 (96.4) |
480 (96.8) |
0.87 |
0 (0.0) |
| P2Y12 inhibitor |
54 (98.2) |
473 (95.4) |
0.33 |
0 (0.0) |
| Warfarin |
4 (7.3) |
34 (6.9) |
0.91 |
0 (0.0) |
| DOAC |
3 (5.5) |
20 (4.0) |
0.62 |
0 (0.0) |
ACE, angiotensin-converting enzyme; ARB, angiotensin II receptor blocker; BMI, body mass index; CABG, coronary artery bypass grafting; CTO, chronic total occlusion; DOAC, direct oral anticoagulant; LAD, left anterior descending coronary artery; LCX, left circumflex coronary artery; LMT, left main trunk; PCI, percutaneous coronary intervention; peak CK, peak creatine kinase; peak CK-MB, peak creatine kinase myocardial band; RCA, right coronary artery; STEMI, ST-segment elevation myocardial infarction.
The 5-year follow-up was achieved in 306 (55.5%) patients, and the mean follow-up period was 2.08 years. Figure 1 shows the Kaplan-Meier curves for the primary outcome. Log-rank test showed that the cumulative probability of the primary outcome was significantly higher in the cancer group than in the non-cancer group. Figure 2 shows the Kaplan-Meier curves for the secondary outcomes. Log-rank test showed that the cumulative probability of the secondary outcomes, including all-cause death, cardiac death, recurrent type 1 MI, and heart failure hospitalization, was significantly higher in the cancer group than in the non-cancer group. In contrast, no significant difference was observed in the cumulative probability of other secondary outcomes including post-discharge coronary revascularization and stroke. Table 2 presents the HR and 95% CI for the primary and secondary outcomes by univariate and the multivariable Cox proportional hazards models. The univariate Cox proportional hazards model revealed that the risk of the primary and secondary outcomes, including all-cause death, cardiac death, recurrent type 1 MI and heart failure hospitalization, was significantly higher in the cancer group than in the non-cancer group. The multivariable Cox proportional hazards model showed the same results as the univariate model, except for recurrent type 1 MI and heart failure hospitalization.
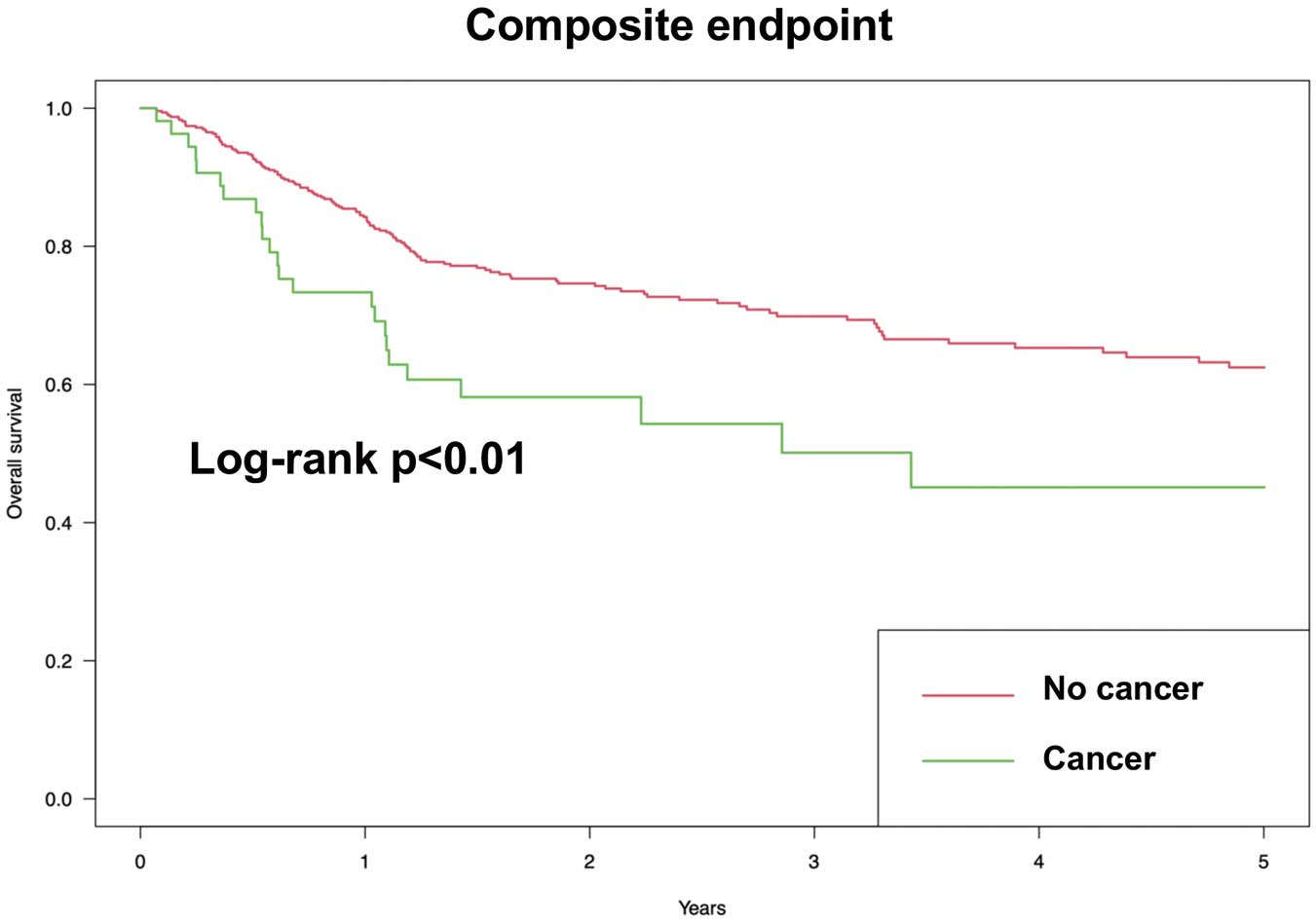
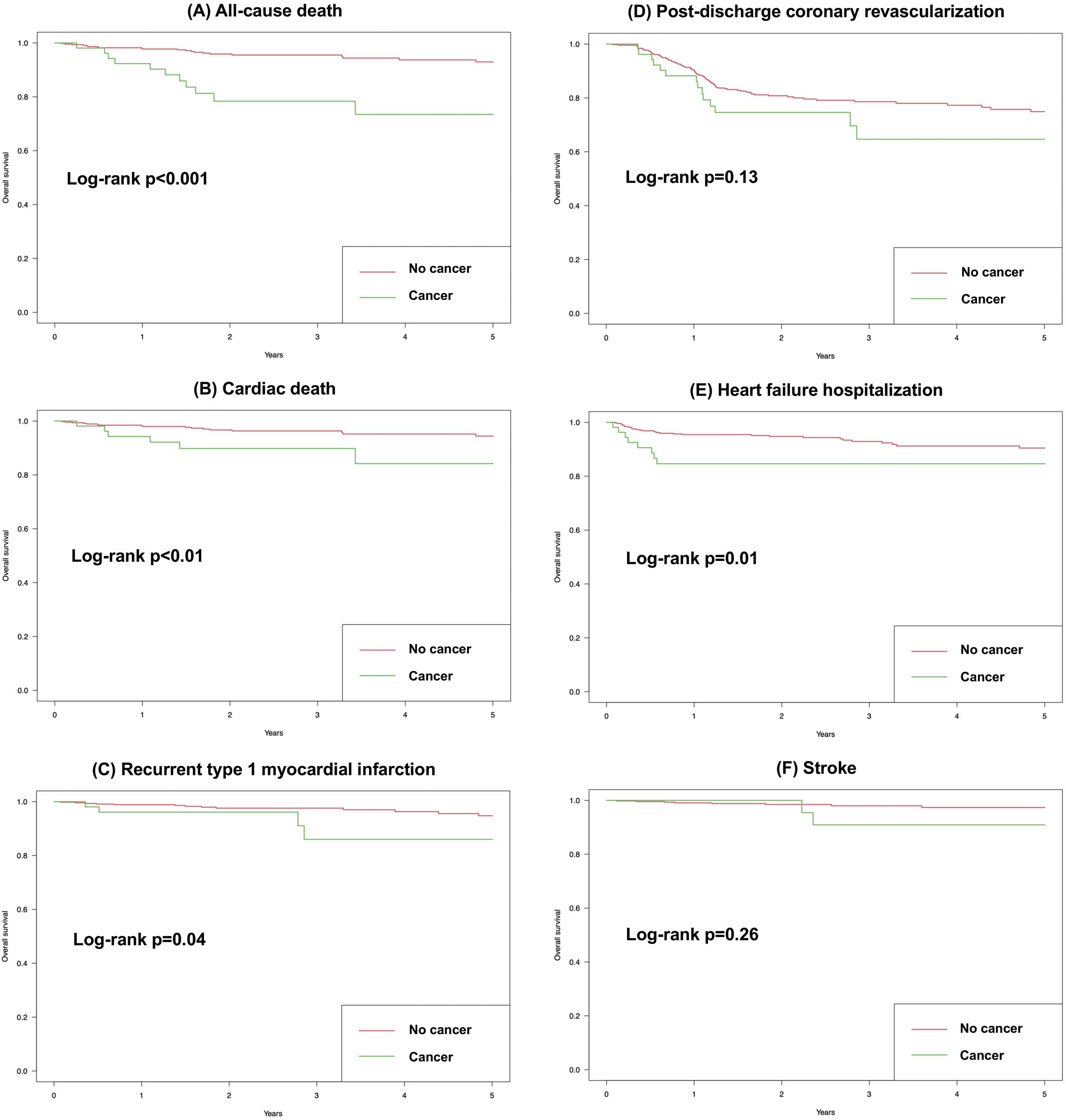
Table 2. HR Using Cox Proportional Hazards Models
| Outcome |
No. of
events |
Univariate model |
Multivariable modelb |
| HR |
95%CI |
HR |
95%CI |
| Primary outcomea |
| Composite |
152 |
1.91 |
1.24–2.96 |
1.78 |
1.13–2.82 |
| Secondary outcome |
| All-cause death |
32 |
4.81 |
2.32–9.98 |
4.96 |
2.14–11.49 |
| Cardiac death |
23 |
3.26 |
1.28–8.27 |
4.00 |
1.39–11.53 |
| Recurrent type 1 MI |
17 |
3.08 |
1.00–9.50 |
2.91 |
0.86–9.77 |
| Post-discharge coronary revascularization |
100 |
1.55 |
0.88–2.72 |
1.56 |
0.85–2.83 |
| HF hospitalization |
38 |
2.58 |
1.18–5.64 |
1.87 |
0.83–4.19 |
| Stroke |
10 |
2.38 |
0.50–11.22 |
2.12 |
0.40–11.17 |
aPrimary outcome was a composite of cardiac death, recurrent type 1 MI, post-discharge coronary revascularization, HF hospitalization, and stroke. bMultivariable Cox proportional hazards model was adjusted for age, history of smoking, history of diabetes mellitus, culprit lesion: LAD (yes/no), no. of diseased vessels (1/2/3), CTO (yes/no), prescription of statin at the hospital discharge (yes/no), prescription of P2Y12 antagonists at the hospital discharge (yes/no). CI, confidence interval; CTO, chronic total occlusion; HF, heart failure; HR, hazard ratio; LAD, left anterior descending coronary artery; MI, myocardial infarction.
Table 3 summarizes the detailed characteristics of the cancer group. The top three prevalent types of cancer were gastric cancer (11 patients), prostate cancer (8 patients), and colon cancer (7 patients). The distribution of cancer types in the cancer group is given in Supplementary Table 1. Advanced cancer was observed in 8 (15.3%) patients. The number of patients who were diagnosed as AMI 6 months or later after the cancer diagnosis was 49 (89.1%). The baseline characteristics were not statistically different according to the period from cancer diagnosis to MI (Supplementary Table 2). For patients who were treated with chemotherapy, detailed treatment information is summarized in Supplementary Table 3. Table 4 presents the results of the univariate and multivariable Cox proportional hazards models for the primary outcome in the cancer group. The risk of the primary outcome was significantly higher in the patients who were diagnosed as AMI within 6 months after the cancer diagnosis than those who were diagnosed at 6 months or later after the cancer diagnosis.
Table 3. Baseline Characteristics of Patients in the Cancer Group
| |
n (%) |
Missing, n (%) |
| Clinical stage at cancer diagnosis |
|
3 (5.5) |
| 0 |
2 (3.8) |
|
| I |
28 (53.8) |
|
| II |
14 (26.9) |
|
| III |
6 (11.5) |
|
| IV |
2 (3.8) |
|
| Metastasis |
|
1 (1.8) |
| Localized |
46 (85.2) |
|
| Metastasis |
8 (14.8) |
|
| Treatments for cancer |
| Surgery |
22 (40.0) |
0 (0.0) |
| Chemotherapy |
8 (14.5) |
0 (0.0) |
| Radiation |
7 (12.7) |
0 (0.0) |
| Endoscopy |
14 (25.9) |
1 (1.8) |
| Endocrine therapy |
4 (7.3) |
0 (0.0) |
| Period from cancer diagnosis to myocardial infarction |
|
0 (0.0) |
| <6 months |
6 (10.9) |
|
| 6–12 months |
2 (3.6) |
|
| 1–2 years |
9 (16.4) |
|
| >2 years |
38 (69.1) |
|
Table 4. HR Using Cox Proportional Hazards Models in the Cancer Group
| |
Univariate model |
Multivariable modela |
| HR |
95% CI |
HR |
95% CI |
| Advanced cancer vs. non-advanced cancer |
1.07 |
0.32–3.61 |
|
|
| Metastasis + vs. − |
1.11 |
0.33–3.75 |
|
|
| Type of cancer |
| Gastric cancer + vs. − |
1.32 |
0.52–3.34 |
|
|
| Prostate cancer + vs. − |
0.45 |
0.11–1.91 |
|
|
| Colon cancer + vs. − |
1.87 |
0.69–5.02 |
|
|
| Treatments for cancer |
| Surgery + vs. − |
0.91 |
0.40–2.05 |
|
|
| Chemotherapy + vs. − |
0.97 |
0.29–3.27 |
|
|
| Radiation + vs. − |
1.65 |
0.39–7.02 |
|
|
| Endoscopy + vs. − |
0.68 |
0.29–1.60 |
|
|
| Endocrine therapy + vs. − |
1.92 |
0.26–14.26 |
|
|
| Period from cancer diagnosis to myocardial infarction |
| ≥6 months |
Ref |
|
|
|
| <6 months |
3.09 |
1.05–9.13 |
5.43 |
1.55–19.07 |
aMultivariable Cox proportional hazards model was adjusted for age, sex and history of diabetes mellitus. CI, confidence interval; HR, hazard ratio; Ref, reference.
Discussion
The present study targeting patients with AMI who underwent revascularization showed that (1) a history of cancer was associated with a higher risk of CV events after the hospital discharge, and (2) among patients with AMI and a history of cancer, a higher risk of CV was observed for patients who were diagnosed as AMI within 6 months after the cancer diagnosis compared with those who were diagnosed ≥6 months after the cancer diagnosis. In the present study, ≈10% of patients had a history of cancer, which was similar to previous studies. We had detailed information on both cancer and AMI, which enabled us to evaluate the factors associated with the various types of CV events among patients with AMI and a history of cancer. The mean follow-up period in the present study was around 2 years; however, the number of patients in whom follow-up did not reach 5 years was 245 (44.5%) and the remaining 306 (55.5%) patients achieved 5 years follow-up. Follow-up was longer than in some of the previous studies,5,9,11,16 which enabled us to evaluate the effect of a history of cancer on long-term CV events.
Several previous studies evaluated the effect of a history of cancer on CV events among patients with AMI who underwent revascularization, but the results are inconsistent. Some concluded that a history of cancer was associated with a higher risk of CV events,5–11 while others showed a similar risk between patients with and without a history of cancer.12–16 This inconsistency among results may be explained in part by the definition of a history of cancer and the limited types of CV events. In some of the previous studies, a history of cancer was defined by questionnaire14 or self-reporting by the patients,15 which may have resulted in failure to identify some patients with a history of cancer and thus affecting the results of the study. In the present study, a history of cancer was confirmed in the in-hospital cancer registry, enabling us to accurately identify patients with a history of cancer. In addition, some of the previous studies did not include some of the major types CV events, other than cardiac death, including recurrent MI, stent thrombosis, and heart failure hospitalization due to lack of detailed information on MI, which may have resulted in failure to find a significant association of a history of cancer with CV events. We evaluated various types of CV events including cardiac death, recurrent type 1 MI, coronary revascularization after discharge, heart failure hospitalization, and stroke.
In this study, patients who were diagnosed as AMI within 6 months after cancer diagnosis carried a higher risk of CV events than those who were diagnosed ≥6 months after the cancer diagnosis, which was similar to previous results.5,9 This may be explained in part by the hyper-coagulation state caused by active cancer. Previous studies have shown tumor cells can trigger the coagulation cascade, which may result in an increase in CAD.9,22–24 We speculate that such a hyper-coagulable state strongly influences the occurrence of MI within 6 months after the cancer diagnosis, although we could not provide supportive data because detailed hematological information was not available in the present study.
We did not find a significant association between the risk of CV events and each cancer therapy among patients with MI and a history of cancer, which was consistent with the previous studies that showed a lack of association between a cancer history and an increase in CV events among MI patients.12–16 However, other studies have shown that some cancer therapies increased CV events among these patients.3,4 For example, radiotherapy has been shown to be associated with thrombosis, endothelial injury and plaque rupture, which may have resulted in an increase in CAD among patients with MI who underwent radiotherapy for cancer.25 This inconsistency in study results may be explained in part by the limited information on both cancer and CV events in the previous studies. We had an advantage of obtaining detailed information on both cancer and MI and were able to examine in detail the association between each cancer-related factor, such as treatments and types of cancer, and CV events. Further studies are needed to evaluate this association, although it is difficult to merge data on MI with cancer registry data in Japan especially,6,13,14 related to strict legal restrictions on clinical investigations.
We included both STEMI and non-STEMI (NSTEMI) patients because we wanted to evaluate the effect of a cancer history on CV events among type 1 AMI patients who underwent revascularization. However, in the clinical setting, it is sometimes difficult to distinguish type 1 and type 2 MI among patients with NSTEMI. Patients with a history of cancer have a high risk of anemia,26,27 and previous studies have also reported that several chemotherapies, including 5-fluorouracil, were associated with coronary artery spasm.28,29 The possibility of misclassification is one of the limitations of the present study, although the main results were the same when the analyses were limited to patients with STEMI treated with PCI.
As we described in the Methods section, cases of patients diagnosed or treated at other centers and where neither diagnosis nor treatment of the tumor was performed at Osaka National Hospital were not registered in the in-hospital cancer registry, which may have affected the classification of the patient having a history of cancer, and is one of the limitations of the in-hospital cancer registry. However, because we reviewed the in-hospital medical records of all the patients included in the present study and confirmed that there was no record of cancer-related diseases before the index MI among patients in the non-cancer group, we believe biases in cancer type or stage of cancer were relatively small.
Specific chemotherapies such as immune checkpoint inhibitors, anti-HER2 antibodies, VEGF inhibitors, and MEK inhibitors have been suggested to cause myocardial damage.30–34 Previous studies also report that immune checkpoint inhibitors are associated with progression of atherosclerosis.30,33–34 Although the mechanism is not fully understood, it is thought that immune checkpoint inhibitors target proteins that negatively regulate atherosclerosis, thereby accelerating atherosclerosis among patients with a history of cancer.33 In the present study, 1 patient was treated with molecular-targeted therapy (Supplementary Table 3), but the limited number of patients in the cancer group did not allow us to examine the effect of each type of chemotherapy on CV events. Further studies are needed to elucidate the effect of these chemotherapies on CV events among patients with AMI who undergo revascularization.
Previous studies have shown that patients with a history of cancer have a higher risk of bleeding that patients without such a history,6,7,9,11 suggesting that the adequacy and compliance with antiplatelet therapy after discharge should be investigated in these patients. We were not able to collect accurate information on the duration of post-discharge antiplatelet therapy because not all medications were necessarily prescribed by Osaka National Hospital after hospital discharge and information on prescriptions from other hospitals or clinics was not available. As far as prescriptions at hospital discharge were concerned, there was no significant difference between the cancer and non-cancer groups in the percentage of patients who were prescribed antiplatelet medication (Table 1).
Study Limitations
We did not have information on cancer after its diagnosis, so changes in disease status such as clinical stage and metastasis were not reflected in the present study, which may have affected the study results. The duration from the end of each type of cancer treatment and the diagnosis of MI was unknown because that information was not available. Baseline cardiac function before the onset of MI might be associated with the incidence of heart failure hospitalization. However, information on left ventricular ejection fraction before the onset of MI was not available. This was a single-center clinical study, so generalization of the results should be made with caution. Unmeasured confounding factors may have affected the results of the study, although we assume a small effect because the e-value of the primary outcome was 2.28.35
Conclusions
A history of cancer was associated with an increased risk of CV events among patients with AMI who received revascularization.
IRB Information
The present study was approved by the Osaka National Hospital Institutional Review Board #2 (Approval No. 21045).
Disclosures
Y.U.: Research grants from Abbott, Pfizer, Daiichi-Sankyo, Astellas, Sanofi, Nihon Kohden, Amgen, AstraZeneca, Boehringer Ingelheim, and Novartis. Lecture fees from Bayer, Daiichi-Sankyo, MSD, Nipro-Goodman, Mochida, Takeda, Teijin, Astellas, Bristol-Myers Squibb, Eisai, AstraZeneca, Japan Lifeline, Novartis, Ono, Boehringer Ingelheim, and Amgen. H.A.: Research grant from Boehringer Ingelheim. Others: None.
Supplementary Files
Please find supplementary file(s);
https://doi.org/10.1253/circj.CJ-22-0838
References
- 1.
Koene RJ, Prizment AE, Blaes A, Konety SH. Shared risk factors in cardiovascular disease and cancer. Circulation 2016; 133: 1104–1114.
- 2.
Johnson CB, Davis MK, Law A, Sulpher J. Shared risk factors for cardiovascular disease and cancer: Implications for preventive health and clinical care in oncology patients. Can J Cardiol 2016; 32: 900–907.
- 3.
Zamorano JL, Lancellotti P, Rodriguez Muñoz D, Aboyans V, Asteggiano R, Galderisi M, et al. 2016 ESC Position Paper on cancer treatments and cardiovascular toxicity developed under the auspices of the ESC Committee for Practice Guidelines: The Task Force for cancer treatments and cardiovascular toxicity of the European Society of Cardiology (ESC). Eur Heart J 2016; 37: 2768–2801.
- 4.
Lenneman CG, Sawyer DB. Cardio-oncology: An update on cardiotoxicity of cancer-related treatment. Circ Res 2016; 118: 1008–1020.
- 5.
Velders MA, Boden H, Hofma SH, Osanto S, van der Hoeven BL, Heestermans AA, et al. Outcome after ST elevation myocardial infarction in patients with cancer treated with primary percutaneous coronary intervention. Am J Cardiol 2013; 112: 1867–1872.
- 6.
Nakatsuma K, Shiomi H, Morimoto T, Watanabe H, Nakagawa Y, Furukawa Y, et al. Influence of a history of cancer on long-term cardiovascular outcomes after coronary stent implantation (an Observation from Coronary Revascularization Demonstrating Outcome Study-Kyoto Registry Cohort-2). Eur Heart J Qual Care Clin Outcomes 2018; 4: 200–207.
- 7.
Guo W, Fan X, Lewis BR, Johnson MP, Rihal CS, Lerman A, et al. Cancer patients have a higher risk of thrombotic and ischemic events after percutaneous coronary intervention. JACC Cardiovasc Interv 2021; 14: 1094–1105.
- 8.
Velders MA, Hagström E, James SK. Temporal trends in the prevalence of cancer and its impact on outcome in patients with first myocardial infarction: A nationwide study. J Am Heart Assoc 2020; 9: e014383.
- 9.
Ueki Y, Vögeli B, Karagiannis A, Zanchin T, Zanchin C, Rhyner D, et al. Ischemia and bleeding in cancer patients undergoing percutaneous coronary intervention. JACC CardioOncol 2019; 1: 145–155.
- 10.
Iannaccone M, D’Ascenzo F, Vadalà P, Wilton SB, Noussan P, Colombo F, et al. Prevalence and outcome of patients with cancer and acute coronary syndrome undergoing percutaneous coronary intervention: A BleeMACS substudy. Eur Heart J Acute Cardiovasc Care 2018; 7: 631–638.
- 11.
Kwok CS, Wong CW, Kontopantelis E, Barac A, Brown SA, Velagapudi P, et al. Percutaneous coronary intervention in patients with cancer and readmissions within 90 days for acute myocardial infarction and bleeding in the USA. Eur Heart J 2021; 42: 1019–1034.
- 12.
Wang F, Gulati R, Lennon RJ, Lewis BR, Park J, Sandhu GS, et al. Cancer history portends worse acute and long-term noncardiac (but not cardiac) mortality after primary percutaneous coronary intervention for acute ST-segment elevation myocardial infarction. Mayo Clin Proc 2016; 91: 1680–1692.
- 13.
Hess CN, Roe MT, Clare RM, Chiswell K, Kelly J, Tcheng JE, et al. Relationship between cancer and cardiovascular outcomes following percutaneous coronary intervention. J Am Heart Assoc 2015; 4: e001779.
- 14.
Takeuchi T, Hikoso S, Hattori S, Kitamura T, Nakatani D, Mizuno H, et al. The effect of a cancer history on patients with acute myocardial infarction after percutaneous coronary intervention. Int Heart J 2021; 62: 238–245.
- 15.
Ederhy S, Cohen A, Boccara F, Puymirat E, Aissaoui N, Elbaz M, et al. In-hospital outcomes and 5-year mortality following an acute myocardial infarction in patients with a history of cancer: Results from the French registry on Acute ST-elevation or non-ST-elevation myocardial infarction (FAST-MI) 2005 cohort. Arch Cardiovasc Dis 2019; 112: 657–669.
- 16.
Iglesias-Garriz I, Delgado I, Prieto-Salvador I, Garrote C, García-Palomo A, Fernández-Vazquez F. Previously diagnosed cancer and mortality after ST-segment elevation acute myocardial infarction treated with primary angioplasty. Catheter Cardiovasc Interv 2020; 95: 1269–1274.
- 17.
Landes U, Kornowski R, Bental T, Assali A, Vaknin-Assa H, Lev E, et al. Long-term outcomes after percutaneous coronary interventions in cancer survivors. Coron Artery Dis 2017; 28: 5–10.
- 18.
Kosugi S, Shinouchi K, Ueda Y, Abe H, Sogabe T, Ishida K, et al. Clinical and angiographic features of patients with out-of-hospital cardiac arrest and acute myocardial infarction. J Am Coll Cardiol 2020; 76: 1934–1943.
- 19.
Thygesen K, Alpert JS, Jaffe AS, Chaitman BR, Bax JJ, Morrow DA, et al. Fourth universal definition of myocardial infarction (2018). J Am Coll Cardiol 2018; 72: 2231–2264.
- 20.
Kitano T, Sasaki T, Gon Y, Todo K, Okazaki S, Kitamura T, et al. The effect of chemotherapy on stroke risk in cancer patients. Thromb Haemost 2020; 120: 714–723.
- 21.
Hicks KA, Mahaffey KW, Mehran R, Nissen SE, Wiviott SD, Dunn B, et al. 2017 Cardiovascular and stroke endpoint definitions for clinical trials. J Am Coll Cardiol 2018; 71: 1021–1034.
- 22.
Rickles FR. Mechanisms of cancer-induced thrombosis in cancer. Pathophysiol Haemost Thromb 2006; 35: 103–110.
- 23.
ten Cate H, Falanga A. Overview of the postulated mechanisms linking cancer and thrombosis. Pathophysiol Haemost Thromb 2008; 36: 122–130.
- 24.
Donnellan E, Kevane B, Bird BR, Ainle FN. Cancer and venous thromboembolic disease: From molecular mechanisms to clinical management. Curr Oncol 2014; 21: 134–143.
- 25.
Lin PY, Cheng PC, Hsu WL, Lo WC, Hsieh CH, Shueng PW, et al. Risk of CVD following radiotherapy for head and neck cancer: An updated systematic review and meta-analysis. Front Oncol 2022; 12: 820808.
- 26.
Knight K, Wade S, Balducci L. Prevalence and outcomes of anemia in cancer: A systematic review of the literature. Am J Med 2004; 116(Suppl 7A): 11S–26S.
- 27.
Wassie M, Aemro A, Fentie B. Prevalence and associated factors of baseline anemia among cervical cancer patients in Tikur Anbesa Specialized Hospital, Ethiopia. BMC Womens Health 2021; 21: 36.
- 28.
Gros R, Hugon V, Thouret JM, Peigne V. Coronary spasm after an injection of vincristine. Chemotherapy 2017; 62: 169–171.
- 29.
Klag T, Cantara G, Ong P, Kaufmann M, Sechtem U, Athanasiadis A. Epicardial coronary artery spasm as cause of capecitabine-induced tako tsubo cardiomyopathy. Clin Res Cardiol 2014; 103: 247–250.
- 30.
Drobni ZD, Alvi RM, Taron J, Zafar A, Murphy SP, Rambarat PK, et al. Association between immune checkpoint inhibitors with cardiovascular events and atherosclerotic plaque. Circulation 2020; 142: 2299–2311.
- 31.
Nemeth BT, Varga ZV, Wu WJ, Pacher P. Trastuzumab cardiotoxicity: From clinical trials to experimental studies. Br J Pharmacol 2017; 174: 3727–3748.
- 32.
Totzeck M, Mincu RI, Mrotzek S, Schadendorf D, Rassaf T. Cardiovascular diseases in patients receiving small molecules with anti-vascular endothelial growth factor activity: A meta-analysis of approximately 29,000 cancer patients. Eur J Prev Cardiol 2018; 25: 482–494.
- 33.
Suero-Abreu GA, Zanni MV, Neilan TG. Atherosclerosis with immune checkpoint inhibitor therapy: Evidence, diagnosis, and management: JACC: CardioOncology State-of-the-Art Review. JACC CardioOncol 2022; 4: 598–615.
- 34.
Chitturi KR, Xu J, Araujo-Gutierrez R, Bhimaraj A, Guha A, Hussain I, et al. Immune checkpoint inhibitor-related adverse cardiovascular events in patients with lung cancer. JACC CardioOncol 2019; 1: 182–192.
- 35.
Mathur MB, Ding P, Riddell CA, VanderWeele TJ. Web site and R package for computing E-values. Epidemiology 2018; 29: e45–e47.



