Abstract
Background: This prospective ANAFIE Registry substudy investigated the relationship between the echocardiographic parameters of left atrial (LA) structure and function and clinical outcomes at 2 years among atrial fibrillation (AF) patients aged ≥75 years.
Methods and Results: Outcomes of 1,474 elderly non-valvular AF (NVAF) patients who underwent transthoracic echocardiography at baseline were analyzed by categories of maximum LA volume index (max. LAVi) and LA emptying fraction (LAEF) total. Baseline mean±standard deviation LAEF total and max. LAVi were 28.2±14.9% and 54.2±25.9 mL/m2, respectively. Proportions of oral anticoagulant (OAC), direct OAC, and warfarin use were 92.7%, 68.7%, and 24.0%, respectively. Patients with LAEF total ≤45.0% (n=1,213) vs. >45.0% (n=224) were at higher risk of cardiovascular events (hazard ratio [HR]: 2.19, P=0.021) and heart failure (HF) hospitalization (HR: 2.25, P=0.045). Risk of all-cause death was higher with max. LAVi >48.0 mL/m2
(n=656) vs. ≤48.0 mL/m2
(n=621) (HR: 1.69, P=0.048). Subgroups with abnormal LA function and structure had increased incidence of cardiac/cardiovascular events and HF hospitalization. No significant interaction was observed between echocardiographic parameters and OAC type.
Conclusions: Elderly Japanese patients with NVAF and LAEF total ≤45.0% were at higher risk of cardiovascular events and HF hospitalization, and those with max. LAVi >48.0 mL/m2
were at higher risk of all-cause death.
Atrial fibrillation (AF), the most common cardiac arrhythmia, increases the risk of stroke, heart failure (HF), and cardiovascular (CV) morbidity.1,2 AF and HF commonly occur together, and morbidity and mortality rates tend to increase when these conditions coexist.3 Previous studies have linked AF and HF to an increased risk of acute ischemic stroke, stroke recurrence, increased mortality, and disability associated with acute ischemic stroke.4–9 There is a well-known but not well-understood bidirectional association between these pathologies: AF exacerbates HF manifestations, and larger atria secondary to HF are more vulnerable to AF.10 Both AF and HF are predicted to increase globally with the increasing aging of populations over the coming decades. Further research must be conducted to improve the overall understanding, detection, and treatment of AF and HF.11
Echocardiography is a useful diagnostic modality for identifying underlying structural and functional changes associated with CV diseases. Changes in echocardiographic markers due to AF, particularly those evaluating left atrial (LA) structure and function,12,13 may predict clinical outcomes.14 Previous studies, including the ENGAGE AF-TIMI 48 study,12,14–17 reported that increased LA volume index (LAVi) and decreased LA function (i.e., lower LA emptying fraction [LAEF] and LA expansion index) translated into a higher risk for the composite of CV death and HF hospitalization in AF patients. However, related data are limited, especially among elderly patients with AF aged ≥75 years in real-world settings.
The All Nippon Atrial Fibrillation In the Elderly (ANAFIE) Registry was a prospective, multicenter, observational study that collected real-world data on the clinical status and prognosis of >30,000 Japanese patients aged ≥75 years with non-valvular AF (NVAF).18 Based on the ANAFIE data, we analyzed the baseline echocardiographic parameters to identify cardiac structural and functional characteristics among elderly Japanese patients with NVAF and found that 51.5% of patients had LA enlargement (max. LAVi ≥48 mL/m2). Additionally, LA enlargement correlated with impaired LA reservoir function.19 The present ANAFIE Registry substudy investigated the relationships between echocardiographic parameters of LA structure and function and clinical outcomes at 2 years in elderly patients with NVAF.
Methods
Study Design
The study design (Supplementary File, Supplementary Figure) and rationale of the ANAFIE Registry have been described previously.20 Briefly, the ANAFIE Registry was a multicenter, prospective, observational cohort study. Patients were followed up for 2 years. This study was purely observational, and patients did not undergo any study-mandated therapeutic interventions. Each patient’s treating physician indicated treatment according to current clinical practice and the physician’s judgment.
The Ethics Committee of The Cardiovascular Institute approved the study protocol. The Registry complied with the Declaration of Helsinki, local regulations, and ethical guidelines in Japan. All patients provided written informed consent before participation and could discontinue participation at any time during the study. The ANAFIE Registry was registered in the UMIN Clinical Trials Registry under the identifier UMIN000024006.
Patients
The details of the inclusion and exclusion criteria of the ANAFIE Registry have been described previously.20 Briefly, enrolled patients were elderly outpatients aged ≥75 years who were diagnosed with NVAF via ECG. Patients diagnosed with mitral stenosis; with a history of artificial heart valve replacement with either mechanical or tissue valve prostheses; a history of CV events including stroke, myocardial infarction (MI), cardiac intervention, HF requiring hospitalization, or any bleeding leading to hospitalization within 1 month before enrollment; a life expectancy <1 year; participating or planning to participate in a clinical study; or deemed inappropriate for study participation were excluded. The only specific criteria for enrollment were the provision of written informed consent for substudy participation and undergoing transthoracic echocardiography at baseline or within 2 months after enrollment.19
Echocardiography
The American Society of Echocardiography (ASE) guidelines19,21 informed the collection and evaluation of echocardiographic data, including LA size and left ventricular structure and function. The operators received a detailed manual for measurements and specific training according to the ASE guidelines before conducting the patient evaluations. Image acquisition was performed using the DICOM format. All echocardiographic measurements and images underwent external validation at a central office. As indicated in the ANAFIE Echocardiographic Substudy and per the protocol,19 1 heartbeat measurement was deemed adequate and image data were gathered for a duration of 5 s. The collected data were then verified against randomly sampled values. Formulae for calculating the echocardiographic parameters19 are provided in the Supplementary Methods. Findings were also confirmed by board-certified cardiologists.19
Max. LAVi of 48 mL/m2
was used as a cutoff value per the criteria for a severely dilated LA according to the ASE guidelines.21 LAEF total of 45.0% was used as a cutoff value, defined as reduced LAEF in the echocardiography substudy of ENGAGE TIMI-48.22
Data Collection and Study Measures
Definitions and assessment items have been previously described.19 The echocardiographic variables assessed in this substudy comprised the following subgroups: max. LAVi subgroup including LAVi >48.0 mL/m2
and LAVi ≤48.0 mL/m2; LAEF total subgroup including LAEF >45.0% and LAEF ≤45.0%; and max. LAVi and LAEF total subgroup including max. LAVi ≤48.0 mL/m2
and LAEF total ≤45.0%, max. LAVi ≤48.0 mL/m2
and LAEF total >45.0%, max. LAVi >48.0 mL/m2
and LAEF total ≤45.0%, and max. LAVi >48.0 mL/m2
and LAEF total >45.0%.
Outcomes assessed were stroke/systemic embolic events (SEE), CV events (composite of stroke, MI, cardiac intervention, HF hospitalization, CV death), cardiac events (composite of MI, cardiac intervention, HF hospitalization, and CV death), HF hospitalization, and all-cause death. A subgroup analysis was conducted to compare the incidence of the above events by the effects of max. LAVi, LAEF total, or direct oral anticoagulants (DOACs) vs. warfarin.
Statistical Analysis
Details of the statistical analysis and measures applied in the ANAFIE Registry have been described.18 More than 4% of the entire ANAFIE population was eligible for this substudy, which was considered sufficient for exploratory analyses. For comparisons of continuous variables, a 2-sample t-test was used, and for categorical variables, a chi-square test was used. The probability of event occurrence was estimated using the Kaplan-Meier method. Incidence rates per 100 person-years with 95% confidence intervals (CIs) were also estimated.
Cox proportional hazards models were used to evaluate the association between the max. LAVi subgroup or LAEF total subgroup and the clinical outcomes, and to estimate hazard ratios (HRs) and 95% CIs after adjusting for anticoagulant type, history of HF, left ventricular ejection fraction, creatinine clearance, B-type natriuretic peptide, sex, age, and body mass index. Additionally, for the comparison of DOACs vs. warfarin, the main effect and interaction with max. LAVi or LAEF total subgroup were also evaluated after adjustment for prognostic factors.
A P value <0.05 was considered statistically significant, and all tests were two-sided.
Statistical analyses were performed using SAS version 9.4 (SAS Institute, Tokyo, Japan).
Results
Baseline Characteristics
Of the 32,725 patients included in the main analysis set of the ANAFIE Registry, 1,474 consented to be involved in this echocardiographic substudy and underwent transthoracic echocardiography at baseline or within 2 months after enrollment.
Regarding the baseline characteristics of patients in this substudy, the mean age was 80.7 years, 59.2% were male, the mean body mass index was 23.3 kg/m2
(Table 1), the mean (±standard deviation [SD]) LAEF total was 28.2±14.9%, the mean max. LAVi was 54.2±25.9 mL/m2, and 41.0% of patients were in sinus rhythm (Table 2). Paroxysmal AF was predominant (45.9%), the mean CHA2DS2-VASc score was 4.3, and the mean HAS-BLED score was 1.9. A history of HF was reported in 32.2% of patients. Most patients (92.7%) received oral anticoagulants (DOACs, 68.7%; warfarin, 24.0%; Table 1).
Table 1.
Baseline Characteristics of Patients
| |
Total
(N=1,474) |
LAEF total, % |
Max. LAVi, mL/m2 |
>45.0
(n=224) |
≤45.0
(n=1,213) |
P value |
>48.0
(n=656) |
≤48.0
(n=621) |
P value |
| Men |
873 (59.2) |
138 (61.6) |
709 (58.5) |
0.378 |
354 (54.0) |
396 (63.8) |
<0.001 |
| Age, years |
80.7±4.6 |
79.9±4.4 |
80.9±4.6 |
0.002 |
81.3±4.7 |
80.0±4.3 |
<0.001 |
| ≥85 |
331 (22.5) |
36 (16.1) |
286 (23.6) |
– |
175 (26.7) |
108 (17.4) |
– |
| BMI, kg/m2 |
23.3±3.5 |
23.6±3.7 |
23.2±3.4 |
0.125 |
23.0±3.5 |
23.5±3.4 |
0.012 |
| Systolic BP, mmHg |
127.5±18.4 |
129.9±19.6 |
127.0±18.1 |
0.058 |
126.4±17.7 |
129.2±18.5 |
0.010 |
| Creatinine clearance, mL/min |
47.5±18.1 |
52.9±17.6 |
46.6±18.0 |
<0.001 |
44.2±16.9 |
51.2±17.2 |
<0.001 |
| <50 |
701 (47.6) |
82 (36.6) |
599 (49.4) |
– |
387 (59.0) |
272 (43.8) |
– |
| CHA2DS2-VASc score |
4.3±1.4 |
4.0±1.3 |
4.4±1.4 |
<0.001 |
4.5±1.4 |
4.1±1.3 |
<0.001 |
| HAS-BLED score |
1.9±0.8 |
1.8±0.8 |
1.9±0.8 |
0.269 |
1.9±0.8 |
1.8±0.8 |
0.019 |
| History of major bleeding |
64 (4.3) |
7 (3.1) |
56 (4.6) |
0.316 |
35 (5.3) |
19 (3.1) |
0.043 |
| AF type |
| Paroxysmal |
677 (45.9) |
189 (84.4) |
469 (38.7) |
<0.001 |
182 (27.7) |
408 (65.7) |
<0.001 |
| Persistent |
463 (31.4) |
24 (10.7) |
423 (34.9) |
– |
241 (36.7) |
153 (24.6) |
– |
| Permanent |
334 (22.7) |
11 (4.9) |
321 (26.5) |
– |
233 (35.5) |
60 (9.7) |
– |
| Oral anticoagulants |
1,366 (92.7) |
188 (83.9) |
1,143 (94.2) |
<0.001 |
613 (93.4) |
572 (92.1) |
0.356 |
| DOAC |
1,012 (68.7) |
165 (73.7) |
817 (67.4) |
<0.001 |
418 (63.7) |
464 (74.7) |
<0.001 |
| Warfarin |
354 (24.0) |
23 (10.3) |
326 (26.9) |
<0.001 |
195 (29.7) |
108 (17.4) |
<0.001 |
| TTR |
75.6±29.7 |
84.6±30.9 |
74.7±29.7 |
0.171 |
74.7±29.4 |
75.8±31.4 |
0.786 |
| Comorbidities |
| Hypertension |
1,076 (73.0) |
160 (71.4) |
888 (73.2) |
0.582 |
500 (76.2) |
438 (70.5) |
0.021 |
| Diabetes mellitus |
394 (26.7) |
46 (20.5) |
339 (27.9) |
0.021 |
175 (26.7) |
170 (27.4) |
0.779 |
| Chronic kidney disease |
286 (19.4) |
34 (15.2) |
244 (20.1) |
0.086 |
153 (23.3) |
103 (16.6) |
0.003 |
| Myocardial infarction |
91 (6.2) |
16 (7.1) |
72 (5.9) |
0.489 |
32 (4.9) |
36 (5.8) |
0.465 |
| Heart failure |
474 (32.2) |
48 (21.4) |
415 (34.2) |
<0.001 |
272 (41.5) |
135 (21.7) |
<0.001 |
| Cerebrovascular disease |
284 (19.3) |
30 (13.4) |
246 (20.3) |
0.016 |
133 (20.3) |
108 (17.4) |
0.188 |
| Gastrointestinal disease |
384 (26.1) |
64 (28.6) |
309 (25.5) |
0.331 |
174 (26.5) |
144 (23.2) |
0.168 |
| Active cancer |
257 (17.4) |
40 (17.9) |
206 (17.0) |
0.750 |
125 (19.1) |
93 (15.0) |
0.053 |
| Dementia |
78 (5.3) |
13 (5.8) |
64 (5.3) |
0.747 |
36 (5.5) |
36 (5.8) |
0.811 |
| Fall within 1 year |
102 (6.9) |
16 (7.1) |
85 (7.0) |
0.813 |
41 (6.3) |
52 (8.4) |
0.153 |
Data are n (%) or mean±standard deviation. AF, atrial fibrillation; BMI, body mass index; BP, blood pressure; DOAC, direct oral anticoagulant; LAEF, left atrial emptying fraction; LAVi, left atrial volume index; TTR, time in the therapeutic range.
Table 2.
Echocardiographic Parameters According to LAEF Total and Max. LAVi Subgroups
| |
Total
(N=1,474) |
LAEF total, % |
Max. LAVi, mL/m2 |
| >45.0 (n=224) |
≤45.0 (n=1,213) |
>48.0 (n=656) |
≤48.0 (n=621) |
| LV structure and function |
| LVDd, cm |
4.6±0.6 |
4.5±0.6 |
4.6±0.6 |
4.7±0.7 |
4.5±0.6 |
| LVDs, cm |
3.0±0.6 |
2.9±0.5 |
3.1±0.7 |
3.2±0.7 |
2.9±0.5 |
| IVSTD, cm |
1.0±0.2 |
1.0±0.2 |
1.0±0.2 |
1.0±0.2 |
1.0±0.2 |
| PWTD, cm |
1.0±0.2 |
0.9±0.1 |
1.0±0.2 |
1.0±0.2 |
0.9±0.2 |
| LVEDV index, mL/m2 |
48.3±18.3 |
46.2±15.2 |
48.6±18.8 |
51.9±21.0 |
44.3±13.9 |
| LVESV index, mL/m2 |
19.9±13.4 |
27.3±13.4 |
31.5±20.9 |
22.5±16.4 |
17.0±8.5 |
| LVEF (Disk), % |
60.7±10.0 |
63.9±8.8 |
60.1±10.0 |
59.3±10.8 |
62.6±8.6 |
| SV index, mL/m2 |
37.3±10.9 |
42.3±10.8 |
36.4±10.7 |
36.4±10.7 |
38.2±11.0 |
| LV mass index, g/m2 |
98.8±28.9 |
90.9±27.4 |
100.4±29.2 |
106.9±32.2 |
90.3±22.2 |
| LA structure and function |
| LAD, cm |
4.4±0.8 |
3.8±0.7 |
4.5±0.8 |
4.8±0.7 |
4.0±0.6 |
| Max. LAVi, mL/m2 |
54.2±25.9 |
36.7±13.3 |
57.5±26.4 |
72.1±24.3 |
35.3±7.9 |
| Min LAVi, mL/m2 |
40.6±24.6 |
17.6±7.1 |
44.6±24.0 |
56.4±23.5 |
23.4±8.4 |
| LAEF total, % |
28.2±14. 9 |
52.4±6.1 |
23.8±11.3 |
22.7±12.1 |
34.7±15.0 |
| Active LAEF, % |
19.3±10.6 |
26.5±11.3 |
16.0±8.4 |
15.8±9.1 |
20.3±10.8 |
| Passive LAEF, % |
19.2±10.4 |
26.0±11.4 |
16.0±8.2 |
17.8±9.1 |
20.0±10.8 |
| Mitral regurgitation (>moderate) |
200 (13.6) |
18 (8.0) |
180 (14.8) |
131 (20.0) |
48 (7.7) |
| Tricuspid regurgitation (>moderate) |
331 (22.5) |
17 (7.6) |
309 (25.5) |
217 (33.1) |
74 (11.9) |
| Rhythm at baseline |
| Sinus, n (%) |
605 (41.0) |
187 (83.5) |
399 (32.9) |
140 (21.3) |
394 (63.4) |
Data are n (%) or mean±standard deviation. IVSTD, interventricular septal thickness in diastole; LA, left atrial; LAD, left atrial diameter; LAEF, left atrial emptying fraction; LAVi, left atrial volume index; LV, left ventricular; LVDd, left ventricular dimension end-diastole; LVDs, left ventricular dimension end-systole; LVEDV, left ventricular end-diastolic volume; LVEF, left ventricular ejection fraction; LVESV, left ventricular end-systolic volume; Pre-A, pre-atrial; PWTD, posterior wall thickness in diastole; SV, systolic volume.
Differences in baseline characteristics by LAEF and LAVi are shown in Table 1. LAEF was observed in 1,437 patients and LAVi in 1,277 patients due to measurement errors or missing data at the time of treatment. In the LAEF total ≤45.0% subgroup, patients were significantly older, had lower creatinine clearance, and had higher CHA2DS2-VASc scores compared with the LAEF total >45.0% subgroup. The proportions of patients with persistent and permanent AF and HF, and receiving warfarin were higher in the LAEF total ≤45.0% subgroup than in the LAEF total >45.0% subgroup. The subgroup with max. LAVi >48.0 mL/m2
showed similar trends for baseline characteristics compared with the LAVi <48.0 mL/m2
subgroup.
Table 2 summarizes the echocardiographic parameters. Mean (±SD) min LAVi was 40.6±24.6 mL/m2, active LAEF was 19.3±10.6%, and passive LAEF was 19.2±10.4%. The proportion of patients in sinus rhythm was 83.5% and 32.9% by LAEF total subgroup and 63.4% and 21.3% by max. LAVi subgroup.
Incidence of Clinical Outcomes According to Max. LAVi and LAEF Total Subgroups
In the subgroup with LAEF total ≤45.0%, cumulative incidences of stroke/SEE, cardiac events, CV events, and HF hospitalization increased significantly and progressively during the study period, except for all-cause death (Figure 1). In the subgroup with max. LAVi >48 mL/m2, cumulative incidences of stroke/SEE, cardiac events, CV events, HF requiring hospitalization, and all-cause death increased significantly and progressively during the study period (Figure 2).
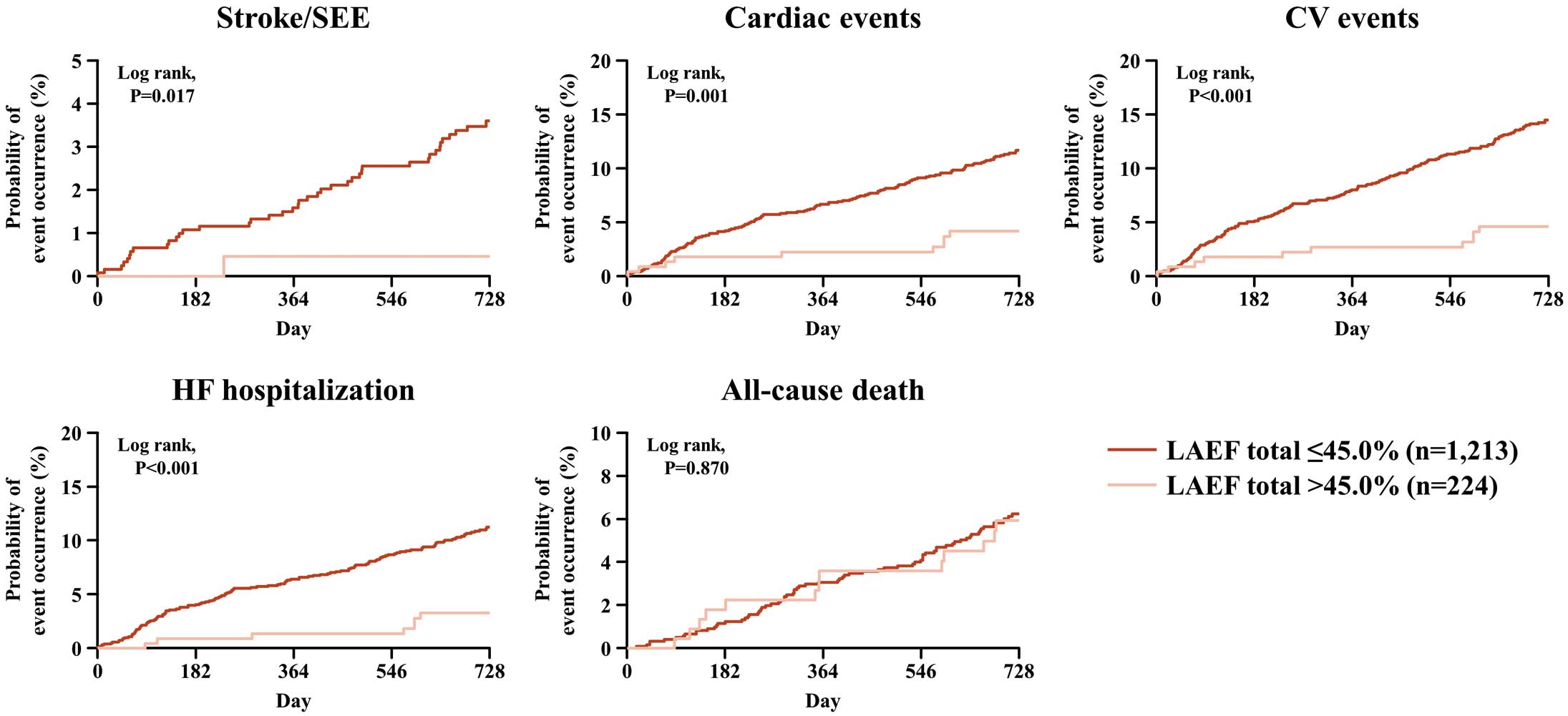
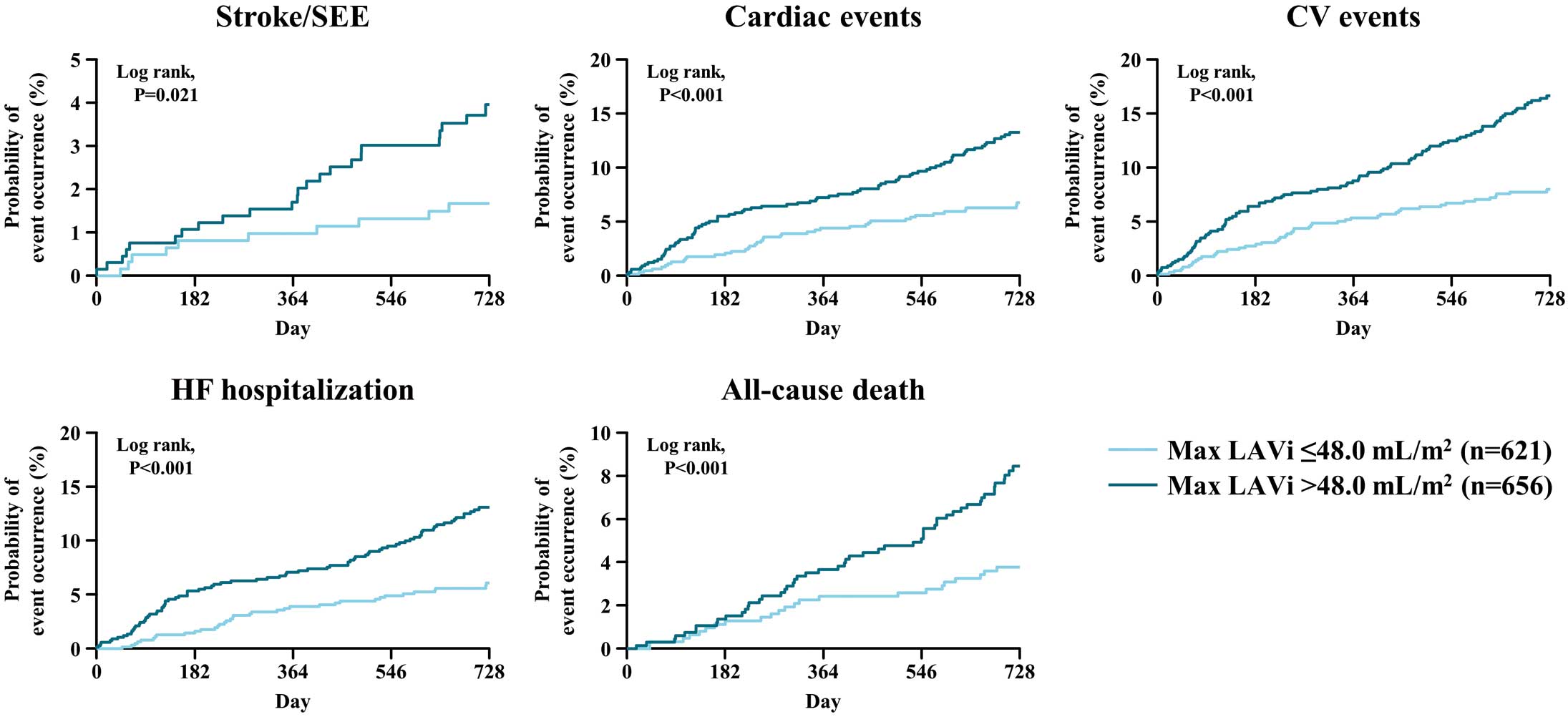
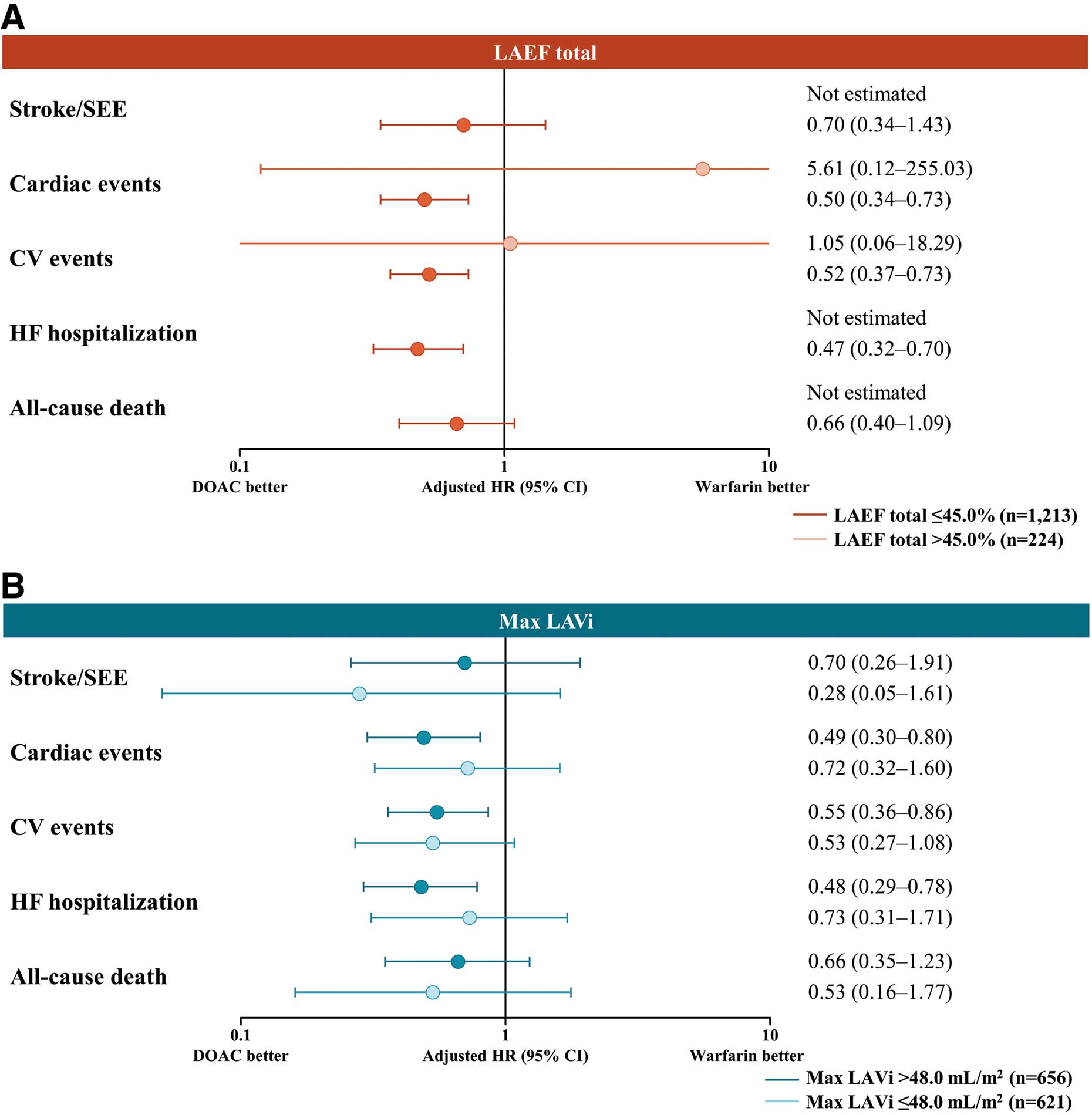
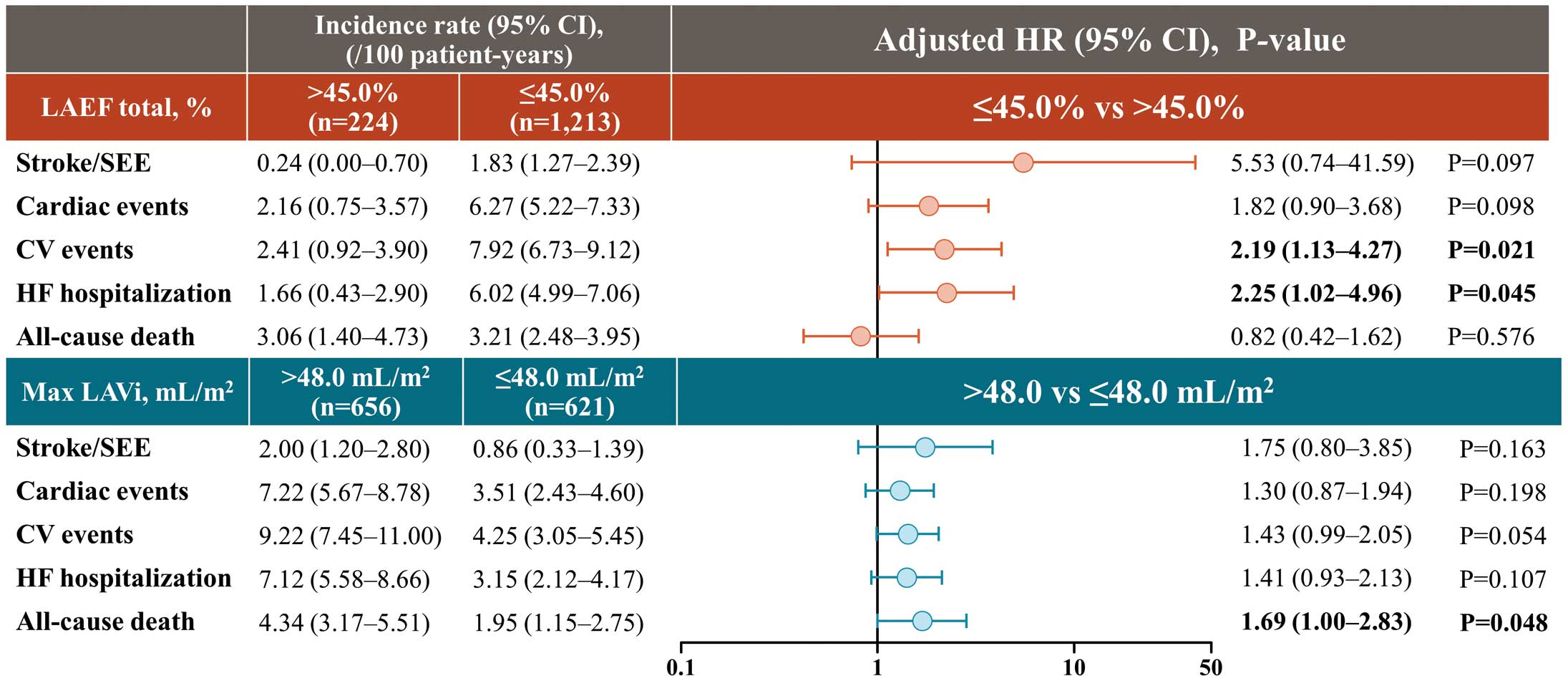
Table 3 summarizes the incidence rates of clinical outcomes according to LAEF total and max. LAVi subgroups. For multivariate analyses, the reference was sufficient structure and function indicated by max. LAVi ≤48.0 mL/m2
and LAEF total >45.0%. Compared with patients with LAEF total >45.0%, those with LAEF total ≤45.0% were at a higher risk of CV events (HR: 2.19, 95% CI: 1.13–4.27, P=0.021) and HF hospitalization (HR: 2.25, 95% CI: 1.02–4.96, P=0.045). Risk of all-cause death was higher in patients with max. LAVi >48.0 mL/m2
than in those with max. LAVi ≤48.0 mL/m2
(HR: 1.69, 95% CI: 1.00–2.83, P=0.048) (Figure 3). In the multivariate analyses, mitral regurgitation (MR) was a risk factor for cardiac events (HR: 2.13, 95% CI: 1.43–3.16, P<0.001), CV events (HR: 1.73, 95% CI: 1.19–2.51, P=0.004), and HF hospitalization (HR: 2.18, 95% CI: 1.46–3.26, P<0.001). Tricuspid regurgitation (TR) was a risk factor for cardiac events (HR: 1.55, 95% CI: 1.06–2.27, P=0.023), HF hospitalization (HR: 1.58, 95% CI: 1.07–2.33, P=0.020), and all-cause death (HR: 1.76, 95% CI: 1.08–2.84, P=0.022) (Supplementary Table 1). Furthermore, incorporating MR and TR into the multivariate model shown in Figure 3 revealed that LAEF total ≤45.0% was a risk factor only for CV events (HR: 2.09, 95% CI: 1.07–4.09, P=0.031) and that max. LAVi >48.0 mL/m2 was not a risk factor for any of the 5 events (Supplementary Table 2).
Table 3.
Incidence of Clinical Outcomes According to LAEF Total and Max. LAVi Subgroups
| Clinical outcomes |
Item |
Per 100
person-years |
Incidence per 100
person-years, 95% CI |
| Lower limit |
Upper limit |
| Stroke/systemic embolic events |
LAEF total |
≤45.0% |
1.83 |
1.27 |
2.39 |
| >45.0% |
0.24 |
0.00 |
0.70 |
| Max. LAVi |
≤48.0 mL/m2 |
0.86 |
0.33 |
1.39 |
| >48.0 mL/m2 |
2.00 |
1.20 |
2.80 |
| Stroke |
LAEF total |
≤45.0% |
1.79 |
1.23 |
2.34 |
| >45.0% |
0.24 |
0.00 |
0.70 |
| Max. LAVi |
≤48.0 mL/m2 |
0.86 |
0.33 |
1.39 |
| >48.0 mL/m2 |
1.92 |
1.13 |
2.70 |
| Ischemic stroke |
LAEF total |
≤45.0% |
1.70 |
1.16 |
2.24 |
| >45.0% |
0.24 |
0.00 |
0.70 |
| Max. LAVi |
≤48.0 mL/m2 |
0.77 |
0.27 |
1.27 |
| >48.0 mL/m2 |
1.83 |
1.07 |
2.60 |
| Hemorrhagic stroke |
LAEF total |
≤45.0% |
0.09 |
0.00 |
0.21 |
| >45.0% |
0.00 |
– |
– |
| Max. LAVi |
≤48.0 mL/m2 |
0.08 |
0.00 |
0.25 |
| >48.0 mL/m2 |
0.08 |
0.00 |
0.24 |
| Systemic embolic events |
LAEF total |
≤45.0% |
0.09 |
0.00 |
0.21 |
| >45.0% |
0.00 |
– |
– |
| Max. LAVi |
≤48.0 mL/m2 |
0.00 |
– |
– |
| >48.0 mL/m2 |
0.16 |
0.00 |
0.39 |
| Major bleeding |
LAEF total |
≤45.0% |
1.02 |
0.60 |
1.44 |
| >45.0% |
0.47 |
0.00 |
1.13 |
| Max. LAVi |
≤48.0 mL/m2 |
0.68 |
0.21 |
1.16 |
| >48.0 mL/m2 |
0.99 |
0.43 |
1.55 |
| All bleedinga |
LAEF total |
≤45.0% |
5.54 |
4.55 |
6.54 |
| >45.0% |
3.68 |
1.82 |
5.54 |
| Max. LAVi |
≤48.0 mL/m2 |
4.39 |
3.16 |
5.62 |
| >48.0 mL/m2 |
5.52 |
4.17 |
6.87 |
| Intracranial hemorrhage |
LAEF total |
≤45.0% |
0.62 |
0.30 |
0.94 |
| >45.0% |
0.00 |
– |
– |
| Max. LAVi |
≤48.0 mL/m2 |
0.51 |
0.10 |
0.92 |
| >48.0 mL/m2 |
0.66 |
0.20 |
1.12 |
| Gastrointestinal bleeding |
LAEF total |
≤45.0% |
1.84 |
1.28 |
2.40 |
| >45.0% |
1.67 |
0.43 |
2.91 |
| Max. LAVi |
≤48.0 mL/m2 |
0.94 |
0.39 |
1.50 |
| >48.0 mL/m2 |
2.52 |
1.62 |
3.42 |
| Heart failure requiring hospitalization |
LAEF total |
≤45.0% |
6.02 |
4.99 |
7.06 |
| >45.0% |
1.66 |
0.43 |
2.90 |
| Max. LAVi |
≤48.0 mL/m2 |
3.15 |
2.12 |
4.17 |
| >48.0 mL/m2 |
7.12 |
5.58 |
8.66 |
| CV events |
LAEF total |
≤45.0% |
7.92 |
6.73 |
9.12 |
| >45.0% |
2.41 |
0.92 |
3.90 |
| Max. LAVi |
≤48.0 mL/m2 |
4.25 |
3.05 |
5.45 |
| >48.0 mL/m2 |
9.22 |
7.45 |
11.00 |
| CV death |
LAEF total |
≤45.0% |
0.84 |
0.46 |
1.21 |
| >45.0% |
0.71 |
0.00 |
1.51 |
| Max. LAVi |
≤48.0 mL/m2 |
0.34 |
0.01 |
0.67 |
| >48.0 mL/m2 |
1.07 |
0.49 |
1.64 |
| All-cause death |
LAEF total |
≤45.0% |
3.21 |
2.48 |
3.95 |
| >45.0% |
3.06 |
1.40 |
4.73 |
| Max. LAVi |
≤48.0 mL/m2 |
1.95 |
1.15 |
2.75 |
| >48.0 mL/m2 |
4.34 |
3.17 |
5.51 |
| Net clinical outcome |
LAEF total |
≤45.0% |
5.26 |
4.31 |
6.22 |
| >45.0% |
3.80 |
1.94 |
5.66 |
| Max. LAVi |
≤48.0 mL/m2 |
3.27 |
2.23 |
4.31 |
| >48.0 mL/m2 |
6.45 |
5.01 |
7.90 |
aAll bleeding was major bleeding, clinically significant bleeding, or minor bleeding. CI, confidence interval; CV, cardiovascular; LA, left atrial; LAEF, left atrial emptying fraction; LAVi, left atrial volume index.
There was no significant interaction between HF status and the risk of any of the clinical outcomes assessed in this analysis. The distribution of max. LAVi and LAEF total was as follows: max. LAVi ≤48.0 mL/m2
and LAEF total ≤45.0%, 35.6%; max. LAVi ≤48.0 mL/m2
and LAEF total >45.0%, 13.0%; max. LAVi >48.0 mL/m2
and LAEF total ≤45.0%, 48.7%; and max. LAVi >48.0 mL/m2
and LAEF total >45.0%, 2.7%. The multivariate analyses of clinical outcomes associated with max. LAVi and LAEF total subgroups are shown in Supplementary Table 3. Multivariate analysis of clinical outcomes associated with max. LAVi and LAEF total subgroups in the Cox proportional hazards model by sinus rhythm and non-sinus rhythm are shown in Supplementary Table 4. There was no significant interaction between max. LAVi or LAEF with any clinical outcome by sinus and non-sinus rhythm.
Incidence of Clinical Outcomes in Patients Using DOACs vs. Warfarin by LAEF Total and Max. LAVi Subgroups
Figure 4 shows the adjusted HRs of clinical outcomes according to the anticoagulant used by LAEF total and max. LAVi. There was no significant interaction between the echocardiographic parameters and type of anticoagulant treatment, and the results were consistent.
Discussion
Previous studies have shown that relevant associations exist between echocardiographic cardiac structure and function and the prognosis of patients with AF;12,14–17 however, they have not investigated these relationships among elderly patients with NVAF in the real world. Thus, the present prespecified, prospective substudy investigated the relationship between echocardiographic parameters of LA structure and function and clinical outcomes in patients from the ANAFIE Registry.
The main findings of this substudy including >1,400 elderly NVAF patients (aged >75 years) were that patients with NVAF and LAEF total ≤45.0% were at a significantly higher risk of CV events (HR: 2.19; P=0.021) and HF hospitalization (HR: 2.25; P=0.045) compared with those with LAEF total >45.0%. Additionally, the risk of all-cause death was significantly higher (HR: 1.69; P=0.048) in patients with max. LAVi >48.0 mL/m2
vs. max. LAVi ≤48.0 mL/m2. Additionally, the presence or absence of HF did not increase the risk of events, suggesting that the echocardiographic parameters of LA function and structure may be more useful as predictors of clinical outcomes than a history of HF among elderly patients with NVAF. These findings also suggest that LAEF total and max. LAVi should be part of the echocardiographic analysis of elderly patients with NVAF, given their predictive value. Furthermore, the present findings build on our previous analysis of the cardiac structural and functional characteristics of elderly Japanese patients with NVAF from the ANAFIE Registry.19
Nearly half (48.7%) of the patients included in this analysis had abnormal LA function and structure (i.e., max. LAVi >48.0 mL/m2
and LAEF total ≤45.0%), whereas those with sufficient LA function and structure (i.e., max. LAVi ≤48.0 mL/m2
and LAEF >45.0%) accounted for only 13%. As expected, the number of events tended to be higher when either LA function or structure was abnormal.
LA dysfunction (i.e., lower LAEF) has been reported to be associated with an increased risk of CV death and HF hospitalization;12 thus, our findings in elderly patients align with previous reports.12,23 Recent studies investigating the predictive value of LA functional measures among patients with HF and other CV diseases have shown that LAEF is an independent predictor of all-cause death and incident HF, and has superior prognostic value over LAVi.24,25 However, LAVi is also an important predictor of all-cause death among patients with AF and HF.26 The echocardiographic evaluation of max. LAVi alone may not be sufficient to predict clinical outcomes. However, the evaluation of LAEF total in conjunction with max. LAVi may contribute to better prediction. Thus, the combination of LAEF total and max. LAVi might be useful in the management of AF patients. Of note, the higher risk differs between patients with LAEF total ≤45.0% and those with max. LAVi >48.0 mL/m2; those with LAEF total ≤45.0% are at a higher risk of CV events and HF hospitalization, and those with max. LAVi >48.0 mL/m2
are at a higher risk of all-cause death.
A recent study of the LA structure and function in the general population over a median examination period >10 years showed that both maximum and minimum LA volumes increased over time and that aging and presence of AF were the most impactful accelerators of LA remodeling,27 which in turn increases the risk of HF, stroke, stroke recurrence, and death, among other outcomes.23 A recent study of Japanese patients with AF reported that patients who had HF events had a larger LAVi than patients without HF events. Furthermore, in that study, LA volume was not only found to be a significant predictor of future HF development but also had an incremental predictive effect for HF development over other conventional risk factors.28
The main limitations of the ANAFIE Registry have been published18 and are closely related to its observational design and the inclusion of only elderly Japanese patients with NVAF, which limits the generalizability of the results. The present substudy has additional limitations associated with using only baseline echocardiographic data and the lack of serial changes, as previously reported.19 Using the LAEF total threshold of the ENGAGE TIMI-48 study22 as the LAEF total threshold in this study is a limitation, and determining an appropriate threshold remains a topic that requires further attention in the future. Furthermore, the possibility that sinus rhythm was involved in the small number of events in patients with small atria and good contractility cannot be denied. It may also be the case that structural and functional parameters differed between paroxysmal and persistent/chronic AF. This was not explored in the current study and will be an interesting topic to explore in future research.
Conclusions
In elderly Japanese patients with NVAF, those with LAEF ≤45.0% were at higher risk of CV events and HF hospitalization, and those with LAVi >48.0 mL/m2
were at higher risk of all-cause death.
Acknowledgments
The authors thank all individuals (physicians, nurses, institutional staff and patients) involved in the ANAFIE Registry. They also thank IQVIA Services, Japan K.K. and EP-CRSU for their partial support in the conduct of this Registry, and Keyra Martinez Dunn, MD, of Edanz (www.edanz.com) for providing medical writing support, which was funded by Daiichi Sankyo Co. Ltd., in accordance with Good Publication Practice (GPP 2022) guidelines (https://www.ismpp.org/gpp-2022). In addition, the authors thank Daisuke Chiba of Daiichi Sankyo Co. Ltd. for support in the preparation of the manuscript.
Funding
This research was supported by Daiichi Sankyo.
Conflicts of Interest
K.H. received remuneration from Daiichi Sankyo, Nippon Boehringer Ingelheim, Pfizer, Bristol-Myers Squibb, Bayer, and Otsuka Pharmaceutical. H.K. declares no conflicts of interest associated with this article. H.I. received remuneration from Daiichi Sankyo, Bayer, Bristol-Myers Squibb, and a consultancy fee from Daiichi Sankyo. T. Yamashita received research funding from Bristol-Myers Squibb, Bayer, and Daiichi Sankyo, manuscript fees from Daiichi Sankyo and Bristol-Myers Squibb, and remuneration from Daiichi Sankyo, Bristol-Myers Squibb, Bayer, Ono Pharmaceutical, Novartis, Otsuka Pharmaceutical, Toa Eiyo. M.A. received research funding from Bayer and Daiichi Sankyo, and remuneration from Bristol-Myers Squibb, Nippon Boehringer Ingelheim, Bayer, and Daiichi Sankyo. H.A. received remuneration from Daiichi Sankyo. T.I. received research funding from Daiichi Sankyo and Bayer, and remuneration from Daiichi Sankyo, Bayer, Nippon Boehringer Ingelheim, and Bristol-Myers Squibb. Y.K. received remuneration from Daiichi Sankyo, Bristol-Myers Squibb, and Nippon Boehringer Ingelheim. K.O. received remuneration from Nippon Boehringer Ingelheim, Daiichi Sankyo, Johnson & Johnson, and Medtronic. W.S. received research funding from Bristol-Myers Squibb, Daiichi Sankyo, and Nippon Boehringer Ingelheim, and remuneration from Daiichi Sankyo, Pfizer Japan, Bristol-Myers Squibb, Bayer, and Nippon Boehringer Ingelheim. S.S. received remuneration from Bristol-Myers Squibb and Daiichi Sankyo. K.T. received remuneration from Daiichi Sankyo, Bayer, Bristol-Myers Squibb, Otsuka, Novartis, and Abbott Medical. A.H. participated in a course endowed by Boston Scientific Japan, has received research funding from Daiichi Sankyo and Bayer, and remuneration from Bayer, Daiichi Sankyo, Bristol-Myers Squibb, and Nippon Boehringer Ingelheim. M.Y. received remuneration from Nippon Boehringer Ingelheim, Daiichi Sankyo. T. Yamaguchi acted as an Advisory Board member of Daiichi Sankyo and received remuneration from Daiichi Sankyo and Bristol-Myers Squibb. S.T. received research funding from Nippon Boehringer Ingelheim and remuneration from Daiichi Sankyo, Sanofi, Chugai Pharmaceutical, Solasia Pharma, Bayer, Sysmex, Nipro, NapaJen Pharma, Atworking, Kringle Pharma, and Kaneka. T.K., Y.M., and A.T. are employees of Daiichi Sankyo. H.T. received research funding from Mitsubishi Tanabe Pharma, Nippon Boehringer Ingelheim, IQVIA Services Japan, MEDINET, Medical Innovation Kyushu, Kowa, Daiichi Sankyo, Johnson & Johnson and NEC, and consulting fees from Novartis Pharma, Ono Pharmaceutical, Bayer, Nippon Boehringer Ingelheim, and remuneration from Kowa, Teijin Pharma, Nippon Boehringer Ingelheim, Mitsubishi Tanabe Pharma, Pfizer Japan, Ono Pharmaceutical, Daiichi Sankyo, Novartis Pharma, Bayer, Otsuka Pharmaceutical, AstraZeneca, Nippon Rinsho, and had a leadership or fiduciary role in the Japanese Heart Failure Society.
T. Yamashita, T.I., W.S., A.H. and H.T. are members of Circulation Journal’s Editorial Team.
IRB Information
Ethics approval was obtained from all relevant institutional review boards, and all patients provided written informed consent and were free to withdraw from the Registry at any time. The principal ethics committee was The Ethics Committees of The Cardiovascular Institute (Tokyo, Japan; Approval no. 299).
Data Availability
The datasets used in the current analysis are available from the corresponding author upon reasonable request and after review by a committee led by the study sponsor.
1. Will the individual deidentified participant data (including data dictionaries) be shared?
→Yes
2. What data in particular will be shared?
→Individual participant data that underlie the results reported in this article, after deidentification (text, tables, figures, and appendices).
3. Will any additional, related documents be available? If so, what is it? (e.g., study protocol, statistical analysis plan, etc.)
→Study protocol
4. When will the data become available and for how long?
→Ending 36 months following article publication.
5. By what access criteria will the data be shared (including with whom)?
→The access criteria for data sharing (including requests) will be decided by a committee led by Daiichi Sankyo.
6. For what types of analyses, and by what mechanism will the data be available?
→Any purpose: Proposals should be directed to yamt-tky@umin.ac.jp To gain access, data requestors will need to sign a data access agreement.
Supplementary Files
Please find supplementary file(s);
https://doi.org/10.1253/circj.CJ-23-0084
References
- 1.
Essa H, Hill AM, Lip GYH. Atrial fibrillation and stroke. Card Electrophysiol Clin 2021; 13: 243–255.
- 2.
Schumacher K, Kornej J, Shantsila E, Lip GYH. Heart failure and stroke. Curr Heart Fail Rep 2018; 15: 287–296.
- 3.
Obeidat M, Burgess M, Lip GYH. Atrial fibrillation in heart failure: Focus on antithrombotic management. Heart Fail Clin 2020; 16: 107–120.
- 4.
Pongmoragot J, Lee DS, Park TH, Fang J, Austin PC, Saposnik G; Investigators of the Registry of the Canadian Stroke Network; University of Toronto Stroke Program for the Stroke Outcomes Research Canada (SORCan—www. sorcan.ca) Working Group. Stroke and heart failure: Clinical features, access to care, and outcomes. J Stroke Cerebrovasc Dis 2016; 25: 1048–1056.
- 5.
Kim WJ, Nah HW, Kim DH, Cha JK. Association between left ventricular dysfunction and functional outcomes at three months in acute ischemic stroke. J Stroke Cerebrovasc Dis 2016; 25: 2247–2252.
- 6.
Sharma JC, Fletcher S, Vassallo M, Ross I. Cardiovascular disease and outcome of acute stroke: Influence of pre-existing cardiac failure. Eur J Heart Fail 2000; 2: 145–150.
- 7.
Hart RG, Coull BM, Hart D. Early recurrent embolism associated with nonvalvular atrial fibrillation: A retrospective study. Stroke 1983; 14: 688–693.
- 8.
Paciaroni M, Agnelli G, Falocci N, Tsivgoulis G, Vadikolias K, Liantinioti C, et al. Early recurrence and major bleeding in patients with acute ischemic stroke and atrial fibrillation treated with non-vitamin-K oral anticoagulants (RAF-NOACs) Study. J Am Heart Assoc 2017; 6: e007034.
- 9.
Katsanos AH, Parissis J, Frogoudaki A, Vrettou AR, Ikonomidis I, Paraskevaidis I, et al. Heart failure and the risk of ischemic stroke recurrence: A systematic review and meta-analysis. J Neurol Sci 2016; 362: 182–187.
- 10.
Denham NC, Pearman CM, Caldwell JL, Madders GWP, Eisner DA, Trafford AW, et al. Calcium in the pathophysiology of atrial fibrillation and heart failure. Front Physiol 2018; 9: 1380.
- 11.
Hu CY, Wang CY, Li JY, Ma J, Li ZQ. Relationship between atrial fibrillation and heart failure. Eur Rev Med Pharmacol Sci 2016; 20: 4593–4600.
- 12.
Inciardi RM, Giugliano RP, Claggett B, Gupta DK, Chandra A, Ruff CT, et al. Left atrial structure and function and the risk of death or heart failure in atrial fibrillation. Eur J Heart Fail 2019; 21: 1571–1579.
- 13.
Rosenberg MA, Gottdiener JS, Heckbert SR, Mukamal KJ. Echocardiographic diastolic parameters and risk of atrial fibrillation: The Cardiovascular Health Study. Eur Heart J 2012; 33: 904–912.
- 14.
Hoit BD. Left atrial size and function: Role in prognosis. J Am Coll Cardiol 2014; 63: 493–505.
- 15.
Hoit BD. Assessment of left atrial function by echocardiography: Novel insights. Curr Cardiol Rep 2018; 20: 96.
- 16.
Osranek M, Bursi F, Bailey KR, Grossardt BR, Brown RD Jr, Kopecky SL, et al. Left atrial volume predicts cardiovascular events in patients originally diagnosed with lone atrial fibrillation: Three-decade follow-up. Eur Heart J 2005; 26: 2556–2561.
- 17.
Lang RM, Badano LP, Mor-Avi V, Afilalo J, Armstrong A, Ernande L, et al. Recommendations for cardiac chamber quantification by echocardiography in adults: An update from the American Society of Echocardiography and the European Association of Cardiovascular Imaging. J Am Soc Echocardiogr 2015; 28: 1–39.e14.
- 18.
Koretsune Y, Yamashita T, Akao M, Atarashi H, Ikeda T, Okumura K, et al. Baseline demographics and clinical characteristics in the All Nippon AF in the Elderly (ANAFIE) Registry. Circ J 2019; 8: 1538–1545.
- 19.
Hiasa KI, Kaku H, Kawahara G, Inoue H, Yamashita T, Akao M, et al. Echocardiographic structure and function in elderly patients with atrial fibrillation in Japan: The ANAFIE Echocardiographic Substudy. Circ J 2022; 86: 222–232.
- 20.
Inoue H, Yamashita T, Akao M, Atarashi H, Ikeda T, Okumura K, et al. Prospective observational study in elderly patients with non-valvular atrial fibrillation: Rationale and design of the All Nippon AF In the Elderly (ANAFIE) Registry. J Cardiol 2018; 72: 300–306. Corrigendum in J Cardiol 2022; 80: 375–376.
- 21.
Nagueh SF, Smiseth OA, Appleton CP, Byrd BF 3rd, Dokainish H, Edvardsen T, et al. Recommendations for the evaluation of left ventricular diastolic function by echocardiography: An Update from the American Society of Echocardiography and the European Association of Cardiovascular Imaging. J Am Soc Echocardiogr 2016; 29: 277–314.
- 22.
Gupta DK, Shah AM, Giugliano RP, Ruff CT, Antman EM, Grip LT, et al. Left atrial structure and function in atrial fibrillation: ENGAGE AF-TIMI 48. Eur Heart J 2014; 35: 1457–1465.
- 23.
Pana TA, McLernon DJ, Mamas MA, Bettencourt-Silva JH, Metcalf AK, Potter JF, et al. Individual and combined impact of heart failure and atrial fibrillation on ischemic stroke outcomes. Stroke 2019; 50: 1838–1845.
- 24.
Modin D, Sengeløv M, Jørgensen PG, Olsen FJ, Bruun NE, Fritz-Hansen T, et al. Prognostic value of left atrial functional measures in heart failure with reduced ejection fraction. J Card Fail 2019; 25: 87–96.
- 25.
Modin D, Pedersen S, Fritz-Hansen T, Gislason G, Biering-Sørensen T. Left atrial function determined by echocardiography predicts incident heart failure in patients with STEMI treated by primary percutaneous coronary intervention. J Card Fail 2020; 26: 35–42.
- 26.
Ramu B, Elwan AM, Coleman CI, Silverman DI, Gluck JA. The association between baseline left atrial volume index and all-cause mortality in patients with heart failure: A meta-analysis. Conn Med 2015; 79: 469–475.
- 27.
Olsen FJ, Johansen ND, Skaarup KG, Lassen MCH, Ravnkilde K, Schnohr P, et al. Changes in left atrial structure and function over a decade in the general population. Eur Heart J Cardiovasc Imaging 2021; 23: 124–136.
- 28.
Taniguchi N, Miyasaka Y, Suwa Y, Harada S, Nakai E, Kawazoe K, et al. Usefulness of left atrial volume as an independent predictor of development of heart failure in patients with atrial fibrillation. Am J Cardiol 2019; 124: 1430–1435.