論文ID: CJ-23-0059
論文ID: CJ-23-0059
Background: Robot-assisted valve surgery represents the latest development in the field of minimally invasive approaches. Robotic assistance may provide greater visualization, enhanced dexterity, and greater precision than traditional mini-thoracotomy aortic valve replacement.
Methods and Results: Aortic valve replacement operations using the da Vinci Xi Surgical System (Intuitive Surgical Inc., Sunnyvale, CA, USA) were performed on 2 patients, 1 with severe aortic insufficiency and the other with aortic stenosis. Both patients had an uneventful postoperative course and were discharged without any adverse events.
Conclusions: Robot-assisted assisted aortic valve replacement appears feasible and safe in limited cases.
Aortic valve replacement (AVR) using conventional bioprostheses remains the gold standard surgical treatment for severe aortic valve disease. However, since the introduction of transcatheter aortic valve replacement (TAVR), there have been marked advances in the treatment of aortic valve diseases. TAVR has opened the door towards less invasive treatment, even for patients with high surgical risk.1 The excellent mid-term results of TAVR have been clarified; hence, TAVR has been approved even for low-risk patients in the US.2,3
Conversely, there are still some situations in which surgical AVR (SAVR) is preferable, such as cases of pure aortic insufficiency (AI), severe annular calcification, and bicuspid aortic valve. Although SAVR with sternotomy was considered the standard procedure in these cases, minimally invasive cardiac surgeries have emerged in recent years as alternative approaches to conventional sternotomy, and minimally invasive SAVR with mini-thoracotomy has become prevalent.4,5
Robot-assisted valve surgery represents the latest development in the field of minimally invasive approaches. Generally, robotic assistance can provide enhanced dexterity and greater visualization and precision, which would provide considerable advantages for AVR with robotic assistance over traditional mini-thoracotomy AVR. Here, we report our experience with robot-assisted AVR using the da Vinci Xi Surgical System (Intuitive Surgical Inc., Sunnyvale, CA, USA). To the best of our knowledge, this is the first clinical report of robot-assisted AVR in Japan.
Two patients, one with with severe AI and the other with aortic stenosis (AS), underwent robot-assisted AVR using the da Vinci Xi Surgical System via a right lateral mini-thoracotomy. The procedure was approved by the Evaluation Committee for Highly Difficult New Medical Technologies at Tottori University Hospital. Both patients provided written informed consent for the procedure.
The robot-assisted aortic valve platform was identical to that used in mitral valve surgery (Figure 1). A 7- to 8-cm working incision was made, and a right lateral mini-thoracotomy was performed in the fourth intercostal space (ICS; Figure 2). Cardiopulmonary bypass (CPB) was established via arterial cannulation of the right common femoral artery and venous drainage of the right femoral and jugular veins. The da Vinci Xi Surgical System was used with the camera port through the working incision in arm 2. Three additional ports were placed via stab incisions: (1) DeBakey forceps in arm 1 in the third ICS; (2) a large needle driver or monopolar curved scissors in arm 4 in the sixth ICS on the right anterior axillary line; and (3) long-tip forceps in arm 3 in the fourth ICS below the edge of the right areola. Aortotomy, administration of selective cardioplegia, placement of non-everting mattress sutures on the aortic annulus, and aortic closure were all performed using robotic assistance. Specifically, the aortotomy was performed using monopolar curved scissors and DeBakey forceps, after which the operative field was secured using long-tip forceps. Selective crystalloid cardioplegia was delivered through the direct coronary ostia using a large needle driver and DeBakey forceps (Figure 3). Monopolar curved scissors were used as much as possible for resecting calcified leaflets. Subsequently, the patient-side surgeon resected the leaflets completely. The patient-side surgeon debrided the calcified aortic annulus using the SONOPET ultrasonic surgical aspirator (Stryker, Portage, MI, USA). Interrupted 2–0 polyester pledgeted non-everting mattress sutures were placed using a large needle driver and DeBakey forceps. The aortotomy was closed using robotic assistance with 4–0 polypropylene sutures, first with horizontal mattress suturing and then over-and-over suturing, using a large needle driver and DeBakey forceps (Figure 3).

The robot-assisted aortic valve replacement (AVR) platform. A 7- to 8-cm lateral working incision was made in the fourth intercostal space (ICS), 2 stab incisions were made in the third and sixth ICSs at the level of the anterior axillary line, and a stab incision was made in the fourth ICS above the edge of the right areola.
Operative incisions in (A) Case 1 and (B) Case 2.
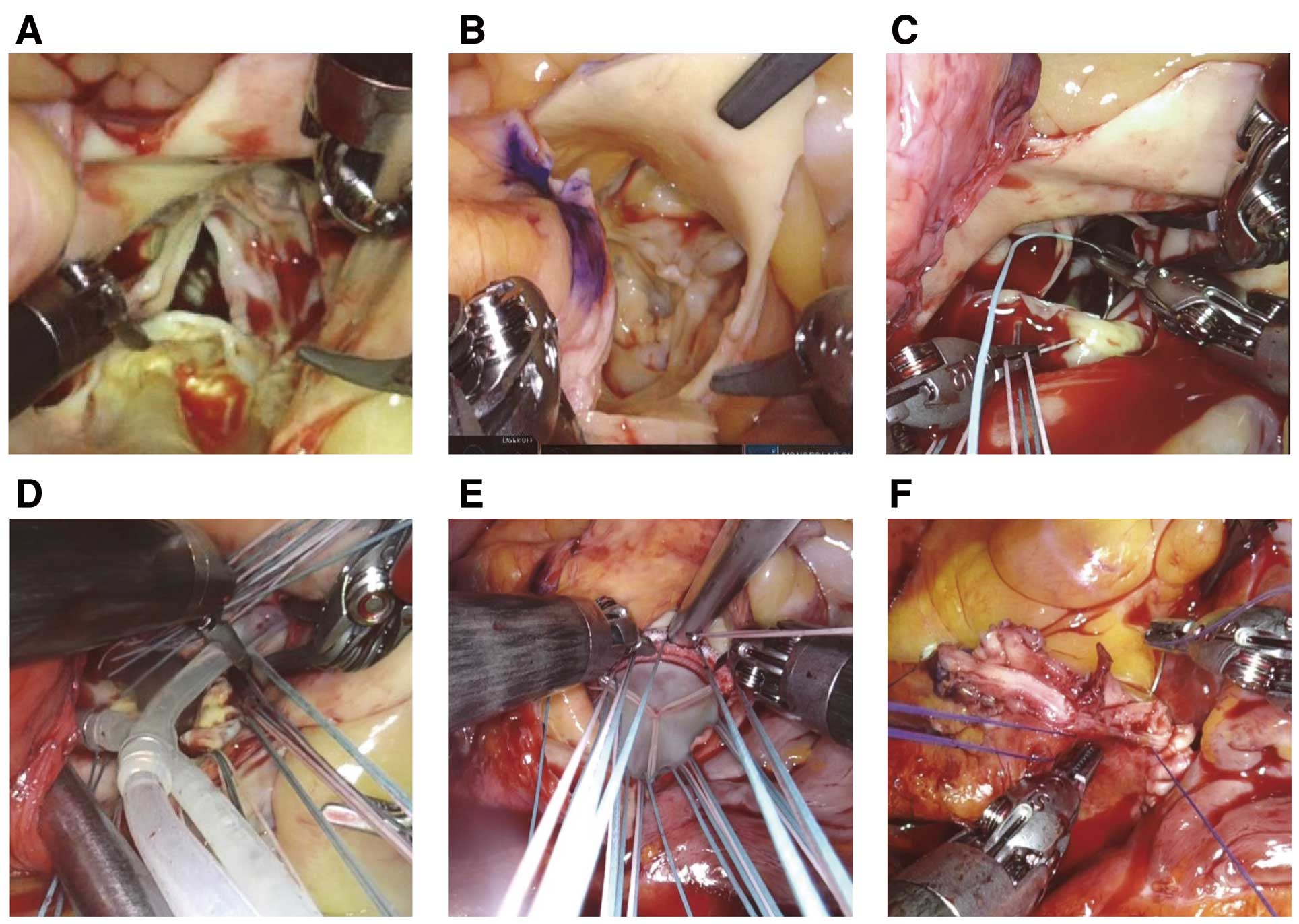
Surgical findings. (A,B) The aortic valve in Case 1 (A) and Case 2 (B). (C,D) Robot-assisted suturing of the non-coronary annulus (C) and administration of selective cardioplegia (D). (E) Suture ligation using the COR-KNOT® DEVICE (LSI Solutions, Victor, NY, USA). (F) Robot-assisted closure of the aortotomy.
A 78-year-old man developed severe AI. The patient had anatomies considered suitable for robot-assisted AVR (Figure 4). Briefly, regarding the surgery, after CPB was established, the da Vinci Xi system was docked, and cardiac arrest was obtained via selective cardioplegia. The aortic valve leaflets were tricuspid with a redundant right coronary leaflet. After non-everting mattress sutures were placed, a 23-mm AvalusTM aortic bioprosthesis (Medtronic, Minneapolis, MN, USA) was placed using a COR-KNOT® DEVICE (LSI Solutions, Victor, NY, USA) for faster suture securement (Figure 3). The total operative time was 264 min; the CPB and aortic cross-clamp times were 176 and 119 min, respectively. The times for aortotomy, valvectomy, annular suture placement, suture tying, and aortotomy closure were 1, 4, 29, 8, and 35 min, respectively. The intraoperative bleeding volume was 60 mL, and 6 units of red cell transfusions were required. The patient was extubated 2 h after admission to the intensive care unit (ICU). The postoperative bleeding volume from the chest tubes was 76 mL, and no transfusion was needed postoperatively. Postoperative echocardiography revealed a well-seated prosthetic valve without perivalvular leakage. The patient was discharged home on postoperative day 9 and return to full activity after 3 weeks.

Preoperative computed tomography scans with contrast. (A) Case 1. The distance from the sternum to the vertebrae was 10.5 cm, and the distance between the aortic valve and the distal end of the ascending aorta was 9.8 cm. (B) Case 2. The distance from the sternum to the vertebrae was 11.4 cm, and the distance between the aortic valve and the distal end of the ascending aorta was 9.1 cm.
A 70-year-old woman with severe AS underwent robot-assisted AVR using a 21-mm INSPIRIS RESILIATM aortic bioprosthesis (Edwards Lifesciences LLC, Irvine, CA, USA). All aortic valves were tricuspid and severely calcified (Figure 3). The total operative time was 324 min; the CPB and aortic cross-clamp times were 228 and 161 min, respectively. The times for aortotomy, valvectomy, annular suture placement, suture tying, and aortotomy closure were 2, 16, 32, 12, and 50 min, respectively. The intraoperative bleeding volume was 125 mL, and 4 units of red cell transfusions were required. The patient was extubated 4 h after admission to the ICU. The postoperative bleeding volume from the chest tubes was 197 mL, and no transfusion was needed postoperatively. Postoperative echocardiography revealed a well-seated prosthetic valve without perivalvular leakage. The patient was discharged on postoperative day 13 and returned to full activity after 3 weeks.
To the best of our knowledge, this is the first report on robot-assisted AVR for patients with AI or AS in Japan. Several reports have come from the US regarding the feasibility and safety of robot-assisted AVR.6–11 However, the procedure has not been performed in Japan for several reasons. First, only valve repair is reimbursed by the Japanese government’s health insurance system. Currently, only traditional mini-thoracotomy AVR with or without thoracoscopy is covered by insurance in Japan, and robot-assisted cardiac surgery is not. Therefore, the patient or hospital has to bear the cost of robot-assisted AVR. This insurance limitation has inhibited the introduction and widespread use of robot-assisted AVR in Japan. In the present cases, the procedure was approved by the Evaluation Committee for Highly Difficult New Medical Technologies at Tottori University Hospital. All surgical and hospital fees were paid by a clinical grant from Tottori University Hospital.
Second, robot-assisted AVR is more technically demanding than robot-assisted mitral valve repair. Robot-assisted mitral valve repair is well established, with excellent long-term outcomes, and is the standard approach in some institutes worldwide.12,13 Conversely, robotic approaches to AVR have been intermittently attempted,6–11 and reports of robot-assisted AVR are scarce. This is probably due to the anatomical features of the aortic valve and the lack of specific robotic instruments for AVR.
Regarding the anatomical features of the aortic valve, it is closer to the chest wall than the mitral valve, and collision among the robotic arms is anticipated. Therefore, to avoid collisions among the robotic arms, good candidates for this approach would have a deep chest (large anteroposterior diameter) and a relatively long aortic root.8 In the present cases, both patients had sufficiently deep chests and long aortic roots (Figure 4). Furthermore, robotic instruments are unsuitable for resecting severely calcified leaflets and debriding the calcified aortic annulus. In the present cases, the patient-side surgeon completed the resection of the calcified remnants and debrided the calcified annulus using an ultrasonic surgical aspirator after the calcified leaflets were partially cut using robotic scissors. In patients with severe AS, an experienced patient-side surgeon who can perfectly manage the calcification on the aortic valve and the annulus is required. Moreover, aortotomy closure is the most delicate and time-consuming procedure, compared with aortotomy, valvectomy, and annular suture placement.10,11 In the present cases, aortotomy closure took the most time during the surgery. During early experience with robot-assisted AVR, it is critical not to select cases with aortic calcification or a small aortic root.
Recently, sutureless and rapid-deployment valves have been introduced to shorten the aortic cross-clamp time; this could help robot-assisted AVR in patients with AS. Several researchers have reported this method;8,9 however, these valves have a relatively high incidence of de novo pacemaker implantation and perivalvular leakage.14 Therefore, although sutureless and rapid-deployment valves could be useful for robot-assisted AVR in some patients, it is very important to select the ideal prosthesis for individual patients considering lifelong management.
Robot-assisted AVR would be superior to traditional mini-thoracotomy SAVR in terms of providing more stable and better visualization, as well as outstanding instrument manipulation. However, recent advances in non-robotic 3-dimensional visualization have contributed greatly to enhanced visualization during mini-thoracotomy SAVR.15 This approach is technically demanding because of the limited range of the human hand or wrist. Nonetheless, with outstanding instrument manipulation, this robotic technology would be promising for improving the performance of minimally invasive SAVR. In particular, optimal suture management becomes possible with either the robotic left or right hand under optimal visualization controlled by the console surgeon. In addition, Badhwar et al reported that a relatively short learning curve could be anticipated, especially for those with robotic experience, because the lateral thoracotomy approach is the same as that in robot-assisted mitral valve repair.10 In the present case series, a relatively large incision was used for safety reasons; however, the incision made could be smaller with experience.
In conclusion, robot-assisted AVR using a conventional prosthesis was performed safely and successfully in 2 patients, 1 with severe AS and 1 with severe AI. We observed that robotic assistance enables excellent exposure of the aortic valve with precise instrument manipulation.
None.
All authors have no conflicts of interest related to this manuscript.
The robot-assisted AVR procedure was approved by the Evaluating Committee for Highly Difficult New Medical Technologies of Tottori University Hospital (Reference no. 04-1).