論文ID: CJ-23-0147
論文ID: CJ-23-0147
Background: Although right ventricular (RV) enlargement may affect RV diastolic dysfunction assessed by end-diastolic forward flow (EDFF) in patients with repaired tetralogy of Fallot (TOF), EDFF may also be modified by left ventricular (LV) hemodynamics. We hypothesized that EDFF is affected by LV hemodynamics, not limited to RV diastolic stiffening.
Methods and Results: Among 145 consecutive patients with repaired TOF who underwent catheterization, hemodynamic properties in 47 with consistent EDFF and 75 without EDFF were analyzed. Compared with patients without EDFF, those with EDFF had a large RV volume with a high regurgitant fraction. Although cardiac index and central venous pressure (CVP) were similar, contrast injection augmented CVP and LV end-diastolic pressure (EDP) in patients with vs. those without EDFF, suggesting compromised diastolic reserve. In patients with EDFF, the velocity–time integral (VTI) of EDFF was positively correlated with LVEDP and systemic vascular resistance, in addition to RV EDP. EDFF-VTI was correlated with hepatic venous wedge pressure and markers of hepatic dysfunction. Subanalysis of the older (≥6 years) half of the study cohort revealed that EDFF was associated with bi-atrial enlargement independent of RV volume, highlighting the pronounced role of EDFF on the diastolic property in the aged cohort.
Conclusions: EDFF-VTI in patients with repaired TOF reflects RV diastolic dysfunction, affected by the left heart system. EDFF-VTI indicates blood stagnation, which may be attributed to end-organ damage.
Although most patients with tetralogy of Fallot (TOF) survive for more than 20 years, significant morbidity is reported in middle-aged patients.1–3 Unsatisfactory outcomes may be attributed to augmented biventricular afterload,4,5 unstable cerebral circulation,6 excessive right ventricular load due to pulmonary regurgitation (PR), and residual pulmonary stenosis.7,8 In particular, PR in patients with repaired TOF is associated with cardiovascular events, including hospitalization with chronic heart failure, sudden cardiac arrest, and ventricular arrhythmias.1,3,9
End-diastolic forward flow (EDFF) is a marker for an uncompliant right ventricle (RV) acting as a conduit during atrial contraction, producing forward flow in the pulmonary artery.10 Although EDFF was originally used to assess RV restrictive physiology postoperatively in patients after TOF repair, recent meta-analyses have revealed that EDFF is predominantly observed in patients with RV eccentric remodeling induced by PR.11 Although EDFF may be observed when the RV volume becomes large enough to steepen the end-diastolic pressure (EDP)-volume relationship, the RV diastolic property is possibly affected by other hemodynamic factors, including innate RV stiffness regardless of volume load, left ventricular (LV) diastolic property, and other loading conditions specific for repaired TOF, such as augmented afterload or arterial stiffening.5 In this study we tested the hypothesis that EDFF in patients with repaired TOF reflects not only secondary RV restrictive physiology due to volume loading, but also the other hemodynamic burdens.
We enrolled 145 consecutive patients with repaired TOF who underwent cardiac catheterization between 2011 and 2021 at the Division of Pediatric Cardiology, Department of Pediatrics, Iwate Medical University. EDFF was measured using an archived echocardiogram adjacent to cardiac catheterization, and its interaction with catheter-derived hemodynamic data was analyzed. In the Division of Pediatric Cardiology, echocardiograms for congenital heart diseases during the relevant period were acquired by trained sonographers specializing in pediatric cardiology. EDFF was measured in the main pulmonary artery at end-diastole, as described previously, and considered to be positive when maximum forward flow velocity in 3 consecutive cardiac cycles was at least 30 cm/s and the flow velocities of the remaining beats were a positive number (E-TOF).10,12,13 The velocity, time, and velocity-time integral (VTI) during 3 consecutive beats were averaged for analysis. To assess the significance of EDFF, patients with E-TOF were compared to patients with repaired TOF in whom forward flow during 3 consecutive beats was absent (N-TOF). Because the EDFF may be affected by ventilation and imaging quality, patients with obscure diastolic forward flow, including those with a maximum velocity <30 cm/s, those with a maximum EDFF velocity >30 cm/s but not consistently positive for 3 consecutive beats, and those for whom 3 consecutive beats were unavailable were excluded from this study to minimize reference bias, as well as selection bias, that could originate from individual variations in imaging quality due to the study design.
Because EDFF may be affected by the strength of the atrial contraction, the surrogate for transtricuspid blood flow volume during atrial contraction was calculated by multiplying the VTI of the tricuspid A-wave by the tricuspid annular area. The tricuspid annular area was calculated as follows, under the assumption of an ellipse:
RA systolic blood flow = tricuspid A-wave VTI × d1 × d2 × π/4
where d1 and d2 are the tricuspid valve diameters measured in the 4-chamber view and RV longitudinal view, respectively, using echocardiography.
Cardiac catheterization was performed under a standard protocol using general anesthesia by anesthesiologists specializing in congenital heart disease. After standardized hemodynamic assessments, LVEDP and inferior vena cava pressure were simultaneously measured before and after contrast imaging of the LV, and pressure changes were recorded. RV and LV volumes were measured using Graham’s and the area-length methods, respectively, and the volume was indexed to body surface area.
This study was approved by the Ethics Committee of Iwate Medical University (MH2020-238).
Statistical AnalysisContinuous data are presented as the mean±SD and categorical data are presented as numbers and percentages. For comparisons between 2 groups, Student t-tests or Mann-Whitney U tests were used as appropriate. A Chi-squared test was used for categorical data. The relationship between EDFF and the hemodynamic index was analyzed using linear regression analysis. Two-tailed P<0.05 was considered as statistically significant. All statistical analyses were performed using JMP Pro 16.2.0 (SAS Institute, Inc., Cary, NC, USA).
Among 145 consecutive patients with repaired TOF, EDFF was positive in 47 patients (E-TOF) and was absent in 75 patients (N-TOF). The remaining 23 patients (16%) were excluded from the study due to either inconsistencies over 3 consecutive beats or the unavailability of 3 consecutive beats. There were no differences in age, body size, mean blood pressure, heart rate, cardiothoracic ratio on chest radiography, and QRS duration on the electrocardiogram (ECG) between the 2 groups (Table 1). E-TOF patients were more likely to be repaired with a transannular patch using an expanded polytetrafluoroethylene (ePTFE) graft, whereas those with N-TOF were more likely to be repaired with the Rastelli procedure using a handmade ePTFE valved conduit.
| E-TOF | N-TOF | P value | |
|---|---|---|---|
| No. patients | 47 | 75 | |
| Age at catheterization (years) | 6.3 [2.9–15.8] | 6.7 [2.4–15.1] | 0.78 |
| Age at repair (years) | 1.3 [0.8–2.6] | 1.4 [0.8–3.1] | 0.41 |
| No. females | 26 | 27 | 0.06 |
| Body weight (kg) | 27.0±18.3 | 29.7±22.9 | 0.51 |
| Body surface area (m2) | 0.91±0.44 | 0.97±0.52 | 0.57 |
| Type of repair (n) | |||
| Transannular patch | 12 | 14 | 0.36 |
| Valve sparing | 20 | 22 | 0.13 |
| Rastelli operation | 9 | 29 | 0.02 |
| Reoperation | 3 | 7 | 0.56 |
| Unknown | 3 | 3 | 0.55 |
| Heart rate (beats/min) | 86.2±22.1 | 89.4±19.8 | 0.42 |
| Mean blood pressure (mmHg) | 64.6±13.4 | 62.2±11.0 | 0.29 |
| Natriuretic peptides (pg/mL) | |||
| BNP | 47.6±44.3 | 42.0±36.3 | 0.45 |
| NT-pro BNP (n) | 256.3±251.6 (24) | 336.2±348.8 (29) | 0.35 |
| ANP (n) | 88.6±75.9 (42) | 73.5±44.0 (72) | 0.18 |
| Cardiothoracic ratio (%) | 56.6±4.7 | 55.8±5.4 | 0.4 |
| QRS duration (ms) | 127.7±30.9 | 119.4±46.8 | 0.32 |
| Arrhythmia requiring treatment (n) | 2 | 2 | |
Unless indicated otherwise, data are given as the mean±SD or median [interquartile range]. ANP, atrial natriuretic peptide; BNP, brain or B-type natriuretic peptide; E-TOF, patients with positive end-diastolic forward flow; n, number of patients with available data in case of missing data; N-TOF, patients without positive end-diastolic forward flow; NT-proBNP, N-terminal pro B-type natriuretic peptide.
Blood pressure, cardiac index, RV systolic pressure, and RVEDP were similar between the 2 groups (Table 2), as were LV systolic pressure, LVEDP, stroke volume index (SVI), and LVEDVI. Consistent with previous reports,11 patients in the E-TOF group had a markedly larger RV end-diastolic volume index (RVEDVI) and higher SVI than patients in the N-TOF group, mediated by the high pulmonary regurgitant fraction in E-TOF. Echocardiography revealed a trend for larger bilateral atrial volume in E-TOF than N-TOF, suggesting impaired ventricular diastolic properties in both ventricles (Table 2). Although the age distribution was similar between the 2 groups, we subdivided patients based on age into those aged ≥6 years and those aged <6 years and compared hemodynamics between them (Table 3). Among patients aged ≥6 years, EDFF-positive patients had a larger bilateral atrium with a similar biventricular size, suggesting an association with ventricular stiffening, which is relatively independent of volume loading. Meanwhile, in the patients aged <6 years, EDFF positivity was associated with a markedly larger RVEDVI, suggesting ventricular stiffening associated with RV enlargement.
| E-TOF | N-TOF | P value | |
|---|---|---|---|
| Catheterization | |||
| CVP (mmHg) | 5.4±2.2 | 5.6±1.8 | 0.70 |
| RVSP (mmHg) | 40.0±2.5 | 42.2±2.5 | 0.49 |
| RVEDP (mmHg) | 6.49±3.0 | 7.05±3.1 | 0.32 |
| Pulmonary RF (%) | 31.8±16.7 | 21.6±26.7 | 0.03 |
| RVSVI (mL/m2) | 56.5±2.4 | 48.7±1.9 | 0.01 |
| RVEDVI (mL/m2) | 167.5±7.0 | 141.8±5.5 | 0.0046 |
| RVEF (%) | 54.8±7.3 | 54.6±7.8 | 0.93 |
| LVSP (mmHg) | 83.2±16.9 | 82.3±14.8 | 0.77 |
| LVEDP (mmHg) | 10.1±4.2 | 10.4±4.2 | 0.76 |
| LVSVI (mL/m2) | 54.4±8.9 | 50.8±11.3 | 0.07 |
| LVEDVI (mL/m2) | 136.2±24.6 | 131.4±31.3 | 0.38 |
| LVEF (%) | 59.8±6.3 | 57.4±5.6 | 0.029 |
| CI (L/min/m2) | 3.1±0.8 | 3.0±0.7 | 0.51 |
| Echocardiogram | |||
| TV e’ velocity (cm/s) (n) | 0.11±0.03 (35) | 0.10±0.04 (36) | 0.09 |
| RAVI (mL/m2) (n) | 14.7±2.7 (38) | 13.3±3.2 (41) | 0.04 |
| MV e’ velocity (cm/s) (n) | 0.10±0.02 (35) | 0.09±0.02 (38) | 0.03 |
| LAVI (mL/m2) (n) | 13.7±3.3 (38) | 12.4±2.9 (41) | 0.06 |
Unless indicated otherwise, data are given as the mean±SD. CI, cardiac index; CVP, central venous pressure; E-TOF, patients with positive end-diastolic forward flow; LAVI, left atrial volume index; LVEDP, left ventricular end-diastolic pressure; LVEDVI, left ventricular end-diastolic volume index; LVEF, left ventricular ejection fraction; LVSP, left ventricular systolic pressure; LVSVI, left ventricular stroke volume index; n, number of patients with available data in case of missing data; MV, mitral valve; N-TOF, patients without positive end-diastolic forward flow; RAVI, right atrial volume index; RF, regurgitant fraction; RVEDP, right ventricular end-diastolic pressure; RVEDVI, right ventricular end-diastolic volume index; RVEF, right ventricular ejection fraction; RVSP, right ventricular systolic pressure; RVSVI, right ventricular stroke volume index; TV, tricuspid valve.
| EDFF+ | EDFF− | P value | |
|---|---|---|---|
| Age <6 years | |||
| LVEDVI (mL/m2) | 85.4±13.7 | 81.9±18.7 | 0.36 |
| LVEF (%) | 59.7±5.7 | 57.7±5.2 | 0.20 |
| LAVI (mL/m2) | 14.4±3.3 | 14.0±2.1 | 0.69 |
| LVEDP (mmHg) | 9.3±4.4 | 10.4±4.0 | 0.47 |
| RVEDVI (mL/m2) | 87.4±21.3 | 71.9±19.2 | 0.0068 |
| RVEF (%) | 56.2±6.8 | 58.2±6.9 | 0.30 |
| RAVI (mL/m2) | 15.2±3.0 | 15.3±2.7 | 0.93 |
| RVEDP (mmHg) | 5.9±1.7 | 7.1±2.7 | 0.072 |
| CVP (mmHg) | 5.1±1.8 | 5.7±1.6 | 0.25 |
| Age ≥6 years | |||
| LVEDVI (mL/m2) | 96.3±13.8 | 95.5±21.6 | 0.87 |
| LVEF (%) | 60.0±6.9 | 57.0±6.0 | 0.080 |
| LAVI (mL/m2) | 13.2±3.2 | 11.0±2.7 | 0.017 |
| LVEDP (mmHg) | 10.8±4.0 | 10.4±4.5 | 0.72 |
| RVEDVI (mL/m2) | 120.9±36.6 | 108.4±38.4 | 0.20 |
| RVEF (%) | 53.5±7.6 | 51.5±7.3 | 0.28 |
| RAVI (mL/m2) | 14.4±2.5 | 11.8±2.6 | 0.0015 |
| RVEDP (mmHg) | 7.3±3.3 | 7.0±3.4 | 0.72 |
| CVP (mmHg) | 5.9±2.4 | 5.4±2.1 | 0.39 |
Unless indicated otherwise, data are given as the mean±SD. EDFF, end-diastolic forward flow. Other abbreviations as in Table 2.
Although hemodynamic data obtained at steady state could not discriminate between those with and without EDFF, pressure measurement after contrast imaging using a similar contrast dose revealed, as expected, marked augmentation of both central venous pressure (1.14±0.20 vs. 0.50±0.16 mmHg; P=0.01) and LVEDP (1.03±0.23 vs. −0.06±0.19 mmHg; P<0.01) in E-TOF patients, suggesting impaired biventricular diastolic reserve compared with N-TOF patients (Figure 1).
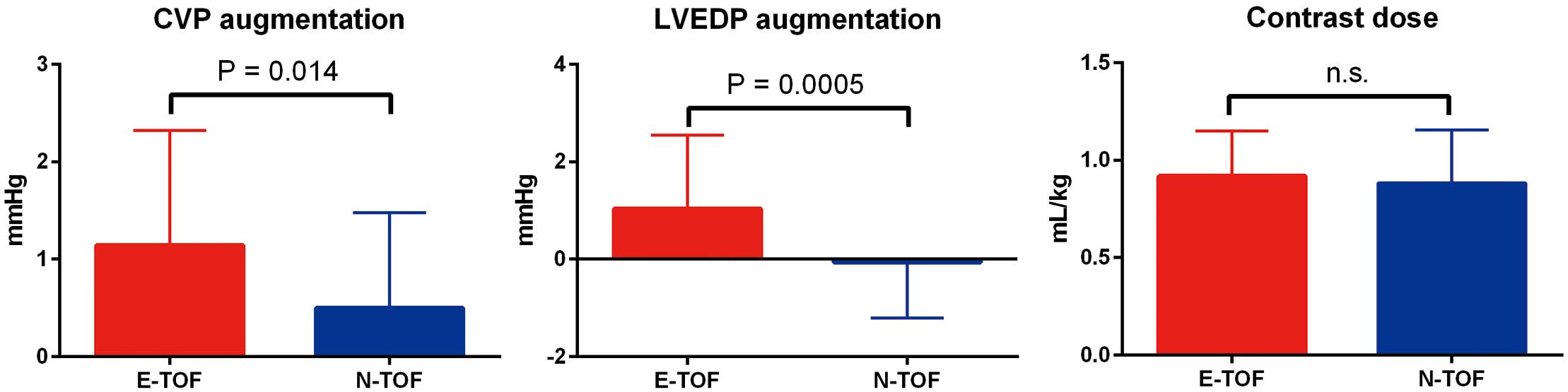
Contrast dose and reactive pressure augmentation of central venous pressure (CVP) and left ventricular end-diastolic pressure (LVEDP). CVP and LVEDP were markedly augmented in patients with (E-TOF) than without (N-TOF) positive end-diastolic forward flow. The contrast dose was similar between the E-TOF and N-TOF groups. Data are the mean±SD.
The relationship between the hemodynamic index and EDFF morphology, including velocity, duration, and the VTI, was analyzed in E-TOF patients. As shown in Figure 2, EDFF velocity and VTI reflected RVEDP independent of RV volume (Figure 2A,C,E). In addition, EDFF velocity and VTI was correlated with LVEDP, suggesting a significant impact of LV diastolic pressure on EDFF morphology (Figure 2B,D,F). This was further delineated by the result that the velocity and VTI of the EDFF were independent of plasma concentrations of atrial natriuretic peptide (ANP), brain or B-type natriuretic peptide (BNP), the surrogate for volume condition (Supplementary Figure). Even after adjusting for RVEDP using analysis of covariance (ANCOVA), LVEDP remained a significant determinant of EDFF-VTI (P=0.046). In addition, EDFF-VTI was closely correlated with systemic vascular resistance (SVR), suggesting an interaction with LV hemodynamics (Figure 3C). Together, the results indicate that the RV diastolic dysfunction assessed by EDFF is also affected by the LV diastolic property, as well as afterload.
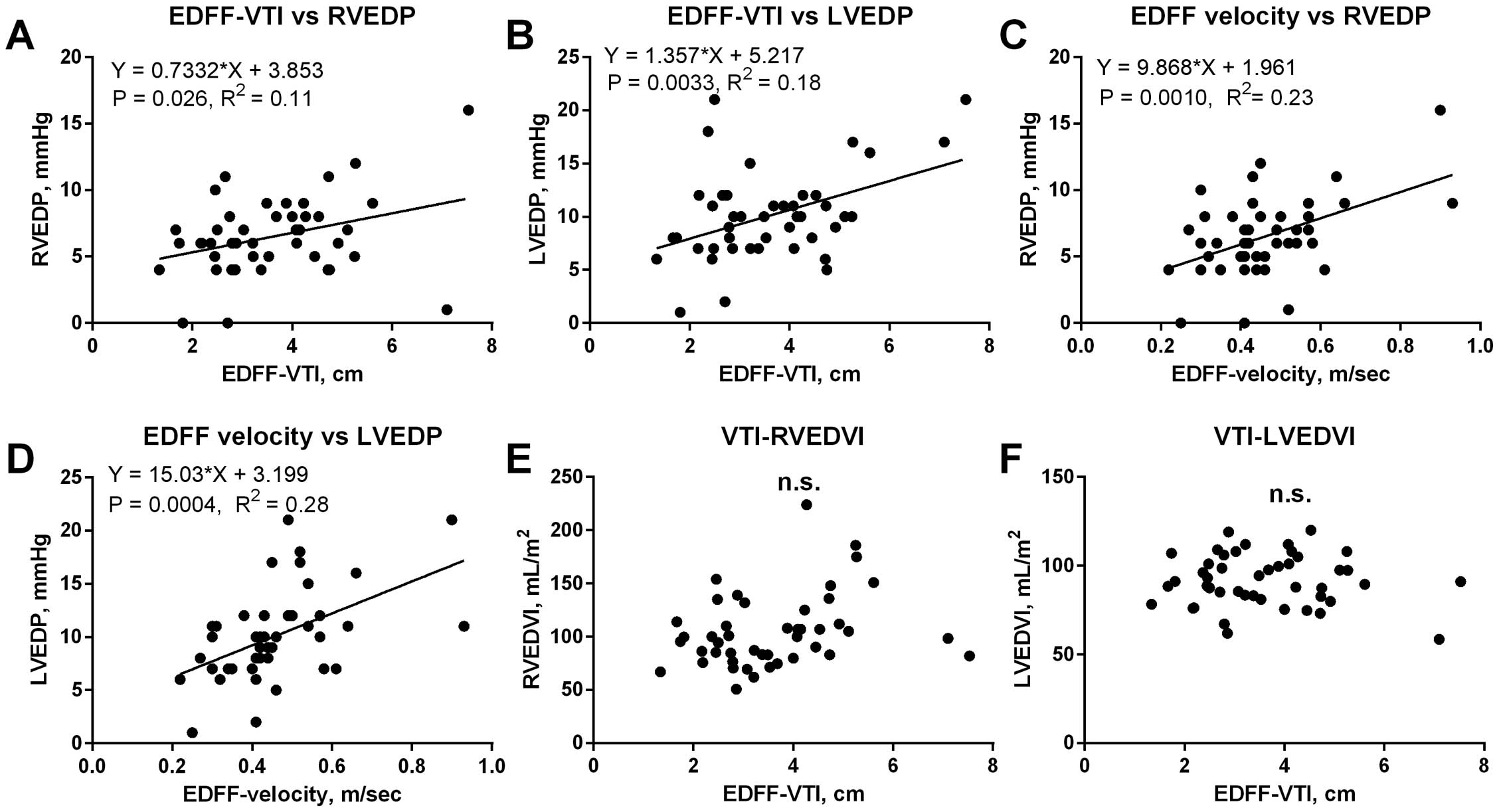
Relationships between the morphology of the end-diastolic forward flow (EDFF) and hemodynamic data. (A–D) The EDFF velocity and velocity-time integral (VTI) were positively correlated with biventricular end-diastolic pressures. (E,F) Although the presence of EDFF was associated with right ventricular end-diastolic volume index (RVEDVI) as indicated in Table 2, EDFF morphology was independent of left ventricular and right ventricular volumes. LVEDP, left ventricular end-diastolic pressure; LVEDVI, left ventricular end-diastolic volume index; RVEDP, right ventricular end-diastolic pressure.

Relationships between end-diastolic forward flow (EDFF) and vascular properties and end organ markers. (A–C) Although the EDFF velocity-time integral (VTI) was independent of central venous pressure (CVP), it was closely correlated with hepatic venous wedge pressure (HWP), a surrogate for hepatic congestion, and systemic vascular resistance (SVR). (D–F) EDFF-VTI was positively correlated with elevated serum creatinine concentrations and the fibrosis-4 (Fib-4) index, and negatively correlated with the platelet count (Plt).
The reverse flow towards the inferior and superior vena cava during an atrial contraction can be an indicator of RV stiffening, assuming a similar grade of venous congestion, although both wide respiratory variation in the diameter and small retrograde flow in the vena cava is hardly measured consistently. Alternatively, it is assumed that an impaired RV preload reserve (diastolic dysfunction) demands that the right atrium increases preload by increasing the atrial contraction volume, whereas the RV cannot fully accept the augmented contraction volume, resulting in increased EDFF. To verify that EDFF-VTI reflects RV stiffness, we analyzed the interaction between EDFF and the surrogate for atrial contraction volume. Owing to the retrospective nature of this study, the amount of available data was limited, but the EDFF and the blood volume flowing into the RV, as well as the tricuspid VTI of atrial contraction, the surrogate of blood flowing into the RV during atrial contraction, were positively correlated with the EDFF, suggesting a large EDFF as a marker for RV diastolic dysfunction which is dependent on RA contraction (Figure 4A,B), but independent of RA volume (Figure 4C). Furthermore, although RVEDVI was positively correlated with the A-wave volume in patients without EDFF (N-TOF), it was independent of A-wave volume in patients with EDFF (E-TOF), implying the existence of EDFF as a reflection of a less-compliant RV (Figure 4D,E).

(A–C) The end-diastolic forward flow (EDFF) velocity-time integral (VTI) was positively correlated with the VTI and volume of transtricuspid flow during the A-wave, but was independent of right atrium (RA) volume, suggesting EDFF-VTI reflects the dependence of right ventricle (RV) volume on atrial contraction. (D,E) Although right ventricular end-diastolic volume index (RVEDVI) increased with the increase in tricuspid A-wave volume in patients without positive EDFF (N-TOF), it was independent in patients with positive EDFF (E-TOF), implying a less compliant RV property in E-TOF patients.
Although EDFF was not associated with plasma concentrations of natriuretic peptides, EDFF-VTI was closely correlated with hepatic venous wedge pressure, a surrogate for venous and hepatic congestion (Figure 3). In agreement with this finding, EDFF-VTI was correlated with a reduced blood platelet count as well as a high fibrosis-4 index, suggesting an interaction with congestive liver damage.
Characteristics of N-TOF PatientsBecause the VTI of the tricuspid A-wave and the transtricuspid blood flow volume during atrial contraction were positively correlated with EDFF-VTI and EDFF velocity (Figure 4), the lack of EDFF in N-TOF patients may be associated with impaired atrial contraction. Similarly, the EDFF may be affected by high pulmonary resistance. Among the 75 patients without EDFF, 3 had diminutive transtricuspid valve flow during right atrial contraction: 1 had a markedly high plasma BNP concentration and 2 had high pulmonary resistance with an RVEDVI >180 mL/m2. These data highlight the limited availability of EDFF in patients with pulmonary hypertension or impaired atrial function.
Improved perioperative care for patients with congenital heart disease has resulted in an excellent 20-year survival rate of more than 90% for patients with TOF,2 which emphasizes the importance of systematic medical care aimed at life-long health. Indeed, Dennis et al found that only 56% of patients with repaired TOF were free of adverse events by the age of 50 years, which included all-cause mortality, heart transplant, heart failure-related hospitalization, arrhythmias requiring therapies, and endocarditis.1 Cuypers et al conducted a cohort study and found that the cumulative survival after 40 years for patients with repaired TOF was 72%.2 The cardiovascular burden over decades may explain the high morbidity and mortality in middle-aged patients with repaired TOF. In addition to RV volume loading due to PR, marked aortic stiffening coupled with progressive aortic dilatation,7,14 augmentation of biventricular afterload,4,5 and fragility of the cerebral circulation6 may synergistically increase the impact of known cardiovascular risk factors. In order to efficiently describe circulatory compromise in patients with repaired TOF, a marker that reflects unfavourable volume and pressure properties of the ventricles is needed.
Our data showed that the morphology of EDFF in patients with repaired TOF reflects RV diastolic dysfunction that is affected by LV diastolic pressure and afterload. Furthermore, EDFF-VTI also indicates blood stagnation, which may be attributed to end organ damage.
EDFF and RV Hemodynamics After TOF RepairOur study analyzed, for the first time, the shape of EDFF and found that VTI and the velocity of EDFF were closely correlated with RVEDP. A meta-analysis conducted by Eynde et al found that RV restrictive physiology in patients with repaired TOF is provoked by the RV volume load secondary to PR, and their EDFF is derived from atrial contraction during end-diastole pumped towards the stiffened ventricle with a volume determined by a steeper end diastolic pressure–volume relationship.11,15 Our study was consistent with this, because patients with EDFF had a larger RVEDVI than those without EDFF. However, detailed morphological analyses of EDFF also indicated that the VTI of EDFF was positively correlated with RVEDP regardless of ventricular volume, suggesting the significance of EDFF in the reflection of RV diastolic stiffening independent of volume load. This was further delineated by the fact that EDFF was independent of natriuretic peptides, markers for fluid condition, which suggests that the resting hemodynamic load was insignificant for our patients, although it became significant when excessive fluid was administered, as shown in Figure 1.16 Importantly, although positive EDFF was more likely to be associated with large RVEDVI in patients <6 years of age, it was more likely to be associated with large bi-atrial volume rather than RVEDVI in patients ≥6 years of age (Table 3), suggesting EDFF serves as a promising indicator of ventricular stiffening independent of RV volume load, particularly in the older cohort.
Impact of LV Hemodynamics on EDFF in After TOF RepairImportantly, our study also found a positive relationship between EDFF and LVEDP, suggesting the involvement of the LV in RV diastolic dysfunction. Further research is needed to analyze the causal relationship between LV hemodynamics and RV stiffening, although diastolic interventricular cross-talk could be a part of the possible explanation. The RV volume load can augment LV diastolic stiffening17,18 and, inversely, as demonstrated by Yu et al, impaired RV diastolic function is observed in patients with compromised LV function, even without LV enlargement or passive pulmonary hypertension.19 The adjustment for RVEDP by ANCOVA revealed the influence of LV on the EDFF, suggesting bidirectional ventricular cross-talk. Indeed, arterial property of repaired TOF increases aortic reflection and characteristic impedance, which can increase LV afterload, then LVEDP, even if it is not obvious at rest.5 Although the impact of LVEDP on the RV diastolic property may vary depending on septal displacement,17,18 our study highlighted the importance of LV involvement in the development of RV restrictive physiology suspected by EDFF. Notably, recent research has revealed the importance of LV diastolic dysfunction as a predictor of all-cause mortality20 and reduced exercise capacity21 in patients with structurally normal hearts. Meanwhile, it is also possible that both systemic and pulmonary arterial stiffening via neurohormonal activation may have developed simultaneously from childhood. As shown in Figure 3, our study also indicated a positive correlation between EDFF-VTI and venous congestion, as well as augmented SVR. This was clinically relevant, because these were further associated with markers of impaired renal function and hepatic insult. Together, the results suggest that therapies, including medications and PVR, guided by EDFF may be protective of systemic organs, which may further ameliorate long-term outcomes in patients with repaired TOF.
Study LimitationsThis study has several limitations. First, our study design poses a risk of reference bias. Our echocardiogram data were acquired by trained sonographers specializing in congenital heart disease, which allowed for detailed analyses of the waveform. However, there is still a risk of insufficient pulmonary blood flow imaging, by which patients with obscure EDFF may have been in the E-TOF or N-TOF groups. To minimize this risk, patients with obscure EDFF, who were on the border between E-TOF and N-TOF, were excluded from the study. Second, the wide age range of our cohort may have resulted in a selection bias. Because elderly patients are more likely to have a lower heart rate, the acquired image of blood flow measurements in the pulmonary artery may be less likely to possess 3 consecutive beats, meeting the exclusion criteria. This may have also masked the important characteristics of cardiac dysfunction, although we used subanalyses to minimize the risk of age distribution, as presented in the Table 3. Third, the data for patient outcomes were limited because not all surgical or medical interventions may have been provided in our institute. Although a few of our cohort experienced reintervention for pulmonary valve and their EDFF diminished postoperatively, we were unable to analyze causal relationships. A prospective cohort study coupled with detailed patient outcomes is warranted to further delineate the significance of EDFF morphology in the outcome of patients with repaired TOF.
The shape of EDFF in patients with repaired TOF reflects the restrictive RV physiology. Its source includes not only RV enlargement-induced stiffening, but also elevation of LVEDP and SVR, which may be provoked by the hemodynamic burdens from youth. Because EDFF represents an impaired ventricular volume reserve of both ventricles, EDFF may be a potential guide for optimizing hemodynamic conditions in children with repaired TOF. Further research is required to evaluate the potential of EDFF for rationalizing diuretics, vasodilators, or even surgical/ transcatheter PVR.
This study was supported by grants from JSPS KAKENHI (Grant no. JP20K08191; to H. Saiki) and from the Miyata Cardiac Research Promotion Foundation (to H. Saiki).
H. Senzaki is a member of Circulation Journal’s Editorial Team. The remaining authors have no conflicts of interest to declare.
This study was approved by the Ethics Committee of Iwate Medical University (MH2020-238).
Please find supplementary file(s);
https://doi.org/10.1253/circj.CJ-23-0147