論文ID: CJ-23-0474
論文ID: CJ-23-0474
Background: Epicardial adipose tissue (EAT) is recognized as a clinical diagnostic marker for cardiometabolic disease. Thicker EAT may be associated with recurrence of ventricular tachycardia after ablation. The association between EAT volume and recurrence of premature ventricular complexes (PVC) following ablation has not been clarified. We investigated the association between EAT volume and PVC recurrence following radiofrequency catheter ablation.
Methods and Results: This retrospective study included 401 patients with PVC undergoing catheter ablation with preprocedural non-contrast computed tomography between 2017 and 2022. The impact of EAT volume in predicting PVC recurrence after ablation was analyzed. The mean (±SD) age of patients was 50.2±13.3 years. Multivariable Cox analysis revealed that a large EAT volume was an independent predictor of PVC recurrence after ablation during a median follow-up of 16.3 months. Kaplan-Meier analysis showed a difference in postablation PVC recurrence between the 2 groups dichotomized around the EAT volume cut-off. The risk of recurrence increased with increasing EAT volume according to restricted cubic spline regression. Furthermore, PVC originating from epicardial locations had larger EAT volumes than those originating from the right ventricular outflow tract.
Conclusions: A large EAT volume was independently associated with PVC recurrence following ablation. Patients with PVC originating from epicardial sites had large EAT volumes. EAT volume may help stratify patients according to their risk of PVC recurrence after ablation.
Radiofrequency catheter ablation (RFCA) is the primary therapy for improving outcomes in patients with premature ventricular complexes (PVC).1 Approximately 80–85% of PVC patients have at least an 80% reduction in PVC burden after ablation.2,3 There has been a heightened emphasis on PVC risk factor modification to reduce recurrence after PVC ablation.4
Epicardial adipose tissue (EAT) accumulates between the visceral epicardium and the myocardium.5 EAT can cover up to 80% of the cardiac surface, is mainly distributed in the atrial and interventricular grooves, and makes up as much as 20% of the heart’s mass.6,7 EAT is closely linked to the heart and directly contributes to the development of arrhythmias.8 Previous studies reported a relationship between EAT thickness and the incidence and severity of atrial fibrillation.9,10 Pericardial fat is linked to the frequency of PVC, which indicates the arrhythmogenic potential of pericardial fat.11 We think that direct evidence of an association between EAT and PVC is still insufficient. In this study, we aimed to investigate the association between preprocedural EAT volume measured by non-contrast computed tomography (CT) and postablation PVC recurrence.
This retrospective study included PVC patients who underwent their first RFCA between January 2017 and March 2022 at the First Affiliated Hospital of Zhengzhou University. The inclusion criteria were as follows: (1) acute procedural success; (2) non-contrast CT; (3) a total PVC count ≥10,000 beats by 24-h Holter monitoring; and (4) the absence of structural heart disease. Patients aged ≤18 years and those with a history of prior RFCA or poor CT image quality were excluded. Patients lost to follow-up before the first follow-up were also excluded. Patients with a diagnosis of PVC were identified in the electronic health record systems of the First Affiliated Hospital of Zhengzhou University using the International Classification of Disease (ICD) code. In the First Affiliated Hospital of Zhengzhou University, non-contrast CT was performed for hospitalized patients who were being examined for various reasons, including smoking history, shortness of breath, or screening for lung diseases. Smokers and drinkers were classified as having a past or current history of smoking and drinking.
This study complied with the Declaration of Helsinki. The study protocol was authorized by the Ethics Committee of the First Affiliated Hospital of Zhengzhou University, and the need for written informed consent was waived.
CT AcquisitionAll patients underwent CT using a dual-source CT system (Somatom Force; Siemens Healthineers, Erlangen, Germany). Consecutive CT scans were performed within a single session without any change in the patient’s body position. Non-contrast CT was performed at 120 kV. The images were reconstructed with a slice thickness of 0.5 mm, a medium soft tissue convolution kernel (B26F), and a reconstructed matrix size of 512×512.
EAT Volume QuantificationEAT volume was measured using semiautomatic software (syngo via Frontier Cardiac Risk Assessment, version 1.2.3; Siemens Healthineers), as shown in Supplementary Figure 1. EAT was defined as a density ranging from −195 to −45 Hounsfield units (HUs) on non-contrast CT.12,13 The software automatically delineated and identified the EAT, with the contours of EAT volume manually adjusted if necessary. EAT volume was measured by 2 radiologists (H.G. and L.R.) who were unaware of the patients’ conditions.
Ablation ProcedureAntiarrhythmic drugs were stopped for 5 half-lives before ablation, if possible. The ablation procedure was performed when the patient was awake. The PVC were spontaneous or isoproterenol induced. Isoproterenol was started at a low dose (1 μg/min) and gradually increased to induce PVC.
The earliest activation site was mapped using an irrigated-tip mapping catheter (ThermoCool; Biosense Webster, Inc., Irvine, CA, USA). The irrigation rate was 17 mL/min with a power of 30–40 W for endocardial-side ablation and 15–30 W for epicardial-side ablation. If radiofrequency energy was applied, it was used for 30–60 s. If the PVC were terminated during the energy application, the application was continued for 120–200 s at the site. If mapping demonstrated a suitable ablation site within the coronary venous system, ablation was attempted within the coronary vein. If ablation at the earliest endocardial site and coronary sinus ablation were ineffective or transiently effective, a subxiphoid puncture was performed for subsequent ablation. Acute procedural success was defined as no occurrence of spontaneous or inducible PVC assessed using an isoproterenol infusion over an at least 30-min observation period after the final ablation lesion and the absence of the predominant PVC in the 24-h period after ablation. The origin of PVC was categorized as the right ventricular outflow tract (RVOT), left ventricular outflow tract (LVOT), cusp, papillary muscle, epicardial, or other.
Outcomes and Follow-upNon-recurrence of PVC was assessed by electrocardiogram (ECG) and 24-h Holter monitoring, and was defined as an at least 80% decrease in PVC burden after ablation compared with before ablation. ECG and Holter monitoring were performed at 1 month, then at 3- to 6-month intervals during the 1-year follow-up, and then at 6- to 12-month intervals thereafter. In addition, patients with PVC symptoms were asked to complete 1 additional outpatient visit. PVC recurrence was detected by ECG, 24-h Holter monitoring, and additional clinical assessments during the follow-up period. Patients were followed up by clinic visits or telephone interviews up to September 2022. The endpoint measure was PVC recurrence following ablation. All patients with successful procedures were able to discontinue antiarrhythmic drugs after RFCA.
Statistical AnalysisContinuous variables are presented as the mean±SD or median with the interquartile range (IQR), and comparisons between groups were made using Student’s t-test or the Mann-Whitney U test, according to whether the data were normally distributed. Categorical variables are presented as numbers and percentages, and were compared using Pearson’s Chi-squared test or Fisher’s exact test. Pearson analysis was used to measure the correlation between EAT volume and continuous variables. Multivariable Cox regression analysis was used to investigate risk factors associated with PVC recurrence after ablation. Variables with P<0.01 in univariable analysis were input into the multivariable Cox regression model, which included age, body mass index (BMI), plasma triglycerides (TG), left ventricular end-diastolic diameter (LVEDD), left ventricular ejection fraction (LVEF), an RVOT origin location, and EAT volume (per 10-mL increase). The cut-off value for EAT volume was defined by a receiver operating characteristic (ROC) curve. Kaplan-Meier curves were drawn to assess the time to PVC recurrence stratified by the EAT volume cut-off (128.80 mL). We used restricted cubic spline (RCS) curves with knots at the 5th, 25th, 75th, and 95th centiles to flexibly model the association between EAT volume and PVC recurrence risk.
Furthermore, we performed subgroup analyses to investigate the association between EAT volume and recurrence in specific patient groups. We also explored the association between EAT volume and the original sites of the PVC, using an RVOT origin as the reference. Statistical analyses were conducted using R Language version 4.0.3 (R Foundation for Statistical Computing, Vienna, Austria). P values presented in this study are the result of 2-tailed tests.
In all, 659 PVC patients who underwent RFCA with pre-ablation non-contrast CT were screened for eligibility. Acute procedural success for PVC was achieved in 85.2% of patients (415/487). In all, 401 patients were included in the final analysis, as shown in Figure 1. The median time from non-contrast CT to RFCA was 2 days (IQR 1–3 days).
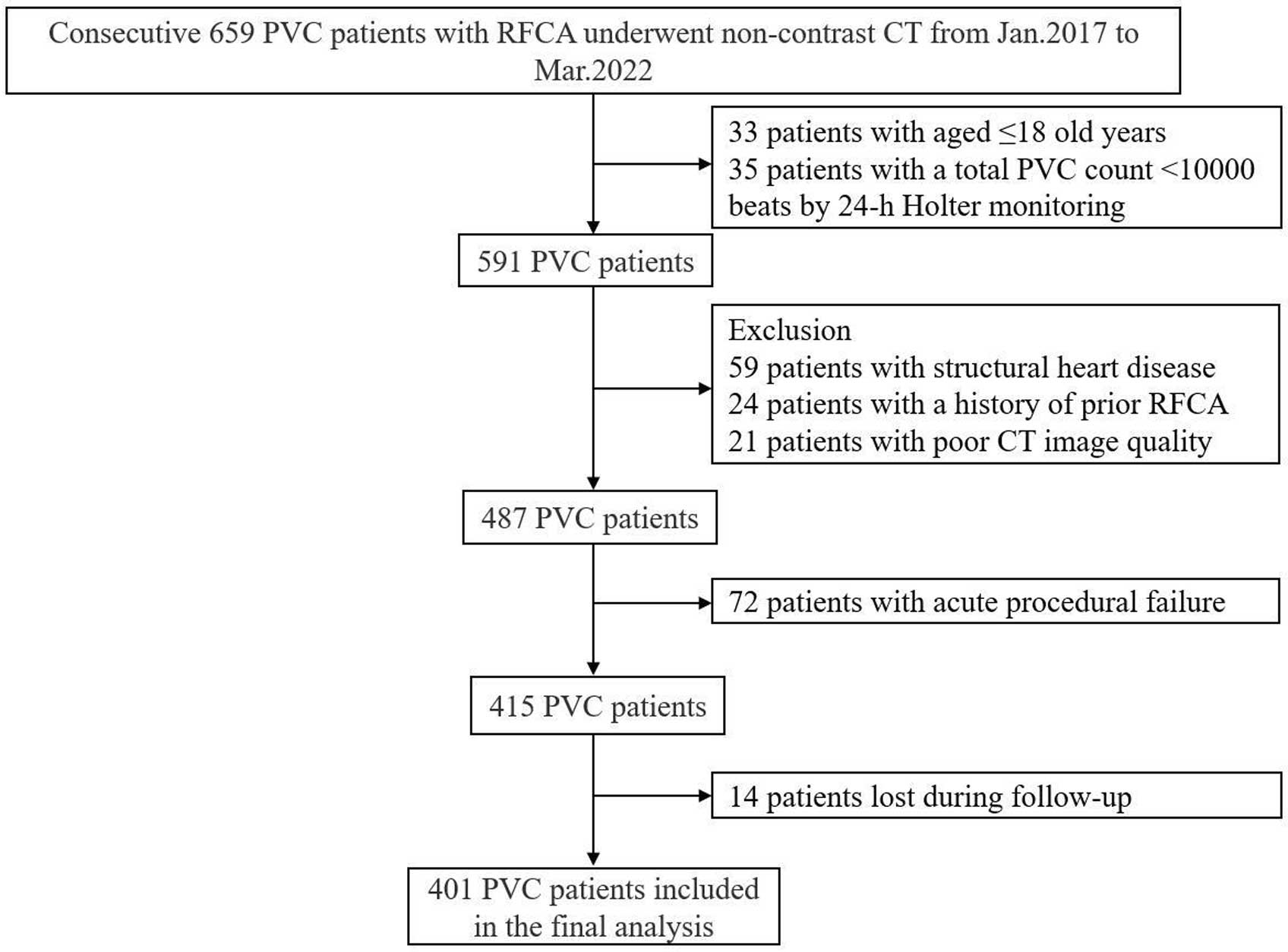
Patient selection and study design. CT, computed tomography; PVC, premature ventricular complexes; RFCA, radiofrequency catheter ablation.
The median follow-up period was 16.3 months (range 10.1–26.6 months), and 18.2% (73/401) of PVC patients experienced recurrence. PVC patients were divided into 2 groups according to whether PVC recurred. Patients with PVC recurrence were more likely to be older (P=0.011) and have a larger BMI (P=0.049) and EAT volume (P<0.001), higher plasma TG concentrations (P=0.034), and lower LVEF (P=0.009) than those without recurrence (Table 1).
Baseline Characteristics in All Patients and According to Postablation Recurrence of PVC
| All | No PVC recurrence |
PVC recurrence |
P value | |
|---|---|---|---|---|
| No. patients | 401 | 328 | 73 | |
| Age (years) | 50.2±13.3 | 49.4±13.2 | 53.8±12.9 | 0.011 |
| Age ≥65 years | 70 (17.5) | 51 (15.5) | 19 (26.0) | 0.033 |
| Female sex | 217 (54.1) | 178 (54.3) | 39 (53.4) | 0.896 |
| BMI (kg/m2) | 24.9±3.5 | 24.8±3.6 | 25.7±3.4 | 0.049 |
| BMI ≥24 kg/m2 | 239 (59.6) | 190 (57.9) | 49 (67.1) | 0.148 |
| Smoker | 75 (18.7) | 57 (17.4) | 18 (24.7) | 0.149 |
| Drinker | 60 (15.0) | 48 (14.6) | 12 (16.4) | 0.696 |
| Hypertension | 135 (33.7) | 108 (32.9) | 27 (37.0) | 0.507 |
| Diabetes | 41 (10.2) | 32 (9.8) | 9 (12.3) | 0.512 |
| Medication on admission | ||||
| ACEI/ARB | 73 (18.2) | 59 (17.8) | 14 (19.2) | 0.622 |
| Statins | 52 (13.0) | 39 (11.9) | 13 (17.8) | 0.173 |
| Laboratory test | ||||
| WBC (mmol/L) | 6.3±1.7 | 6.2±1.7 | 6.5±1.8 | 0.157 |
| LDL-C (mmol/L) | 2.3±0.8 | 2.3±0.7 | 2.4±0.8 | 0.325 |
| HDL-C (mmol/L) | 1.2±0.3 | 1.2±0.3 | 1.1±0.3 | 0.595 |
| TC (mmol/L) | 3.9±0.9 | 3.9±0.8 | 4.0±0.9 | 0.495 |
| TG (mmol/L) | 1.2 [0.9–1.7] | 1.2 [0.9–1.7] | 1.4 [1.0–1.8] | 0.034 |
| FPG (mmol/L) | 5.0±1.2 | 5.0±1.2 | 5.0±1.2 | 0.932 |
| hs-CRP (>2 mg/L) | 88/315 (27.9) | 66/251 (26.3) | 22/64 (34.4) | 0.198 |
| Echocardiographic variables | ||||
| LVEF (%) | 61.7±5.2 | 62.1±4.7 | 59.9±6.6 | 0.009 |
| LVEDD (mm) | 47.3±5.9 | 47.0±5.4 | 48.6±7.4 | 0.085 |
| CT variable | ||||
| EAT volume (mL) | 132.4±57.5 | 125.0±52.6 | 165.5±66.7 | <0.001 |
| Procedural characteristics | ||||
| Total procedure time (min) | 136 [121–162] | 135 [121–161] | 138 [121–169] | 0.142 |
| Total ablation time (s) | 502 [297–724] | 498 [298–723] | 535 [289–753] | 0.385 |
| Origin locations of PVCs | <0.001 | |||
| RVOT | 188 (46.9) | 168 (51.2) | 20 (27.4) | |
| Non-RVOT sites | 213 (53.1) | 160 (48.8) | 53 (72.6) | |
Unless indicated otherwise, data are given as the mean±SD, median [interquartile range], or n (%). ACEI, angiotensin-converting enzyme inhibitor; ARB, angiotensin receptor blocker; BMI, body mass index; CT, computed tomography; EAT, epicardial adipose tissue; FPG, fasting blood glucose; HDL-C, high-density lipoprotein cholesterol; hs-CRP, high-sensitivity C-reactive protein; LDL-C, low-density lipoprotein cholesterol; LVEDD, left ventricular end-diastolic dimension; LVEF, left ventricular ejection fraction; PVC, premature ventricular complexes; RVOT, right ventricular outflow tract; TC, total cholesterol; TG, triglycerides; WBC, white blood cells.
Procedure-Related Characteristics
Eighteen patients had procedure-related complications: 10 (2.5%) with vascular access complications, 1 (0.2%) with atrioventricular block, 1 (0.2%) with pulmonary embolism, 2 (0.5%) with transient ischemic attack, and 4 (1.0%) with pericardial effusion. There were no significant differences in the total procedure time (P=0.14) or total ablation time (P=0.39) between patients with and without PVC recurrence. Of the 401 patients with PVC, 188 (46.9%), 32 (8.0%), 64 (16.0%), 21 (5.2%), 37 (9.2%), and 59 (14.7%) had PVC originating from the RVOT, LVOT, cusp, papillary muscle, epicardial, and other sites, respectively (Supplementary Figure 2). Patients without PVC recurrence more often had an RVOT origin than patients with PVC recurrence (P<0.001). The highest rate of PVC recurrence was in patients with a papillary muscle origin (38.1%); the lowest recurrence rate was in patients with an RVOT origin (10.6%; Supplementary Figure 3).
EAT Volume CharacteristicsEAT volume distribution is shown in Figure 2. The mean EAT volume was 132.4±57.5 mL. The association between EAT volume and clinical characteristics was assessed (Supplementary Table). EAT volume was larger in PVC patients who were aged ≥65 years, male, and had a BMI ≥24 kg/m2, those with diabetes, and smokers (P<0.05; Supplementary Figure 4). In addition, of the 401 patients with PVC included in this study, 146 (36.4%) had non-sustained ventricular tachycardia (VT). There was a no significant increase in patients with PVCs accompanied by non-sustained VT compared with patients not accompanied by non-sustained VT (139.7±55.7 vs. 128.3±58.2 mL, respectively; P=0.056).
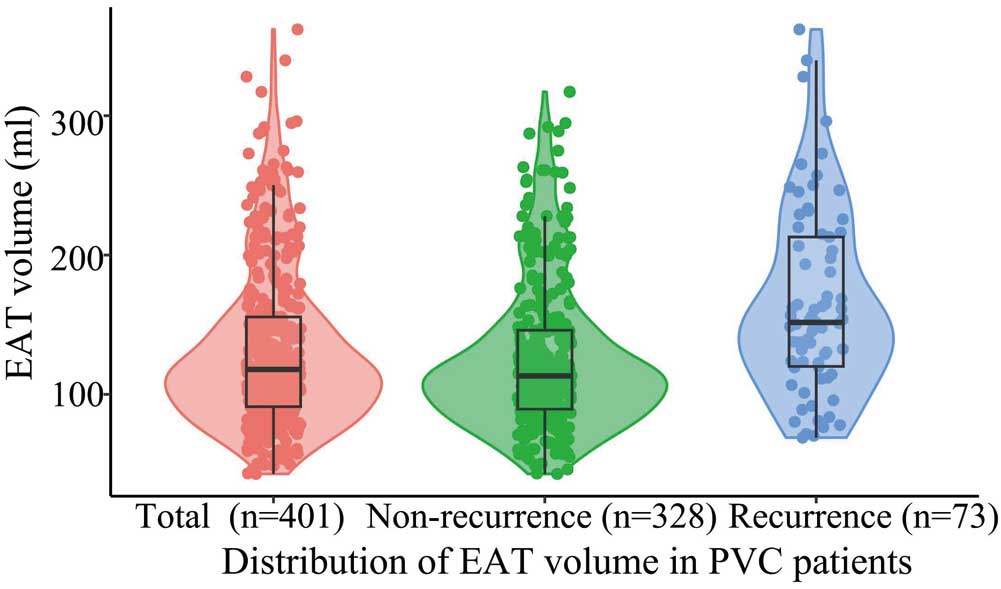
Violin plots showing the distribution of epicardial adipose tissue (EAT) volume in all patients with premature ventricular complexes (PVC) and according to the recurrence of PVC after ablation. Different colored symbols represent measured EAT volume. The boxes indicate the interquartile range, with the horizontal line in each box indicating the median. The vertical lines represent the 95% confidence intervals for the data.
Predictive Value of EAT
Multivariable Cox analysis revealed that EAT volume (per 10-mL increase; hazard ratio [HR] 1.08; 95% confidence interval [CI] 1.04–1.12; P<0.001) and an RVOT origin (HR 0.53; 95% CI 0.31–0.91; P=0.020) were independent predictors for postablation PVC recurrence after adjusting for other factors. From ROC analysis, the EAT volume cut-off value was 128.80 mL (Supplementary Figure 5). When EAT volume was stratified according to the cut-off EAT volume (128.80 mL), a large EAT volume was also independently associated with a high risk of PVC recurrence (Table 2). Kaplan-Meier curves for freedom from PVC recurrence are shown in Figure 3. Recurrence risk increased as EAT volume increased (P for non-linearity=0.360) by RCS analysis, with the cut-off EAT volume (128.80 mL) from ROC analysis as the reference (Supplementary Figure 6).
Multivariate Cox Regression Analysis of Risk Factors for Recurrence of PVC After Ablation
| Variables | Model 1 | Model 2 | ||
|---|---|---|---|---|
| HR (95% CI) | P value | HR (95% CI) | P value | |
| Age | 1.00 (0.98–1.02) | 0.979 | 1.00 (0.98–1.02) | 0.795 |
| BMI | 1.00 (0.94–1.07) | 0.987 | 1.00 (0.93–1.07) | 0.962 |
| TG | 0.98 (0.72–1.34) | 0.907 | 1.00 (0.74–1.35) | 0.988 |
| LVEDD | 0.97 (0.92–1.03) | 0.303 | 0.98 (0.93–1.03) | 0.465 |
| LVEF | 0.95 (0.90–1.00) | 0.052 | 0.96 (0.92–1.02) | 0.165 |
| Origin location of RVOT | 0.53 (0.31–0.91) | 0.020 | 0.49 (0.29–0.83) | 0.008 |
| EAT volume (per 10-mL increase) | 1.08 (1.04–1.12) | <0.001 | ||
| EAT volume (as a categorical variable) | 3.56 (2.02–6.26) | <0.001 | ||
The variables with P<0.01 in univariable analysis were used in the multivariable regression model. Multivariable analysis Model 1 included age, BMI, TG, LVEDD, LVEF, RVOT as the location of origin of PVC, and EAT volume (per 10-mL increase). Multivariable analysis Model 2 included age, BMI, TG, LVEDD, LVEF, RVOT as the location of origin of PVC, and EAT volume as a categorical variable. The cut-off point for EAT volume to predict PVC recurrence was 128.80 mL based on receiver operating characteristic curve analysis. CI, confidence interval; HR, hazard ratio. Other abbreviations as in Table 1.

Kaplan-Meier curves for the recurrence of premature ventricular complexes (PVC) following ablation. From receiver operating characteristic curve analysis, the cut-off point for epicardial adipose tissue (EAT) volume to predict PVC recurrence was 128.80 mL.
Further Analysis of EAT Volume
In the subgroup analysis, a large EAT volume was significantly associated with a high recurrence risk according to different subgroups in terms of age (P<0.05). No significant interaction was observed in the subgroup analysis (P>0.05), as shown in Figure 4. In subgroup analysis according to site of origin, for those with a site of origin in the RVOT, cusp, epicardial, and other site, EAT volume was larger in those with than without PVC recurrence (P<0.05). However, there was no significant difference in EAT volume between those with and without PVC recurrence patients for sites of origin in the LVOT and papillary muscle (P>0.05; Supplementary Figure 7). In addition, EAT volume was significantly larger in patients with PVC originating from an epicardial location than in those with PVC originating from an RVOT location (P=0.001; Figure 5).

Subgroup analysis of the prognostic value of epicardial adipose tissue (EAT) volume (per 10-mL increase) for recurrence after ablation in patients with premature ventricular complexes (PVC). BMI, body mass index; CI, confidence interval; DM, diabetes; HR, hazard ratio.

Comparison of mean (±SD) epicardial adipose tissue (EAT) volume in patients with premature ventricular complexes (PVC) according to the location of origin. The symbols represent measured EAT volume. LVOT, left ventricular outflow tract; RVOT, right ventricular outflow tract.
This study has several findings. First, we first found that large EAT volume was an independent predictor for PVC recurrence after ablation. Second, the risk of PVC recurrence increased as EAT volume increased. Third, patients with PVC originating from the epicardial location had a large EAT volume. These findings indicate that EAT volume may be a valuable marker for predicting the risk of PVC recurrence.
An increasing number of studies are investigating the association between EAT and cardiac arrhythmias.14 A large EAT volume remained independently associated with atrial fibrillation recurrence after ablation.15 Kırış et al reported that EAT thicknesses was greater in 50 patients with PVC than in 50 control subjects.16 One study revealed that the EAT thickness of the right atrioventricular groove is a predictor of VT recurrence after ablation.17 Another cross-sectional study also found that a large EAT thickness, measured by transthoracic echocardiography, is an independent risk factor for ablation success in 106 PVC patients. Compared with the previous cross-sectional study,2 in the present study we measured EAT volume using non-contrast CT rather than thickness using ultrasound. We first revealed that EAT volume was an independent predictor of PVC recurrence after ablation during follow-up.
The recurrence rate is high after ablation for PVC originating from an epicardial location.4 An EAT thickness >7 mm is an important reason for failure of epicardial ablation.18 The presence of fat accumulation has been associated with a decrease in the amplitude of the bipolar electrogram.19 Fragmented and isolated electrograms are also more frequently observed in areas with fat accumulation, which is more likely to induce arrhythmias.20 Our data suggest that EAT volume was larger in patients with PVC originating from an epicardial location, which may indicate that PVC patients with a large EAT volume have a high risk of recurrence after ablation. We considered that ablation by an epicardial approach may acutely terminate PVC but is less effective in the long term than radiofrequency ablation (RFA) of PVC in the endocardium. This difference may be because RFA is less likely to lead to the effective ablation of lesions in PVC patients with accumulated EAT.21 Furthermore, subgroup analysis of different patients according to the origin of PVC revealed that patients with recurrence had a larger EAT volume, except in the case of PVC originating from the LVOT and papillary muscle, which may be due to biased results because of the small sample size in the subgroup analysis or a lack of stability of the catheter to ablate at these sites. A larger EAT volume is also a risk factor for several cardiovascular hazard factors (diabetes, age ≥65 years, overweight, hypertension, and smoking), which can enhance the risk of arrhythmia development.5,22 Clinical cohorts often include multiple PVC risk factors that have been shown to act synergistically to drive the progression of arrhythmia.5,22 EAT volume could be more meaningful for assessing the risk status of PVC patients.
The exact mechanism linking EAT and PVC has not been fully established. Several critical mechanisms can be used to illustrate this regulation. EAT is an essential source of energy for the myocardium during periods of heightened energy demand through lipolysis and fat oxidation.23–25 EAT is also a relevant source of inflammatory cytokines and profibrotic factors, which may affect the surrounding myocardium.26 Fat infiltration into the myocardium contributes to the development of arrhythmias by triggering automaticity and damaging neighboring myocardial cells.27 Research has demonstrated a correlation between EAT and the occurrence of ventricular arrhythmia (VA) in the RVOT. Notably, there is an increased susceptibility to inducible VA when rapid pacing is performed in the EAT-connected RVOT.28 EAT has been found to prolong action potential duration in myocytes, indicating a higher propensity for arrhythmogenic activity compared with adipose tissue from other locations.29 Parisi et al30 found that EAT is a site of catecholamine biosynthesis. Through the secretion of catecholamines, EAT may directly contribute to an increased sympathetic tone and a sympathovagal imbalance, which has been proven to be associated with the incidence and maintenance of arrhythmias.31 EAT may enhance the occurrence of PVC through increased trigger activity. EAT alters cardiac electrophysiology by creating an anatomical barrier, thereby delaying activation. The heterogeneity of conduction is caused by the uneven distribution of EAT throughout the myocardium and the uncoupling of myocardial cells. The resulting heterogeneity in conduction slowing promotes the occurrence of re-entry mechanisms.32 Furthermore, paracrine communication between EAT and the myocardium causes myocardial fibrosis, prolonged action potential duration, and the depolarization of cardiomyocytes.32
In our study we used non-contrast CT to measure EAT volume. Non-contrast CT can be used for rapid and reliable clinical quantification of EAT volume.33 Marwan et al34 found that CT-based EAT volume quantification is feasible compared with contrast coronary CT angiography by adjusting the measured upper limit value. Non-contrast CT scans have good concordance and reproducibility with coronary CT angiography.35 Non-contrast CT has the advantages of convenience, a reduced radiation dose and intensity, and better suitability for the general population.36
Although our findings are significant, several limitations remain in the present study. This was a single-center retrospective study, which may lead to selection bias. Patients enrolled in our study rarely underwent cardiac magnetic resonance imaging, which may have resulted in patients with cardiomyopathy being missed. However, our study follow-up was prospective and enhanced the data quality. We could not assess the potential association between the origin of PVC and the thickness of EAT. To address this issue, further research may be needed using cardiac magnetic resonance imaging to quantify EAT thickness and to investigate the impacts of EAT thickness on the origin of PVC. In addition, the PVC patients included in this study were from a tertiary referral center, and the procedure was performed by experienced operators, which may not be representative of the general population. Our study did not further quantify inflammation parameters by positron emission tomography and detailed flow cytometry profiling of lymphocyte populations, so the relationship between EAT and inflammatory factors needs to be further evaluated. Finally, we examined non-contrast CT images with decreased image quality despite intrinsic movement artifacts. The feasibility and reliability of EAT quantification in non-contrast CT acquisition needs further study.
In our study, a large EAT volume was independently associated with PVC recurrence following RFCA. RFA is more difficult in PVC patients with a large EAT volume and does not easily lead to effective ablation. Patients with PVC originating from epicardial sites had a large EAT volume. EAT may alter the properties of local electrophysiology and provide a way to stratify the risk of PVC patients after ablation.
We are grateful to the Dr. Lichen Ren which measured EAT volume data in this research.
This study was supported by the Capital Health Research and Development of Special Fund (2020-2-4065), National High Level Hospital Clinical Research Fund (2022-NHLHCRF-PY-19) and Beijing Medical and Health Fund (2022-HX-23).
The authors have no conflicts of interest to declare.
Z.W. and Y.S. conceived the study. Z.W., J.L., J.C., H.G., H.H., and S.J. contributed to the data collection. H.G., H.H., and Y.C. contributed to the methodology. Z.W. and J.L. drafted the manuscript. J.D. and Y.S. modified the manuscript. All authors reviewed and endorsed the final manuscript.
The Ethics Committee of The First Affiliated Hospital of Zhengzhou University approved the study protocol (Approval no. 2022-KY-0288).
The deidentified participant data will not be shared.
Please find supplementary file(s);
https://doi.org/10.1253/circj.CJ-23-0474