論文ID: CJ-23-0134
論文ID: CJ-23-0134
Background: It remains unclear which comorbidities, other than lipid parameters, or combination of comorbidities, best predicts cardiovascular events in patients with known coronary artery disease (CAD) treated with statins. Therefore, we aimed to identify the nonlipid-related prognostic factors and risk stratification of patients with stable CAD enrolled in the REAL-CAD study.
Methods and Results: Blood pressure, glucose level, and renal function were considered as risk factors in the 11,141 enrolled patients. The primary endpoint was a composite of cardiovascular death, nonfatal myocardial infarction, nonfatal ischemic stroke, and unstable angina. The secondary composite endpoint was the primary endpoint and/or coronary revascularization. A significantly worse prognosis at the primary endpoint was observed in the estimated glomerular filtration rate (eGFR) ≤60 group, and the combination of eGFR ≤60 and HbA1c ≥6.0 was the worst (hazard ratio (HR) 1.66; P<0.001). However, even in the eGFR >60 group, systolic blood pressure (SBP) ≥140 mmHg met the secondary endpoint (HR 1.33; P=0.006), and the combination of eGFR ≤60 and HbA1c ≥6.0 was also the worst at the secondary endpoint (HR 1.35; P=0.002).
Conclusions: Regarding nonlipid prognostic factors contributing to the incidence of cardiovascular events in statin-treated CAD patients, renal dysfunction was the most significant, followed by poor glucose control and high SBP.
Lipid-lowering therapy with statins is one of the most effective treatment strategies for patients with or at high risk of atherosclerotic cardiovascular disease (ASCVD).1 Statin therapy for the primary and secondary prevention of ASCVD has consistently demonstrated significant reductions in low-density lipoprotein cholesterol (LDL-C), resulting in a decrease in the incidence of major cardiovascular events.2–9 Recent randomized controlled clinical trials (RCTs) have demonstrated the benefits of aggressive LDL-C reduction using high-intensity statins.7–9
However, even in patients treated with high-intensity statins, there is still a high cardiovascular risk called “residual risk”. In the Treating to New Targets (TNT) trial comparing the efficacy of high-dose vs. low-dose statin therapy in patients with stable coronary artery disease (CAD), 8.7% of patients randomized to receive intensive lipid-lowering with atorvastatin 80 mg had a major cardiovascular event during a 5-year period.8 Similar results were obtained in the Incremental Decrease in End Points Through Aggressive Lipid Lowering (IDEAL) trial.7 Thus, factors other than LDL-C reduction contribute to future cardiovascular events, making it important to identify this residual risk for stratification of patients and to determine the expected outcomes for individual patients.
The mechanisms underlying this residual cardiovascular risk in statin-treated patients include lipid- and nonlipid-related factors. Among the nonlipid-related factors, we focused on blood pressure, glucose levels, and renal function, alone or in combination, on residual cardiovascular risk in patients with CAD treated with statins. The aim of this study was to stratify each factor and determined which factors or their combination were associated with cardiovascular events in patients with stable CAD enrolled in the REAL-CAD (Randomized Evaluation of Aggressive or Moderate Lipid Lowering Therapy with Pitavastatin in Coronary Artery Disease) study.10
This study was a subanalysis of the REAL-CAD study, in which 13,054 Japanese patients with stable CAD were randomized to low-dose (1 mg/day) or high-dose (4 mg/day) pitavastatin, and data for 12,413 patients were analyzed.10 Briefly, eligible patients in the REAL-CAD study were 20- to 80-year-old males and females with stable CAD, defined as a history of acute coronary syndrome (ACS) or coronary revascularization >3 months prior or a clinical diagnosis of CAD with angiographically documented coronary artery stenosis of ≥75% diameter narrowing according to the American Heart Association Classification.11 In the current subanalysis, we enrolled 11,141 patients after excluding 1,272 patients because of missing data. The REAL-CAD study was approved by the Public Health Research Foundation ethics review committee (No. 9K0109) and by the ethics committees at all participating sites, and all patients provided written informed consent. The study was conducted in accordance with the Declaration of Helsinki.
Classification of ComorbiditiesHypertension was classified first as use of antihypertensive drugs; second, by systolic blood pressure (SBP): (1) ≤120 mmHg, (2) 120–129 mmHg, (3) 130–139 mmHg, and (4) ≥140 mmHg; and third, by pulse pressure: (1) ≤40 mmHg; (2) 40–49 mmHg, and (3) ≥50 mmHg. Diabetes mellitus (DM) was classified first as use of antidiabetic drugs, and second by HbA1c: (1) ≤6.0%; (2) 6.0–7.9%, and (3) ≥8.0%. Chronic kidney disease (CKD) was classified by estimated glomerular filtration rate (eGFR): (1) ≤30 mL/min/1.73 m2, (2) 30–60 mL/min/1.73 m2, and (3) >60 mL/min/1.73 m2.
EndpointsEndpoints for risk stratification were determined as follows: Event 1, primary endpoint (composite of cardiovascular death, nonfatal myocardial infarction, nonfatal ischemic stroke, and unstable angina requiring emergency hospitalization); Event 2, composite of Event 1 and clinically indicated coronary revascularization excluding target-lesion revascularization at sites of prior percutaneous coronary intervention; Event 3, death from any cause; Event 4, cardiovascular death; Event 5, cardiac death.
Statistical AnalysisThe following statistical analyses were performed to investigate the proposed working hypothesis by examining the relationship between the incidence of the major events (Events 1–5) and risk factors after adjusting for the effects of regulators. Blood pressure, glucose levels, and renal function were considered risk factors. As described in the Classification of Comorbidities, the risk factors were further subclassified and used in the sensitivity analyses. Pitavastatin dose (1 vs. 4 mg), age, sex, body mass index, LDL-C, high-density lipoprotein cholesterol, triglycerides, C-reactive protein, and heart rate were considered as regulatory variables. The 5 major events were analyzed separately using the following exploratory statistical methods.
In order to evaluate effect of risk factors as a whole on event incidence, a classification and regression tree (CART) was used to construct a set of “layers” consisting of an asymmetric combination of risk factors. The hazard ratio (HR) between each layer was examined after controlling for the effects of regulator variables.
Furthermore, from a clinical point of view, sensitivity analyses were also performed in some extracted layers of the model constructed from each data analysis. From the parameter estimates obtained from these statistical analyses, we evaluated the appropriate blood pressure, glucose levels, and renal function, and verified the working hypotheses related to the therapeutic strategy and improvement of poor prognosis. All tests were two-tailed, and a value of P<0.05 was considered statistically significant. All statistical analyses were conducted by statisticians (Hitoshi Obara and Tatsuyuki Kakuma) using StataMP 16 statistical software (StataCorp, College Station, TX, USA).
Among the 11,141 enrolled patients in the present study, there were 1,919 patients without antihypertensive drugs, 9,222 were taking antihypertensive drugs, 8,179 without antidiabetic drugs, and 2,962 were taking antidiabetic drugs (Figure 1). We performed statistical analyses in each group to identify which comorbidities or combination of comorbidities predicted worse prognosis.

Study flowchart. DM, diabetes mellitus; HT, hypertension.
The baseline characteristics of the 11,141 patients are summarized in Table 1. The mean age was 68.1±8.3 years, and 82.9% were male.
| Age, years | 68.1±8.3 |
| Male, n (%) | 9,233 (82.9) |
| Weight, kg | 65.2±11.2 |
| Body mass index, kg/m2 | 24.6±3.4 |
| Current smoking, n (%) | 1,778 (16.0) |
| Heart rate, beats/min | 69.6±11.6 |
| Systolic blood pressure, mmHg | 127.6±16.2 |
| Distribution, n (%) | |
| <120 mmHg | 3,251 (29.2) |
| 120–129 mmHg | 2,941 (26.4) |
| 130–139 mmHg | 2,586 (23.2) |
| ≥140 mmHg | 2,363 (21.2) |
| Diastolic blood pressure, mmHg | 72.9±10.9 |
| Pulse pressure, mmHg | 54.7±13.1 |
| Distribution, n (%) | |
| <40 mmHg | 1,110 (10.0) |
| 40–49 mmHg | 2,812 (25.2) |
| ≥50 mmHg | 7,219 (64.8) |
| Hemoglobin A1c | 5.9±0.9 |
| Distribution, n (%) | |
| <6.0% | 7,409 (66.5) |
| 6.0–7.9% | 3,423 (30.7) |
| ≥8.0% | 309 (2.8) |
| Estimated GFR | 65.9±18.3 |
| Distribution, n (%) | |
| <30 mL/min/1.73 m2 | 186 (1.7) |
| 30–60 mL/min/1.73 m2 | 4,073 (36.6) |
| >60 mL/min/1.73 m2 | 6,882 (61.8) |
| Laboratory tests | |
| Total cholesterol, mg/dL | 167.4±26.0 |
| LDL-C, mg/dL | 87.8±19.0 |
| HDL-C, mg/dL | 50.7±12.5 |
| Triglycerides, mg/dL | 145.0±90.7 |
| High-sensitivity C-reactive protein, mg/L | 1,595.7±4,889.7 |
| Comorbidities, n (%) | |
| Ischemic stroke | 779 (7.0) |
| Atrial fibrillation | 695 (6.2) |
| Peripheral artery disease | 803 (7.2) |
| Malignancies | 600 (5.4) |
| Chronic heart failure | 589 (5.3) |
| Myocardial infarction | 5,771 (51.8) |
| Medications, n (%) | |
| ACEI or ARB | 7,056 (63.3) |
| β-blocker | 4,342 (39.0) |
| Calcium-channel blocker | 4,861 (43.6) |
| Diuretic | 1,975 (17.7) |
| Dipeptidyl peptidase-4 inhibitor | 1,145 (10.3) |
| Glucagon-like peptide 1 receptor agonist | 188 (1.7) |
| Metformin | 777 (7.0) |
| Sodium-glucose cotransporter 2 inhibitor | 4 (0.0) |
| Sulfonylurea | 1,250 (11.2) |
| Thiazolidinedione | 858 (7.7) |
Data are n (%), median (interquartile range), or mean (SD). ACEI, angiotensin-converting enzyme inhibitor; ARB, angiotensin-receptor blocker; eGFR, estimated glomerular filtration rate; HDL-C, high-density lipoprotein cholesterol; LDL-C, low-density lipoprotein cholesterol.
As shown in Table 2 and Figure 2, a significantly worse prognosis in Event 1, primary endpoint occurred in the eGFR ≤60 group, and the combination of eGFR ≤60 and HbA1c ≥6.0 was the worst (HR 1.66; P<0.001). However, even in the eGFR >60 group, SBP ≥140 met Event 2 (HR 1.33; P=0.006), and the combination of eGFR ≤60 and HbA1c ≥6.0 was also the worst in Event 2, secondary endpoint (HR 1.35; P=0.002) (Table 2, Figure 3). Furthermore, eGFR ≤60 or HbA1c ≥6.0 was a significant predictor of all-cause death (Event 3), in which the combination of eGFR ≤60 and HbA1c ≥6.0 was also the worst (Table 2). For cardiovascular death (Event 4), the combination of eGFR ≤60 and HbA1c ≥6.0 and the combination of eGFR ≤60, HbA1c <6.0%, and SBP ≥130 were similarly and significantly worse (Table 2). For cardiac death (Event 5), eGFR ≤60 predicted worse prognosis; however, the combination of eGFR ≤60 and DM medication use was the worst (Table 2). Even in patients with eGFR >60, SBP <120 or the combination of SBP ≥120 and DM medicine use was also significant.
| HR | SE | P value | 95% CI | ||
|---|---|---|---|---|---|
| Event 1 | eGFR >60 & no DM medicine | Ref. | |||
| eGFR >60 & DM medicine | 1.19 | 0.16 | 0.209 | 0.91–1.55 | |
| eGFR ≤60 & HbA1c <6.0 | 1.29 | 0.15 | 0.028 | 1.03–1.62 | |
| eGFR ≤60 & HbA1c ≥6.0 | 1.66 | 0.21 | <0.001 | 1.29–2.13 | |
| Event 2 | eGFR >60 & SBP <140 | Ref. | |||
| eGFR >60 & SBP ≥140 | 1.33 | 0.14 | 0.006 | 1.09–1.62 | |
| eGFR ≤60 & HbA1c <6.0 | 1.14 | 0.1 | 0.135 | 0.96–1.34 | |
| eGFR ≤60 & HbA1c ≥6.0 | 1.35 | 0.13 | 0.002 | 1.12–1.64 | |
| Event 3 | eGFR >60 & HbA1c <6.0 | Ref. | |||
| eGFR >60 & HbA1c ≥6.0 | 1.46 | 0.22 | 0.014 | 1.08–1.97 | |
| eGFR ≤60 & HbA1c <6.0 | 1.42 | 0.2 | 0.013 | 1.08–1.87 | |
| eGFR ≤60 & HbA1c ≥6.0 | 1.97 | 0.3 | <0.001 | 1.47–2.64 | |
| Event 4 | eGFR >60 | Ref. | |||
| eGFR ≤60 & HbA1c ≥6.0 | 1.87 | 0.37 | 0.002 | 1.26–2.76 | |
| eGFR ≤60 & HbA1c <6.0 & SBP <130 | 0.92 | 0.24 | 0.73 | 0.55–1.52 | |
| eGFR ≤60 & HbA1c <6.0 & SBP ≥130 | 1.71 | 0.39 | 0.019 | 1.09–2.66 | |
| Event 5 | eGFR >60 & SBP ≥120 & no DM medicine | Ref. | |||
| eGFR >60 & SBP ≥120 & DM medicine | 2.8 | 1.13 | 0.01 | 1.28–6.16 | |
| eGFR >60 & SBP <120 | 3.32 | 1.2 | 0.001 | 1.64–6.73 | |
| eGFR ≤60 & no DM medicine | 3.4 | 1.12 | <0.001 | 1.78–6.50 | |
| eGFR ≤60 & DM medicine | 5.59 | 1.93 | <0.001 | 2.84–11.01 |
CI, confidence interval; DM, diabetes mellitus; HR, hazard ratio; SBP, systolic blood pressure; SE, standard error. Other abbreviations as in Table 1.
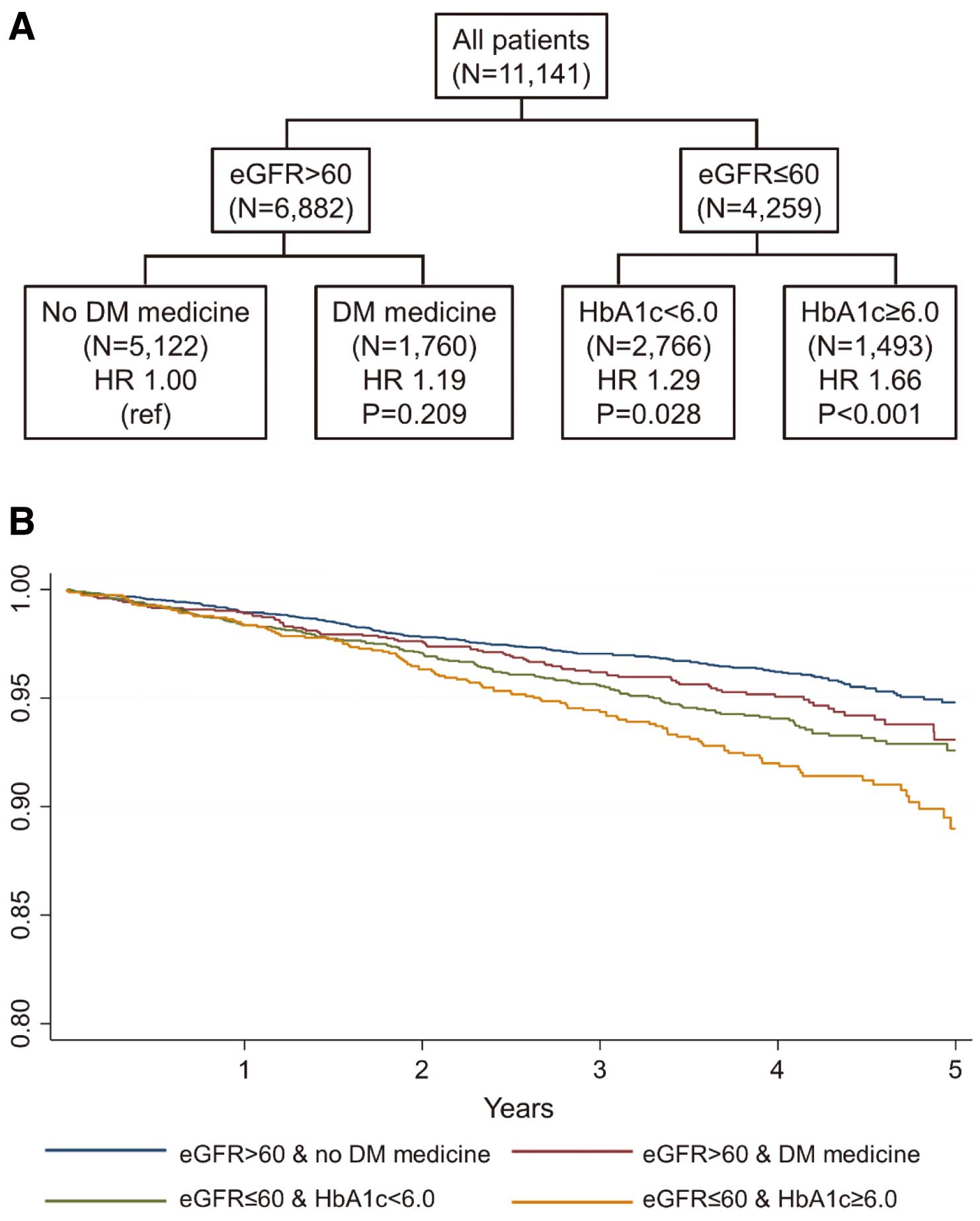
CART analysis for combined risk factors (A) and Kaplan-Meier curves (B) for Event 1 in whole study patients. CART, classification and regression tree; DM, diabetes mellitus; eGFR, estimated glomerular filtration rate; HR, hazard ratio.
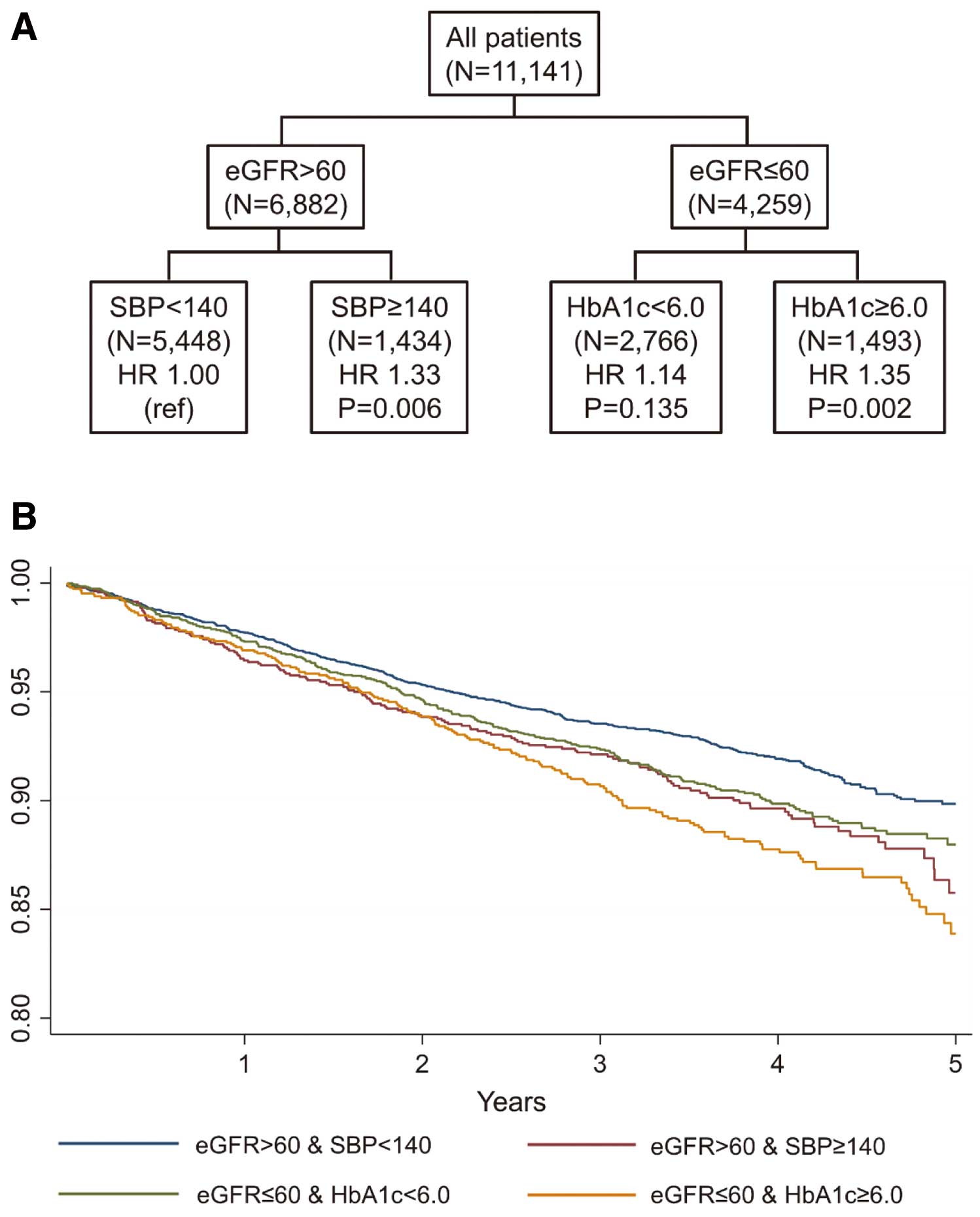
CART analysis for combined risk factors (A) and Kaplan-Meier curves (B) for Event 2 in whole study patients. CART, classification and regression tree; eGFR, estimated glomerular filtration rate; HR, hazard ratio; SBP, systolic blood pressure; HR, hazard ratio.
In the “no antihypertensive drug” group, no significant predictors were detected.
Antihypertensive Drug PopulationIn Event 1, the combination of eGFR ≤60 and HbA1c ≥6.0 was a significant predictor (Supplementary Table 1). In Event 1 and/or coronary revascularization (Event 2), the combination of eGFR >60 and SBP ≥140 was the only significant predictor (Supplementary Table 1). Either eGFR ≤60 or HbA1c ≥6.0 was a significant predictor of all-cause death (Event 3), in which the combination of eGFR ≤60 and HbA1c ≥6.0 was also the worst (Supplementary Table 1). For cardiovascular death (Event 4), eGFR ≤60 was the only predictor (Supplementary Table 1). In cardiac death (Event 5), SBP <120, DM medication use, or eGFR ≤60 predicted worse prognosis; however, the combination of eGFR ≤60 and DM medication use was again the worst (Supplementary Table 1).
No Antidiabetic Drug PopulationIn this population, eGFR ≤60 was the only significant predictor in Events 1 and 3 (Supplementary Table 2), and no predictor was observed in Event 2 (Supplementary Table 2). In cardiovascular death (Event 4) and cardiac death (Event 5), either SBP <120 or eGFR ≤60 was a predictor, in which eGFR ≤60 was the worst predictor (Supplementary Table 2).
Antidiabetic Drug PopulationIn this population, eGFR ≤60 was the only significant predictor in Events 1 and 5 (Supplementary Table 3) and an insignificant predictor in Events 3 and 4 (Supplementary Table 3). No predictor was detected for Event 2.
The REAL-CAD study is currently the largest RCT to compare clinical outcomes between patients with known CAD treated with high- and low-dose pitavastatin therapy. High-dose pitavastatin reduced the primary endpoint (a composite of cardiovascular death, nonfatal myocardial infarction, nonfatal ischemic stroke, and unstable angina requiring emergency hospitalization) by 19% during a median follow-up period of 3.9 years10 when compared with low-dose pitavastin. In the present subanalysis of the REAL-CAD study, we explored nonlipid-related prognostic factors associated with future cardiovascular events in patients with CAD who were treated with pitavastatin and had a modestly controlled level of LDL-C. Using the CART method, we found that poor renal function was the strongest predictor, followed by glucose level or SBP, for future cardiovascular events. The combination of eGFR ≤60 and HbA1c ≥6.0 had the worst incidence of the primary endpoint. Even in the eGFR >60 group, SBP ≥140 met the secondary endpoint, and the combination of eGFR ≤60 and HbA1c ≥6.0 was also the worst in the secondary endpoint. To the best of our knowledge, this is the first study to demonstrate the importance of combined risk stratification of renal function, glucose level, and high blood pressure in CAD patients treated with statin. The present findings seemed to be useful for identifying those patients requiring more aggressive treatment and management.
Importance of Renal Function in Statin-Treated PatientsCKD is a recognized independent predictor of cardiovascular events in patients after myocardial infarction12 or coronary revascularization.13 Although the prevalence of CKD in the present study was 38.2% (based on eGFR ≤60 mL/min/1.73 m2), which is higher than in previous statin trials (range 26.2–32.2%),14,15 our CART analysis consistently identified eGFR ≤60 as the primary discriminator for all event analyses, including all-cause death, cardiovascular death, and cardiac death in statin-treated patients with CAD. Previous large-scale statin trials have revealed that patients with CKD had a significantly higher incidence of cardiovascular events than those without CKD.14,15 Our study provides further support that CKD is potentially a primary residual risk factor of cardiovascular events in patients with CAD treated with statins.
Recently, Wakabayashi et al examined the effect of major risk factors (blood pressure, glucose level, and renal function) on the residual cardiovascular risk in patients with high-dose (4 mg/day) pitavastatin in the REAL-CAD study, and identified eGFR ≤60 as the only significant predictor in patients with CAD receiving high-dose pitavastatin.16 Although our study included patients with low- and high-dose pitavastatin, which more closely reflects real-world clinical practice, we also found eGFR ≤60 to be a primary discriminating factor.
It has been reported that high-intensity statin therapy has favorable effects on cardiovascular outcomes in patients with CAD without CKD; however, there are conflicting results in patients with CAD and CKD. A substudy of the TNT compared clinical outcomes between CAD patients treated with high- and low-dose atorvastatin, showing that high-dose atorvastatin reduced the incidence of cardiovascular events in patients with CAD with and without CKD. However, a substudy of the IDEAL study failed to show the benefit of a high-dose of atorvastatin compared with the usual dose of simvastatin in patients with CAD and CKD. Recently, as a substudy of the REAL-CAD study, our group categorized CAD patients in more detail by eGFR as follows: eGFR ≥60, eGFR ≥45 and <60, and eGFR <45 mL/min/1.73 m2.17 High-dose pitavastatin therapy reduced cardiovascular events in CAD patients irrespective of the eGFR level, although the numerical effect of the reduced events was smaller in patients with lower eGFR. The finding that CAD patients with CKD are less likely to respond to statin therapy than those without CKD is supported by the present study.
Worse cardiovascular outcomes in patients with CKD may be determined by the presence of more vulnerable features of coronary plaques and their unfavorable response to statin therapy. An intravascular imaging study using optical coherence tomography (OCT), demonstrated that patients with CKD had larger lipid profiles and a higher prevalence of calcium, cholesterol crystals, and plaque disruption than those without CKD.18 A substudy of the Providing Regional Observations to Study Predictors of Events in the Coronary Tree (PROSPECT) study showed that patients with CKD had a higher prevalence of necrotic core and dense calcium, and worse clinical outcomes.19 Recently, Minami et al conducted a serial OCT study to explore the clinical predictors of changes in the fibrous cap area of plaque in patients with CAD undergoing statin therapy.20 Their study showed that baseline presentation with CKD independently predicted unfavorable reduction in thin-cap area, indicating a failure to thicken and stabilize fibrous tissue within the fibrous cap in patients with CKD, even after statin treatment.
Importance of Diabetic Condition in Statin-Treated PatientsThere have been few reports on the effect of HbA1c levels on cardiovascular outcomes in statin-treated patients with CAD. Noguchi et al investigated the prognostic risk factors for cardiovascular events after discharge in patients with ACS using a Japanese ACS registry.21 In their study, 78.6% of the patients were treated with statins at discharge, and both DM and nonDM patients were included. Among the risk factors, HbA1c >7.0% was independently associated with an increased incidence of cardiovascular events after ACS, and the optimal cutoff value of HbA1c was determined to be 6.4%. Menon et al evaluated the utility of HbA1c on cardiovascular events in patients with DM and CAD enrolled in the Assessment of Clinical Effects of Cholesteryl Ester Transfer Protein Inhibition with Evacetrapib in Patients at a High Risk for Vascular Outcomes (ACCELERATE) trial.22 Although 96.1% of the study patients were treated with statins, increased baseline HbA1c levels were strongly associated with the occurrence of cardiovascular events. The results of those studies support our findings.
Some caution is needed in the interpretation of our findings showing HbA1c ≥6.0 as a major discriminator, because current guidelines recommend <7.0% as an optimal HbA1c level for secondary prevention.23 The present study included patients with and without DM and did not examine the effect of HbA1c on cardiovascular outcomes in patients with and without DM separately. It is even more important to determine the target glycemic goal individually, taking into consideration other risk factors including age, organ dysfunction, risk of hypoglycemia, and social and familial support systems.23 Further studies are needed to separately examine the significance of HbA1c levels on cardiovascular outcomes in statin-treated CAD patients with and without DM, or those without antidiabetic drugs.
Importance of SBP in Statin-Treated PatientsOur CART analysis identified SBP as an important discriminator of major cardiovascular events in statin-treated patients with CAD. In all study patients, SBP ≥140 met Event 2, even in the eGFR >60 group. In cardiovascular death, the combination of eGFR ≤60, HbA1c <6.0%, and SBP ≥130 was significantly worse. Similarly, even in the subgroup of patients taking antihypertensive drugs, a combination of eGFR >60 and SBP ≥140 was the only significant predictor in Event 2. On the other hand, our study showed that low SBP (SBP <120) was also significant for cardiac death in all study patients, in those taking antihypertensive drugs, and in those not taking antidiabetic drugs. These results indicate that modest SBP control can improve clinical outcomes in CAD patients treated with statins.
The relationship between blood pressure and cardiovascular outcomes has been shown in some studies to follow a J- or U-shaped curve, with an increased event rate at very low and very high blood pressure in patients with stable CAD. In contrast to our findings, 2 subanalyses of statin RCTs in patients with CAD showed the J-curve phenomenon with higher event rates at low and high diastolic and systolic blood pressures for the composite endpoint of major cardiovascular events.24,25 These differences may be explained by differences in blood pressure parameters. We used only baseline blood pressure data as the blood pressure parameter, whereas the 2 subanalyses of statin RCTs used average follow-up blood pressure variables. Bangalore et al analyzed patients enrolled in the Pravastatin or atorvastatin Evaluation and Infection Therapy–Thrombolysis In Myocardial Infarction (PROVE IT-TIMI) 22 trial and examined the relationship between average follow-up blood pressure/baseline blood pressure and cardiovascular outcomes.24 A J- or U-shaped relationship was found with average follow-up blood pressure variables, but not with baseline blood pressure variables. Our current study and the previous statin studies24,25 have shown that SBP lowering prevents major cardiovascular events. Furthermore, we need to be aware of the considerations for CAD patients with low SBP to improve clinical outcomes. Further studies are needed to determine whether high and low blood pressures are causal risk factors for an increased incidence of cardiovascular events in statin-treated patients with CAD.
Improving the Clinical Outcomes in Patients With CAD Treated With StatinAs demonstrated in the REAL-CAD study, patients on low-dose statins should be switched to higher-dose statins. Likewise, physicians need to recognize the importance of renal function, blood pressure, and HbA1c levels in improving clinical outcomes, irrespective of low- or high-dose statins.
Based on the results of the present study, it is unclear whether treatment for renal dysfunction improves clinical outcomes in CAD patients. Our CART analyses indicated that the prevention of worsening renal function by maintaining eGFR >60 is associated with better outcomes.
Optimal medical management of blood pressure and glucose is important to prevent worsening renal function. In patients with CKD, the aim of blood pressure control is to both protect against the cardiovascular risks of hypertension (stroke, heart failure, CAD) and delay progressive loss of eGFR.26 Lifestyle modification (salt restriction, weight normalization, regular exercise, reduction in alcohol intake, and smoking cessation) should be an integral part of the therapy for blood pressure control. Treatment with angiotensin-converting enzyme inhibitors (ACEIs) or angiotensin receptor blockers (ARBs) is also recommended for patients with diabetes, hypertension, and albuminuria.27 Recently, the efficacy of sodium-glucose cotransporter 2 inhibitors on renal outcomes has been reported in nondiabetic patients with CKD28 or heart failure.29 Early initiation of these treatments may prevent worsening renal function in patients with CAD.
A guideline has recommended that patients with stable CAD and hypertension should be treated with β-blockers and ACEIs/ARBs.30 Patients with clinical signs of heart failure or left ventricular ejection fraction (LVEF) <40% should be also treated with guideline-directed medical therapy (GDMT).23 In the current study, ACEIs/ARBs and β-blockers were administered to 63.3% and 39.0% of patients, respectively. In patients who are indicated for the GDMTs, thorough implementation of such treatment may improve clinical outcomes. On the other hand, patients with stable CAD and lower SBP, such as <120 mmHg, especially those with clinical signs of heart failure or reduced LVEF, should be cautious with ACEIs/ARBs and β-blockers that further lower SBP. It may be necessary to check for signs of hypotension or bradycardia, biomarkers such as the B-type natriuretic peptide level, and left ventricular function more frequently in CAD patients with lower SBP treated with the GDMTs to improve clinical outcomes.31
Study LimitationsThere are several to note. First, this was a post-hoc analysis and was not prospectively designed. Second, the results came from the analysis of data obtained at entry of subjects to the main study, and we did not take into consideration possible changes in eGFR, blood pressure, HbA1c, and other covariates during the follow-up period. Third, we did not include other risk factors, such as smoking status, in the CART analysis. We focused on blood pressure, renal function, and glucose level among the various risk factors of patients with CAD, which was approved by the steering committee. Fourth, we identified eGFR ≤60 as the primary discriminator for cardiovascular events. A decreased eGFR strongly correlates with aging. Our analyses were adjusted for age, but it is unclear whether the same results would be obtained for younger and elderly patients. Further large-scale studies are needed to examine the significance of eGFR on cardiovascular outcomes in statin-treated CAD patients, separately for younger and elderly populations. Fifth, low SBP itself may not be bad. Rather, the pathophysiology that causes low SBP may be bad. In the REAL-CAD study, however, patients with poor condition, such as with severe congestive heart failure (EF <30% or NYHA class ≥3), were excluded at study entry. Caution is needed when interpreting the results of the current substudy, in which both low SBP and high SBP affected the clinical outcomes. It is unclear whether modest SBP control improves clinical outcomes in CAD patients treated with statins. Our study was from a randomized trial comparing the effect of high- or low-dose statins in patients with CAD. The management of blood pressure was left to the discretion of the treating physician. Sixth, β-blockers or ACEIs/ARBs are considered suitable to be prescribed for patients with hypertension or heart failure. Because the data did not reveal the purpose of these medications, patients treated with β-blockers or ACEIs/ARBs were categorized as the antihypertensive population. Seventh, we excluded patients on hemodialysis because of safety concerns related to the potential toxic effects of high-intensity statins in patients with reduced renal clearance, particularly because the benefits of statins in patients on hemodialysis are unclear. Finally, the presence of proteinuria or albuminuria is associated with adverse clinical outcomes in patients without reduced eGFR; however, urine measurements were not systematically performed in the present study.
According to our CART analysis, the presence of CKD, the level of HbA1c, and SBP were significant and mutually independent predictors of long-term cardiovascular events in patients with CAD treated with statins.
The authors thank all patients and investigators who participated in this study.
The Randomized Evaluation of Aggressive or Moderate Lipid Lowering Therapy with Pitavastatin in Coronary Artery Disease (REAL-CAD) study was funded by the Comprehensive Support Project for Clinical Research of Lifestyle-Related Disease of the Public Health Research Foundation. The company manufacturing the study drug (Kowa Pharmaceutical Co., Ltd.) was one of the entities providing financial support for Public Health Research Foundation projects but was not involved in the study design, analysis, data interpretation, or manuscript preparation.
Y.J.A., Y.F., S.Y., H.D., H.S., R.N. are members of Circulation Journal’s Editorial Team. Y.J.A. received research grants from Abbott Medical Japan LLC, Amgen Astellas BioPharma, Apex International K.K., Boston Scientific Japan K.K., Daiichi Sankyo Co., Ltd., Edwards Lifescience Co., Fujifilm Toyama Chemical Co., Ltd., MC Inc., Mitsubishi Tanabe Pharma Co., Medtronic Japan Co., Ltd., National Cerebral and Cardiovascular Center, Nippon Boehringer lngelheim Co., Ltd., Nihon Medi-Physics Co., Ltd., Nihon Koden K.K., and Otsuka Pharmaceutical Co., Ltd.; honoraria from Ono Pharmaceutical Co., Ltd., Otsuka Pharmaceutical Co., Ltd., and Novartis Pharma K.K. Y.F. received research grants from Sanofi KK, and Shionogi & Co., Ltd.; honoraria from the Public Health Research Foundation, AstraZeneca KK, Eisai Co., Ltd., Kowa Pharmaceutical Co., Ltd.; research grants and honoraria from MSD KK, Otsuka Pharmaceutical Co., Ltd., Daiichi Sankyo Co., Ltd., Sumitomo Dainippon Pharma Co., Ltd., Teijin Pharma Ltd., Bayer Yakuhin, Ltd., Mochida Pharmaceutical Co., Ltd., Astellas Pharma Inc., Sanwa Kagaku Kenkyusho Co., Ltd., Takeda Pharmaceutical Co., Ltd., Mitsubishi Tanabe Pharma Corp., Pfizer Japan Inc., Ono Pharmaceutical Co., Ltd., and AstraZeneca KK. I.S. received honoraria from GlaxoSmithKline, AstraZeneca, Takeda, Bayer, Pfizer, Bristol-Myers Squibb, Boehringer lngelheim, MSD, Kyowa Hakko Kirin, Daiichi Sankyo, Novartis, Sanofi, Kowa, Shionogi, Kissei, Astellas, Amgen, Ono, Otsuka, Novonordisk, Mochida, Teijin, Sysmex, Nipro, Kyorin, Fuji and Sumitomo Dainippon; research grants from Public Health Research Foundation, Kowa, National Cerebral and Cardiovascular Center and Medical Informatics Study Group. H.D. received honoraria from Amgen Astellas BioPharma KK, Daiichi Sankyo Co., Ltd., Kowa Pharmaceutical Co., Ltd., and MSD KK; research grants from Canon, Glory, and Fujifilm Holdings Co., Ltd; scholarship grants from Eisai Co., Ltd., Bayer Yakuhin, Ltd., and Daiichi Sankyo; courses endowed by Phillips, Resmed, Fukuda Denshi, Asahikasei, Inter-Reha, and Toho Holdings Co., Ltd. H.S. received research grants from Shionogi & Co., Ltd., Teijin Pharma Ltd., Astellas Pharma Inc., and Otsuka Pharmaceutical Co., Ltd.; honoraria from Kowa Pharmaceutical Co., Ltd., Sanofi KK, AstraZeneca KK, and Bayer Yakuhin, Ltd.; research grantd and honoraria from MSD KK, Mitsubishi Tanabe Pharma Corp., and Daiichi Sankyo Co., Ltd.; research grant, other research support, and honoraria from the Public Health Research Foundation. T. Kimura received research grants from Sumitomo Dainippon Pharma Co., Ltd., Astellas Pharma Inc., Otsuka Pharmaceutical Co., Ltd., Mitsubishi Tanabe Pharma Corp., and Takeda Pharmaceutical Co., Ltd.; other research support and honoraria from Kowa Pharmaceutical Co., Ltd., and Bayer Yakuhin, Ltd.; research grant, other research support, and honoraria from MSD KK, Sanofi KK, Mochida Pharmaceutical Co., Ltd., Daiichi Sankyo Co., Ltd., the Public Health Research Foundation, and Amgen Astellas BioPharma KK. S.I. received research grant and honoraria from the Public Health Research Foundation. R.N. received honoraria from Kowa Pharmaceutical Co., Ltd., Takeda Pharmaceutical Co., Ltd., Bayer Yakuhin, Ltd., Daiichi Sankyo Co., Ltd., Shionogi & Co., Ltd., MSD KK, Mitsubishi Tanabe Pharma Corp., Amgen Astellas BioPharma KK, Eisai Co., Ltd., Astellas Pharma Inc., Sumitomo Dainippon Pharma Co., Ltd., and Mochida Pharmaceutical Co., Ltd.; honoraria and expert witness fee from the Public Health Research Foundation. Other coauthors report no conflicts.
The REAL-CAD study was approved by the Institutional Review Board of the Public Health Research Foundation (reference no. 9K0109) and by ethics committees at all participating sites.
The deidentified participant data for this study will not be shared.
Please find supplementary file(s);
https://doi.org/10.1253/circj.CJ-23-0134