Abstract
Background: The Micra leadless pacemaker has demonstrated favorable outcomes in global trials, but its real-world performance and safety in a Japan-specific population is unknown.
Methods and Results: Micra Acute Performance (MAP) Japan enrolled 300 patients undergoing Micra VR leadless pacemaker implantation in 15 centers. The primary endpoint was the acute (30-day) major complication rate. The 30-day and 6-month major complication rates were compared to global Micra studies. All patients underwent successful implantation with an average follow-up of 7.23±2.83 months. Compared with previous Micra studies, Japanese patients were older, smaller, more frequently female, and had a higher pericardial effusion risk score. 11 acute major complications were reported in 10 patients for an acute complication rate of 3.33% (95% confidence interval: 1.61–6.04%), which was in line with global Micra trials. Pericardial effusion occurred in 4 patients (1.33%; 3 major, 1 minor). No procedure or device-related deaths occurred. Frailty significantly improved from baseline to follow-up as assessed by Japan Cardiovascular Health Study criteria.
Conclusions: In a Japanese cohort, implantation of the Micra leadless pacemaker had a high success rate and low major complication rate. Despite the Japan cohort being older, smaller, and at higher risk, the safety and performance was in line with global Micra trials.
Leadless pacemakers were developed to avoid the pocket- and lead-related complications associated with transvenous pacemakers (TV-PM).1,2 The Micra VR leadless pacemaker was first assessed for its safety and performance in the Micra Investigational Device Exemption (IDE) study,2 in which 726 patients underwent an implant attempt between December 2013 and May 2015 at 56 sites across North America, Europe, Asia, and Australia. The primary analysis for the study showed that the Micra VR was implanted with a high success rate (99.2%) and had a 4% complication rate at 6 months post-implant, which was lower than the 7.4% rate for TV-PM systems in the historical control group.2 The Micra Post-Approval Registry (PAR) study followed the IDE and aimed to determine the safety and effectiveness of the Micra VR in a real-world setting.3,4 The PAR trial, still in the process of completing long-term follow-ups, began in 2015 and enrolled 1,810 patients through April 2018 from North America, Europe, the Middle East, and New Zealand. An initial analysis of PAR enrollees further demonstrated the safety and performance of Micra VR, with an implant success rate of 99.1%, a major complication rate of 2.7% at 1-year post-implant, and stable device parameters through 12 months.3
The Micra IDE and PAR studies laid the groundwork for showing the safety benefit of the Micra VR leadless pacemaker over traditional TV-PMs, but, due to low Japanese representation in those trials, its performance in a Japan-specific population is unknown. Therefore, the purpose of this study was to determine its safety and performance in a Japanese cohort and compare the results with those in previous global trials.
Methods
Study Design
The Micra Acute Performance (MAP) Japan trial was a prospective, observational, nonrandomized multicenter study aimed at examining the acute performance and safety of the Micra VR system in a Japan-specific cohort. This post-approval study was conducted across 15 Japanese centers from April 26, 2019 to April 30, 2022. All protocols were approved by an ethics committee at each participating institution. The study was conducted in accordance with the guiding principles of the Declaration of Helsinki, Good Clinical Practice, Ethical Guidelines for Medical and Health Research Involving Humans Subjects, and in accordance with local laws, regulations, and standards in the regions where the study was performed.
Patients and Procedures
Patients who were able to provide written consent, were willing to adhere to regular follow-up, and would be implanted with a Micra VR device during the study period were eligible to be enrolled. Informed written consent was given by all patients before implantation of the device. Following consent, demographics and medical history were collected to construct a risk score for pericardial effusion following Micra VR implant based on a recently published risk scoring system for Micra implants.5 Briefly, the scoring system uses commonly available preprocedural clinical characteristics to categorize patients as low, medium, or high risk for pericardial effusion.
The implant procedure for the Micra VR leadless pacemaker has been described.2,6 In brief, a catheter guides the Micra device through the femoral vein to the right ventricle where it is implanted into the myocardium via tines at the distal end of the device. A tether, which connects the device to the catheter, is then cut to remove the delivery system once adequate pacing parameters are achieved. All patients were followed predischarge and for at least 6 months post-implantation as per site standard of care or as needed for reported adverse events.
Study Endpoints
The primary endpoint of this study was the safety of the Micra VR system in a Japan-specific population as indicated by the acute major complication rate. Acute major complications were defined as adverse events related to the Micra system or implantation procedure that occurred within 30 days of implantation and resulted in ≥1 of the following criteria: (1) death, (2) permanent loss of device function due to mechanical or electrical dysfunction of the device, (3) hospitalization, (4) hospitalization ≥48 h, and (5) system revision (explant, repositioning, replacement, permanently programming device to off). The major complication rate was also calculated at 6 months post-implantation, and both the acute and 6-month complication rates were compared with those from the Micra PAR and IDE studies.2,4 All complications were independently adjudicated and classified by a Clinical Events Committee.
Ancillary endpoints assessed during the study included: device electrical values (pacing capture threshold, sensing amplitude, pacing impedance), implantation procedural details (fluoroscopy duration, procedure duration, implant location, number of attempts), and patient frailty.
Frailty Score
Frailty was assessed by both the Kihon Checklist and Japanese Cardiovascular Health Study (J-CHS) criteria at baseline and 6-month follow-up. The Kihon Checklist is a 25-point questionnaire with “yes” or “no” responses. A score was given to classify the patient as “robust” (0–3 total score), “pre-frail” (4–7), or “frail” (≥8).7 A similar method is used for J-CHS criteria by which a final score is derived from a 5-point checklist to classify subjects as “robust” (total score of 0), “pre-frail” (1–2), or “frail” (≥3).8 A detailed overview of both checklists can be found in Supplementary Table 1.
Statistical Analysis
A sample size of 300 was selected because it would allow a single event time occurring at an underlying rate of 1% to be detected with 95% confidence. Summary statistics are presented as number and percent for categorical variables, and the mean and standard deviation for continuous variables. The exact binomial method was used for computing 95% confidence intervals (CIs) for the acute major complication rate. For comparisons of baseline variables and implant parameters between Micra studies, one-way analysis of variance (ANOVA) or the Kruskal-Wallace test was used for continuous variables, and Fisher’s exact test was used for categorical variables. The Fine-Gray competing risk model was used to compare the major complication rates through 6-months between Micra MAP Japan and the Micra IDE and PAR studies. For this analysis, death unrelated to the Micra system or procedure was considered the competing risk. Logistic regression was used to compare patient and procedural characteristics, as well as pericardial effusion rates, between Micra studies. Mortality rates at 30-days and 6-months post-implantation were calculated using the Kaplan-Meier method. A repeated measures ANOVA test was conducted to assess serial changes in device electrical values from implantation to each follow-up time period. McNemar-Bowker tests were used to compare changes in frailty between baseline and follow-up. All analyses were performed with SAS version 9.4 (SAS Institute Inc., Cary, NC, USA) or the R statistical package (www.r-project.org).
Results
Patients’ Characteristics
A total of 300 patients (48.7% female) were enrolled in the study, with an average age of 82.6±9.7 years. Baseline patient characteristics for MAP Japan are provided in Table 1. Compared with the global IDE and PAR cohorts, the MAP Japan population was significantly older, more frequently female, had a lower body mass index, and had higher rates of renal dysfunction and congestive heart failure. More patients were precluded from having a transvenous system in MAP Japan (27%) compared with IDE (6.2%) and PAR (23.9%) (P<0.001). Bradyarrhythmia with atrial fibrillation was the most common pacing indication in all 3 studies, but the proportion was significantly lower in MAP Japan (42%) than in the IDE (63.9%) and PAR (62.6%) studies (P<0.001) (Table 1).
Table 1. Baseline Characteristics by Study
| Subject characteristics |
MAP Japan
(n=300) |
IDE
(n=726) |
PAR
(n=1,810) |
P value |
| Age (years) |
82.6±9.7 |
75.9±11.0 |
75.6±13.4 |
<0.001 |
| BMI |
21.8±3.7 |
27.6±5.3 |
27.7±5.8 |
<0.001 |
| Weight (kg) |
53.4±11.7 |
79.0±18.4 |
79.3±18.8 |
<0.001 |
| Height (cm) |
155.7±9.9 |
168.7±10.7 |
169.0±10.5 |
<0.001 |
| Sex (female) |
146 (48.7%) |
299 (41.2%) |
702 (38.8%) |
0.005 |
| AF |
177 (59.0%) |
527 (72.6%) |
1,288 (71.2%) |
<0.001 |
| CHF |
80 (26.7%) |
131 (18.0%) |
236 (13.0%) |
<0.001 |
| COPD |
14 (4.7%) |
92 (12.7%) |
177 (9.8%) |
<0.001 |
| CAD |
38 (12.7%) |
205 (28.2%) |
398 (22.0%) |
<0.001 |
| HTN |
171 (57.0%) |
571 (78.7%) |
1,174 (64.9%) |
<0.001 |
| Diabetes |
70 (23.3%) |
207 (28.5%) |
479 (26.5%) |
0.23 |
| Renal dysfunction |
111 (37.0%) |
149 (20.5%) |
388 (21.4%) |
<0.001 |
| Dialysis |
19 (6.3%) |
28 (3.9%) |
143 (7.9%) |
0.001 |
| Prior CIED |
29 (9.7%) |
0 (0.0%) |
275 (15.2%) |
<0.001 |
| Condition that precludes the use of TV-PPM |
81 (27.0%) |
45 (6.2%) |
433 (23.9%) |
<0.001 |
| Effusion risk level (%) |
n=298 |
|
|
|
| Low |
52.7% (157/298) |
72.8% (528/725) |
71.7% (1,268/1,768) |
<0.001 |
| Medium |
21.1% (63/298) |
15.6% (113/725) |
17.0% (301/1,768) |
|
| High |
26.2% (78/298) |
11.6% (84/725) |
11.3% (199/1,768) |
|
| Pacing indication (%) |
| Bradyarrhythmia with AF |
126 (42.0%) |
464 (63.9%) |
1,128 (62.6%) |
<0.001 |
| Sinus node dysfunction |
76 (25.3%) |
126 (17.4%) |
174 (9.6%) |
|
| AV Block |
75 (25.0%) |
109 (15.0%) |
210 (11.6%) |
|
| Syncope |
22 (7.3%) |
16 (2.2%) |
243 (13.5%) |
|
| Other |
1 (0.3%) |
11 (1.5%) |
48 (2.7%) |
|
| LVEF (%) |
61.6±9.5 |
58.8±8.7 |
56.2±9.3 |
<0.001 |
AF, atrial fibrillation; AV, atrioventricular; BMI, body mass index; CAD, coronary artery disease; CIED, cardiac implantable electronic device; CHF, chronic heart failure; COPD, chronic obstructive pulmonary disease; HTN, hypertension; IDE, Investigational Device Exemption [study]; LVEF, left ventricular ejection fraction; MAP, Micra Acute Performance [study]; PAR, Post-Approval Registry [study]; TV-PPM, transvenous pacemaker.
When classified according to the recently published predictive risk score for pericardial effusion (see Methods), the proportion of intermediate- and high-risk (21.1% and 26.2%) patients in the MAP cohort Japan was significantly higher than the proportions in IDE (15.6% and 11.6%) and PAR (17.0% and 11.3%) (P<0.001) (Table 1).
Implantation Characteristics
Micra VR was successfully implanted in all 300 (100%) MAP Japan patients, with an average hospital stay of 7.1±9.8 days, which was significantly longer than in the global Micra studies (Table 2). The median procedure duration was shorter for MAP Japan patients (25.0 min; interquartile range: 14–36 min) compared with IDE (28.0 min; interquartile range: 21–41 min) and PAR (26.0 min; interquartile range: 19–40 min) (P=0.006), but median fluoroscopy time was longer (9.5 min [6–15] vs. 6.0 min [4–10] vs. 6.6 min [4–11], respectively; P<0.001). A majority (66.9%) of MAP Japan patients required only 1 deployment, and the device was implanted within the septum in 87.7% of cases. Intraprocedural intravenous anticoagulation was used in 223 (74.3%) patients, and reversant was used in 43 (14.3%) patients (Table 2). The mean follow-up period for the Japan cohort was 7.23±2.83 months.
Table 2. Procedural Characteristics by Study
| Implantation parameters |
MAP Japan
(n=300) |
IDE
(n=726) |
PAR
(n=1,810) |
P value |
| Implant success |
300 (100.0%) |
720 (99.2%) |
1,793 (99.1%) |
0.29 |
| Days hospitalized following implant |
7.1±9.8 |
2.1±2.7 |
3.3±18.7 |
<0.001 |
| Procedure duration (min) |
| Mean±SD |
29.2±21.6 |
34.8±24.0 |
32.8±25.6 |
0.006 |
| Median |
25.0 |
28.0 |
26.0 |
|
| 25–75th percentile |
14–36 |
21–41 |
19–40 |
|
| Fluoroscopy duration (min) |
| Mean±SD |
14.8±18.1 |
8.9±16.6 |
9.5±17.4 |
<0.001 |
| Median |
9.5 |
6.0 |
6.6 |
|
| 25–75th percentile |
6–15 |
4–10 |
4–11 |
|
| No. of deployments (%) |
| 1 |
200 (66.9%) |
435 (60.1%) |
1,023 (61.0%) |
0.06 |
| 2 |
54 (18.1%) |
132 (18.2%) |
350 (20.9%) |
|
| 3 |
17 (5.7%) |
66 (9.1%) |
145 (8.7%) |
|
| >3 |
28 (9.4%) |
91 (12.6%) |
158 (9.4%) |
|
| Implant location (%) |
| Apex |
26 (8.7%) |
475 (66.0%) |
576 (32.4%) |
<0.001 |
| Septum |
263 (87.7%) |
240 (33.3%) |
1,158 (65.1%) |
|
| RVOT |
7 (2.3%) |
4 (0.6%) |
25 (1.4%) |
|
| Other |
4 (1.3%) |
1 (0.1%) |
20 (1.1%) |
|
| Intraprocedure anticoagulation |
| IV anticoagulation |
223 (74.3%) |
380 (52.3%) |
1,393 (77.8%) |
<0.001 |
| Reversant use |
43 (14.3%) |
70 (9.6%) |
201 (11.3%) |
0.09 |
IV, intravenous; RVOT, right ventricular outflow tract; SD, standard deviation; other abbreviations as in Table 1.
There were 11 major complications reported in 10 patients within 30 days of implantation for an acute complication rate of 3.33% (95% CI: 1.61–6.04%) (Table 3). Of the 11 acute major complications, there were 3 cardiac effusions/perforations, 2 events at the groin puncture site, 2 cases of deep vein thrombosis, and 4 pacing issues (system modification in all 4; see system modifications section below for details). No additional major complications were reported through the remainder of the follow-up period (Table 3). No device-related infections were reported during the study period.
Table 3. Major Complications in MAP Japan
Type of major complication
(no. of patients, %) |
MAP Japan (n=300) |
| Acute |
Total |
| Total major complications |
11 (10, 3.33%) |
11 (10, 3.33%) |
| Thrombosis |
2 (2, 0.67%) |
2 (2, 0.67%) |
| Deep vein thrombosis |
2 (0.67%) |
2 (0.67%) |
| Events at groin puncture site |
2 (1, 0.33%) |
2 (1, 0.33%) |
| Arteriovenous fistula |
1 (0.33%) |
1 (0.33%) |
| Vascular access site |
1 (0.33%) |
1 (0.33%) |
| Cardiac effusion/perforation |
3 (3, 1.00%) |
3 (3, 1.00%) |
| Cardiac perforation |
1 (0.33%) |
1 (0.33%) |
| Cardiac tamponade |
1 (0.33%) |
1 (0.33%) |
| Pericardial effusion |
1 (0.33%) |
1 (0.33%) |
| Pacing issues |
4 (4, 1.33%) |
4 (4, 1.33%) |
| Device dislocation |
1 (0.33%) |
1 (0.33%) |
| Device pacing issue |
3 (1.00%) |
3 (1.00%) |
Abbreviations as in Table 1.
System modification was required in 4 patients due to high pacing thresholds, 1 of which was initially caused by a micro-dislodgement. The device was successfully explanted in 3 of the patients (1–5 days post-implantation) and programmed to OOO in the 4th (39 days post-implantation). All 4 patients subsequently received a transvenous pacing system (1 upgraded to a dual-chamber device, 3 received a single-chamber device).
Cardiac Effusions/Perforations
In total, 4 cardiac perforation/effusion events occurred in 4 patients (1.33%), all within 1 month of implantation. Three of the 4 events met the criteria for a major complication. The minor effusion was noted on a computed tomography scan, resolved without issue, and appeared to be caused by tine interaction with the right ventricular free wall during implantation. The 3 major effusions in 3 patients (1.00%) were discovered within hours of the operation and 2 of the 3 were resolved by pericardiocentesis, while the third resolved without intervention, though it did prolong hospitalization. Supplementary Table 2 provides details on all 4 pericardial effusion events.
Deaths
There were 22 deaths during the study period, with 2 occurring within 30 days of implantation (1 from COVID-19 and 1 from cardiogenic shock). The 30-day and 6-month Kaplan-Meier estimated death rates were 0.7% and 6.9%, respectively. None of the deaths were related to the Micra device or the procedure, and only 2 of the deaths were known to be cardiac-related (cardiogenic shock and heart failure).
Electrical Performance
At implantation, the mean pacing capture threshold was 0.76±0.63 V at 0.24 ms and remained stable at 6 months (0.69±0.58 V; P=0.39) and 12 months (0.63±0.27 V; P=0.96) post-implantation (Figure 1A). The mean R-wave amplitude was 9.6±4.8 mV at implantation and increased to 11.9±5.5 mV at 6 months (P=0.01) and 15.2±6.0 mV at 12 months (P<0.001) post-implantation (Figure 1B). Mean impedance was 771.5±206.1 Ω at implantation and decreased to 524.2±110.5 Ω (P<0.001) and 551.4±117.5 Ω (P<0.001) at 6 and 12 months, respectively (Figure 1C).
Frailty Assessment
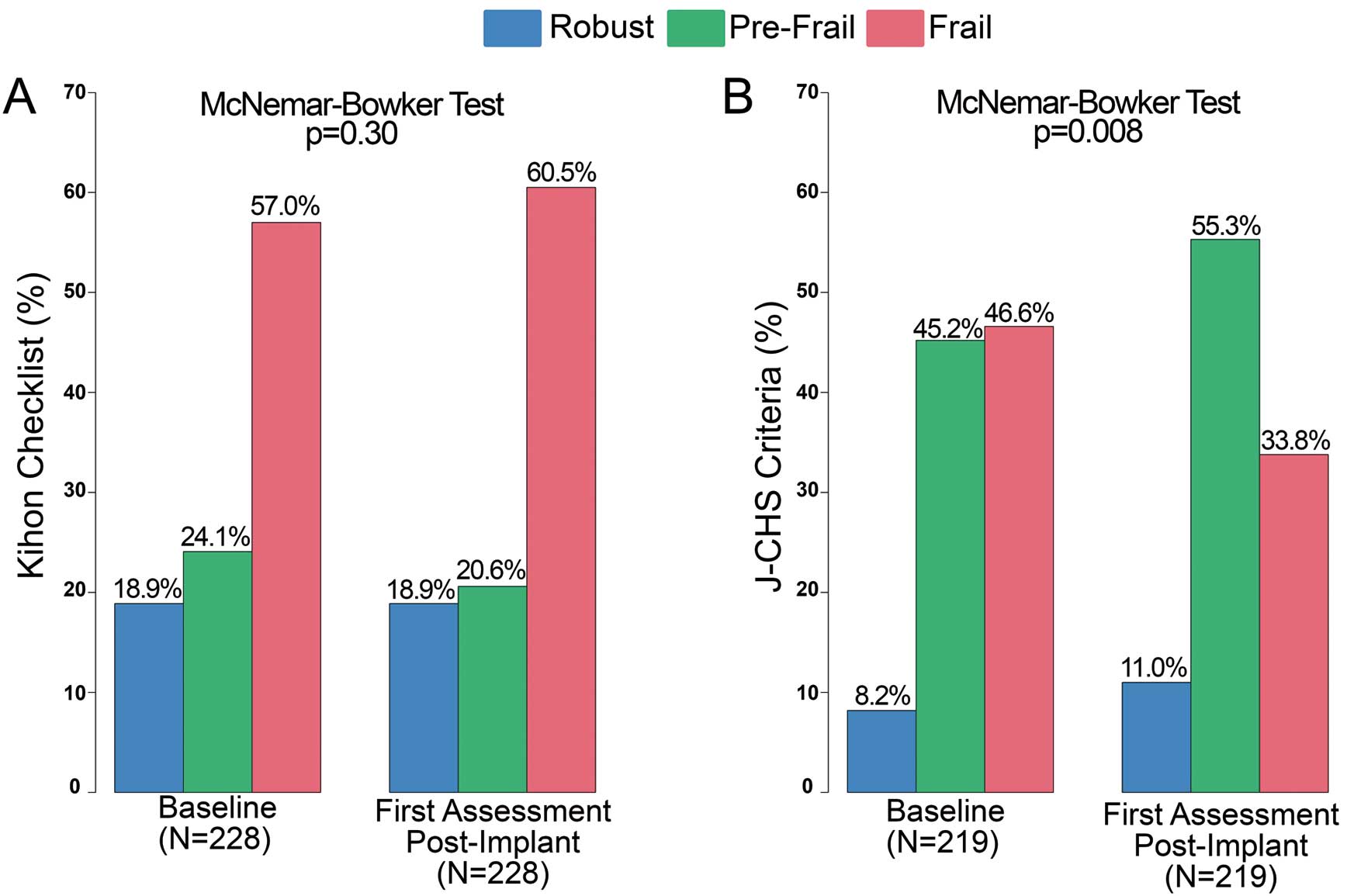
There was no difference in frailty index from baseline to follow-up when using the Kihon Checklist (P=0.3; Figure 2A). However, the J-CHS criteria showed a significant change in frailty as seen by a decrease in the fraction of patients classified as “frail” at follow-up (P=0.008; Figure 2B).
Safety Comparison With IDE and PAR Studies
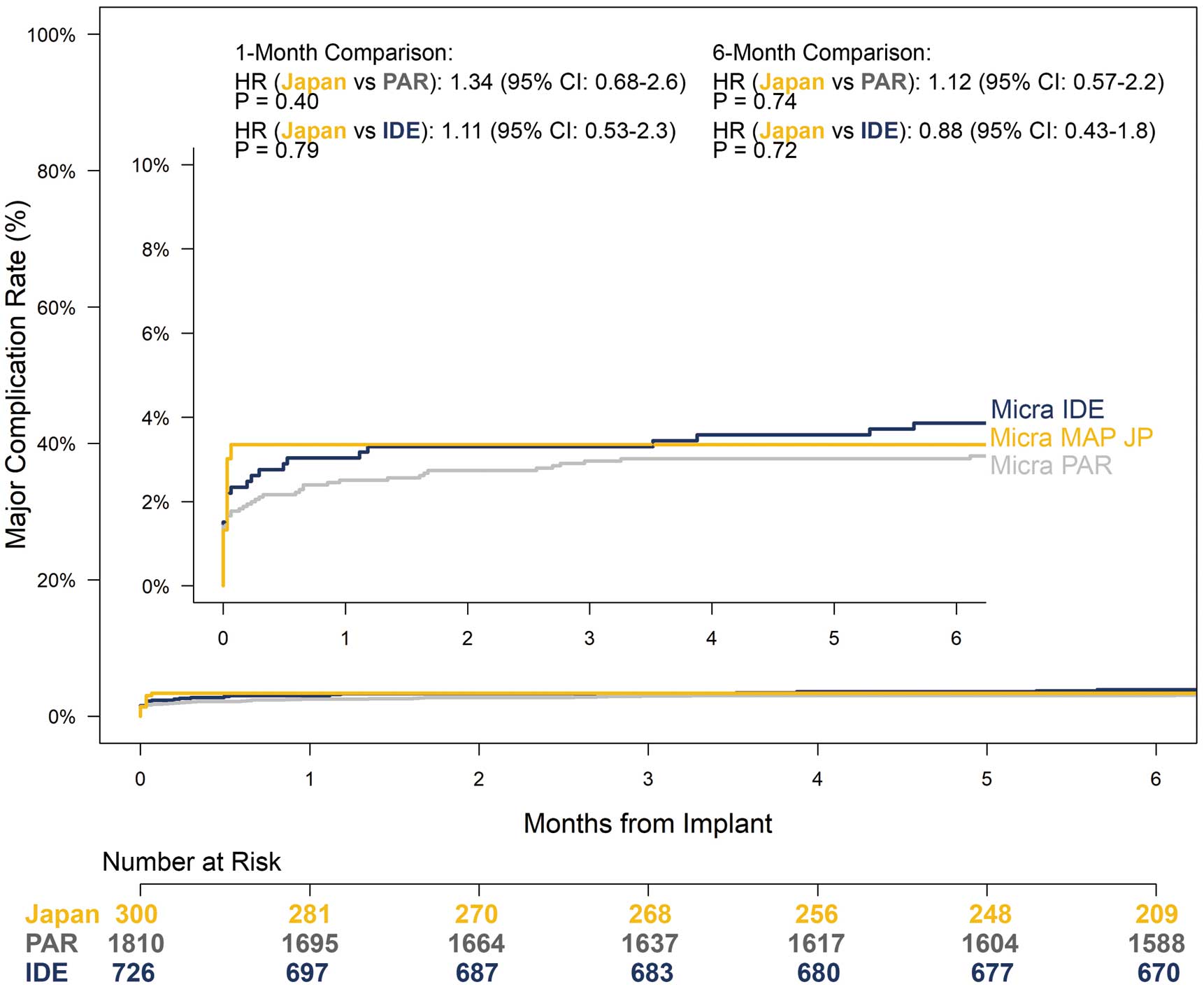
The risk of an acute major complication was not significantly different between MAP Japan and Micra IDE (hazard ratio (HR): 1.11; 95% CI: 0.53–2.3; P=0.79) and Micra PAR (HR: 1.34; 95% CI: 0.68–2.6; P=0.40) studies (Figure 3). At 6 months post-implantation, the estimated MAP Japan major complication risk remained similar to that of Micra IDE (3.86%; HR: 0.88; 95% CI: 0.43–1.8; P=0.72) and PAR (3.02%; HR: 1.12; 95% CI: 0.57–2.2; P=0.74). Supplementary Table 3 provides the details of major complications from each Micra study through 6 months.
The rate of pericardial effusions (both major and minor combined) was not different between MAP Japan and the IDE trial (1.3% vs. 1.8%, P=0.603) or between MAP Japan and the PAR trial (1.3% vs. 0.8%, P=0.396) (Supplementary Figure).
Discussion
This is the first report on the safety and performance of the Micra VR pacemaker in a large, exclusively Japanese cohort. The Micra system was implanted successfully in 100% of MAP Japan patients, which is similar to the success rates in the global IDE (99.2%) and PAR (99.1%) studies. Mean pacing capture threshold remained steady, while average R-wave amplitude increased and mean pacing impedance gradually decreased through follow-up, as is seen with transvenous systems.9 The primary endpoint of the acute major complication rate was 3.33% in the MAP Japan cohort, which remained unchanged through 6 months and was low and in line with the 6-month rates reported in the IDE (3.86%) and PAR (3.02%) trials despite the Japan cohort being older, smaller, and at higher risk for pericardial effusion. Additionally, MAP Japan also compared favorably with the MAP Europe, Middle East and Africa (EMEA) and China studies in terms of implantation success rate (99.9% and 98.8% for MAP EMEA and China, respectively) and major complication rate (3.6% at 12 months and 2.4% at 6 months for MAP EMEA and China study, respectively).10,11 The MAP Japan results also reinforce those from the Japan Transcatheter Pacing study, a retrospective subanalysis of Japanese patients (n=36) from the IDE trial, showing a 100% implantation success rate and no major complications through 12 months.12 As such, Micra’s safety and performance are consistent worldwide and do not appear to differ much by race or region.
Although Micra eliminates lead- and pocket-related complications, cardiac perforation and pericardial effusion remain potential complications. The incidence of cardiac perforation and pericardial effusion (major and minor) in MAP Japan was 1.33%, which was lower than in IDE (1.8%) and higher than in PAR (0.8%). The MAP Japan cohort had a higher risk score for pericardial effusion than IDE or PAR, which would suggest a higher incidence of pericardial perforation and pericardial effusion. On the other hand, MAP Japan used a lower implantation site at the apex (8.7%) compared with IDE (66%) and PAR (32.5%). Apical implantation is considered a risk factor for cardiac perforation or pericardial effusion but is not measured in the risk score for pericardial effusion because the risk score is based on patient demographics and medical history. This may explain why the incidence of cardiac perforation and pericardial effusion in MAP Japan was in line with IDE and PAR despite being a higher risk population. Fortunately, there were no deaths due to cardiac perforation or pericardial effusion in MAP Japan, but adequate preparation should be made for the implantation procedure in medium- to high-risk patients. Proper implanting technique is paramount for reducing pericardial effusion and other complications. This includes using imaging to confirm proper septal placement, being aware of common signs and symptoms associated with pericardial effusion and having access to echocardiography and a pericardiocentesis kit.
One original aspect of this Micra study is that frailty was assessed at both baseline and 6-month follow-up using 2 methods. There was a significant improvement with the J-CHS criteria, with a 12.8% decrease in the number of frail patients from baseline to follow-up. On the other hand, the Kihon Checklist showed no significant difference, which may be because the J-CHS criteria is limited to measuring physical frailty, whereas the Kihon Checklist includes many questions to assess factors such as nutritional status, oral function, confinement, cognitive function, and depressive mood that are not influenced by an improvement in cardiac function within the follow-up period. These results suggest that the J-CHS criteria might be more sensitive for assessing improvements in frailty after a leadless pacemaker procedure.
Of the 22 deaths (7.3%) during the study period, none were related to the Micra device or implantation procedure. The Kaplan-Meier estimated 6-month all-cause mortality rate was 6.9%, which is similar to rates reported in previous global leadless studies despite MAP Japan’s significantly higher average age and higher number of patients precluded from receiving an implant.3,13
The MAP Japan cohort had a significantly higher average age than either IDE or PAR (82.6 vs. 75.9 and 75.6 years, respectively). In Japan, the mean age at pacemaker implantation in the 2010 s was 75.8 years, but has increased every decade since 1980, so the MAP Japan cohort could be partially capturing that trend.14 Patients were hospitalized for longer in MAP Japan compared with the previous Micra trials, which may reflect differences in medical practice given that implantation success and complication rates were similar. Additionally, it has been reported that Japan has the longest average hospital stay among developed countries.15
Study Limitations
The MAP Japan study was not a randomized controlled study, with the performance of the device being compared with prior Micra studies. The patient cohort was limited to Japanese only and therefore the results may not be translatable to other patient populations. Centers that participated in the study may not be a full representation of all implanting institutions in Japan; however, the centers were spread across 6 regions and 9 prefectures. Additionally, there was a short follow-up in this study, but despite that, it is reassuring that the results were consistent with those from the previously published studies.
Conclusions
In a Japanese cohort, the Micra leadless pacemaker was implanted with a high degree of success, improvement in physical frailty, and a low major complication rate that was similar to rates observed in previous Micra studies. Thus, despite the Japan cohort being older, smaller, and at higher risk, the safety and performance results were in line with global Micra trials.
Disclosures
K. Ando: honorarium from Medtronic, Japan Lifeline, Terumo, Bristol Myers Squibb, Abbott Japan, Biotronik; T. Harada: honorarium from Medtronic, Bayer; Y. Yoshida: honorarium from Medtronic, Biotronik, Toray; K. Kusano: honorarium from Medtronic, Daiichi-Sankyo, Boehringer-Ingelheim, Pfizer, and research funding from Medtronic, Hitachi, Biotronik, JSR; S. Sasaki: department endowed by Medtronic, Biotronik, Fukuda Denshi; M. Shoda: honorarium from Medtronic, Philips Japan, Cook Medical Japan, Abbott Japan, and department endowed from Medtronic, Abbott Japan, Biotronik, Boston Scientific Japan; N. Nishii: honorarium from Medtronic, Boston Scientific, Cook Medical Japan, and department endowed from Medtronic; A. Shiose: consultant for Edwards Lifesciences, Terumo, Japan Lifeline, NIPRO, honorarium from Abbott Japan, Edwards Lifesciences, CSL Behring, Century Medical, Terumo, Medtronic, Mallinckrodt Pharmaceuticals, research funding from AIR WATER, Edwards Lifesciences, Corcym Japan, Century Medical, Medtronic, LivaNova, and scholarship funds/donations from ITI, Abbott Japan, Edwards Lifesciences, Kanaya Ikakiki, KISHIYA, JMS, GM Medical, Century Medical, Senko Medical Instrument, Terumo, Medtronic, Heiwa bussan; C. Okai, K. Stromberg, J. Murphy, T.R. Holmes: Medtronic employees; K. Soejima: remuneration from Medtronic and an Associate Editor for the Circulation Journal; K. Inoue, S. Shizuta, T. Onuki, Y. Watari, A. Fukui, J. Hosoda: no conflicts of interest to disclose.
IRB Information
Listing of Site Ethics Committees (EC no., Site name, Ethics Committee). 749, Kyorin University Hospital, Kyorin University Ethics Committee; B19050001, Yokohama City University Hospital, Yokohama City University Ethics Committee; F2018C110, Showa University Fujigaoka Hospital, Showa University Fujigaoka Hospital Institutional Review Board; R19042, National Cerebral and Cardiovascular Center, National Cerebral and Cardiovascular Center Ethics Committee; 19032704, Kokura Memorial Hospital, Kokura Memorial Hospital Ethics Committee; 4378, St. Marianna University School of Medicine Hospital, St. Marianna University School of Medicine Hospital Ethics Committee; 19-024, Sakakibara Heart Institute, Sakakibara Heart Institute Ethics Committee; Teirin No. 19-105, Teikyo University Hospital, Teikyo University Hospital Ethics Committee; 1347, Japanese Red Cross Aichi Medical Center Nagoya Daini Hospital, Japanese Red Cross Aichi Medical Center Nagoya Daini Hospital Ethics Committee; R2102, Kyoto University Hospital, Kyoto University Graduate School and Faculty of Medicine Ethics Committee; 38, Yamagata Prefectural Central Hospital, Yamagata Prefectural Central Hospital Ethics Committee; 1906-010, Okayama University Hospital, Okayama University Graduate School of Medicine, Dentistry and Pharmaceutical Sciences and Okayama University Hospital Ethics Committee; 2019-214, Kyushu University Hospital, Kyushu University Insititutional Review Board for Clinical Research; 2019-1053, Hirosaki University Hospital, Hirosaki University Ethics Committee; H31-4, Tokyo Women’s Medical University Hospital, Tokyo Women’s Medical University Ethics Committee.
Data Availability
The deidentified participant data used in this study will not be made available.
Supplementary Files
Please find supplementary file(s);
https://doi.org/10.1253/circj.CJ-23-0269
References
- 1.
Kirkfeldt RE, Johansen JB, Nohr EA, Jorgensen OD, Nielsen JC. Complications after cardiac implantable electronic device implantations: An analysis of a complete, nationwide cohort in Denmark. Eur Heart J 2014; 35: 1186–1194.
- 2.
Reddy VY, Exner DV, Cantillon DJ, Doshi R, Bunch TJ, Tomassoni GF, et al. Percutaneous implantation of an entirely intracardiac leadless pacemaker. N Engl J Med 2015; 373: 1125–1135.
- 3.
El-Chami MF, Al-Samadi F, Clementy N, Garweg C, Martinez-Sande JL, Piccini JP, et al. Updated performance of the Micra transcatheter pacemaker in the real-world setting: A comparison to the investigational study and a transvenous historical control. Heart Rhythm 2018; 15: 1800–1807.
- 4.
Roberts PR, Clementy N, Al Samadi F, Garweg C, Martinez-Sande JL, Iacopino S, et al. A leadless pacemaker in the real-world setting: The Micra Transcatheter Pacing System Post-Approval Registry. Heart Rhythm 2017; 14: 1375–1379.
- 5.
Piccini JP, Cunnane R, Steffel J, El-Chami MF, Reynolds D, Roberts PR, et al. Development and validation of a risk score for predicting pericardial effusion in patients undergoing leadless pacemaker implantation: Experience with the Micra transcatheter pacemaker. Europace 2022; 24: 1119–1126.
- 6.
Ritter P, Duray GZ, Steinwender C, Soejima K, Omar R, Mont L, et al. Early performance of a miniaturized leadless cardiac pacemaker: The Micra Transcatheter Pacing Study. Eur Heart J 2015; 36: 2510–2519.
- 7.
Satake S, Arai H. The revised Japanese version of the Cardiovascular Health Study criteria (revised J-CHS criteria). Geriatr Gerontol Int 2020; 20: 992–993.
- 8.
Satake S, Shimokata H, Senda K, Kondo I, Toba K. Validity of Total Kihon Checklist Score for predicting the incidence of 3-year dependency and mortality in a community-dwelling older population. J Am Med Dir Assoc 2017; 18: 552.e1–552.e6.
- 9.
Su L, Wang S, Wu S, Xu L, Huang Z, Chen X, et al. Long-term safety and feasibility of left bundle branch pacing in a large single-center study. Circ Arrhythm Electrophysiol 2021; 14: e009261.
- 10.
Chen K, Zhang S, Wu L, Liu X, Su Y, Zhou Y, et al. A prospective, multicenter, single-arm study of performance of the micra transcatheter pacemaker in chinese patients: A comparison to the global experience. Int J Heart Rhythm 2021; 6: 47–53.
- 11.
Roberts PR, Clementy N, Mondoly P, Winter S, Bordachar P, Sharman D, et al. A leadless pacemaker in the real-world setting: Patient profile and performance over time. J Arrhythm 2023; 39: 1–9.
- 12.
Soejima K, Asano T, Ishikawa T, Kusano K, Sato T, Okamura H, et al. Performance of leadless pacemaker in Japanese patients vs. rest of the world: Results from a global clinical trial. Circ J 2017; 81: 1589–1595.
- 13.
Piccini JP, El-Chami M, Wherry K, Crossley GH, Kowal RC, Stromberg K, et al. Contemporaneous comparison of outcomes among patients implanted with a leadless vs. transvenous single-chamber ventricular pacemaker. JAMA Cardiol 2021; 6: 1187–1195.
- 14.
Matsubara T, Sumiyoshi M, Kimura A, Minami-Takano A, Maruyama K, Kimura Y, et al. Trend in age at the initial pacemaker implantation in patients with bradyarrhythmia: A 50-year analysis (1970–2019) in Japan. Circ J 2022; 86: 1292–1297.
- 15.
Kato N, Kondo M, Okubo I, Hasegawa T. Length of hospital stay in Japan 1971–2008: hospital ownership and cost-containment policies. Health Policy 2014; 115: 180–188.