Abstract
Background: This post hoc subanalysis aimed to investigate the impact of polyvascular disease (PolyVD) in patients with acute myocardial infarction (AMI) in the contemporary era of percutaneous coronary intervention (PCI).
Methods and Results: The Japan Acute Myocardial Infarction Registry (JAMIR), a multicenter prospective registry, enrolled 3,411 patients with AMI between December 2015 and May 2017. Patients were classified according to complications of a prior stroke and/or peripheral artery disease into an AMI-only group (involvement of 1 vascular bed [1-bed group]; n=2,980), PolyVD with one of the complications (2-bed group; n=383), and PolyVD with both complications (3-bed group; n=48). The primary endpoint was all-cause death. Secondary endpoints were major adverse cardiovascular events (MACE), including cardiovascular death, non-fatal myocardial infarction, non-fatal stroke, and major bleeding. In the 1-, 2-, and 3-bed groups, the cumulative incidence of all-cause death was 6.8%, 17.5%, and 23.7%, respectively (P<0.001); that of MACE was 7.4%, 16.4%, and 33.8% (P<0.001), respectively; and that of major bleeding was 4.8%, 10.0%, and 13.9% (P<0.001), respectively. PolyVD was independently associated with all-cause death (hazard ratio [HR] 2.21; 95% confidence interval [CI], 1.48–3.29), MACE (HR 2.07; 95% CI 1.40–3.07), and major bleeding (HR 1.68; 95% CI 1.04–2.71).
Conclusions: PolyVD was significantly associated with worse outcomes, including thrombotic and bleeding events, in the contemporary era of PCI in AMI patients.
An acute myocardial infarction (AMI) is one of the most important presentations of atherosclerotic disease. The number of patients with AMI who are hospitalized continues to increase in the aging society in Japan.1 For patients who manifest with ST-segment elevation diagnostic of ST-segment elevation myocardial infarction (STEMI), emergency reperfusion has been the cornerstone of treatment. Antiplatelet therapy is a critical component of the medical regimen in the acute phase following coronary stent implantation, as well as for secondary prevention. Although the antithrombotic benefit of dual anti-platelet therapy (DAPT) has become well established, it is associated with an increased risk of bleeding, in particular when using potent P2Y12
inhibitors.
Polyvascular disease (PolyVD), characterized by atherothrombotic lesions in multiple vascular beds (e.g., cerebrovascular disease, peripheral artery disease [PAD], or coronary artery disease), poses a significant risk for future cardiovascular events.2 In addition to having multiple vascular systems already compromised, it has been reported that a lesser likelihood of receiving appropriate therapies is associated with a poor prognosis.3,4 Therefore, the aim of the present post hoc subanalysis of the Japanese Association of Myocardial Infarction Registry (JAMIR) was to investigate the incidence of PolyVD and its impact on prognosis in the context of current AMI management, in which the use of drug-eluting stents (DES) and optimal medical therapy (OMT) is more established.
Methods
Study Population
The design of the JAMIR study has been described in detail elsewhere.5 Briefly, from December 2015 to May 2017, the JAMIR enrolled consecutive patients from 50 institutions who presented with spontaneous onset of AMI to examine ischemic and bleeding events in Japanese patients with AMI, as well as the association between those events and antiplatelet therapy, including the potent P2Y12
inhibitor prasugrel. AMI was diagnosed according to the universal definition.6,7 Patients were excluded if they were admitted to hospital ≥24 h after onset, if they had no return of spontaneous circulation on admission after an out-of-hospital cardiopulmonary arrest or if they had AMI as a complication of percutaneous coronary intervention (PCI) or coronary artery bypass graft surgery (CABG). Patient management, including the choice of antiplatelet drugs, was at the discretion of the treating physician. Finally, 3,411 patients from 50 institutions in Japan were enrolled in the JAMIR study.
Figure 1 shows patient selection in the present study. Patients were divided into 3 groups based on complications of a prior stroke and PAD as follows: (1) an AMI-only group, with neither complication (involving 1 vascular bed only; 1-bed group); (2) a PolyVD group with one of the complications (2-bed group); and (3) a PolyVD group with both complications (3-bed group). Prior stroke was defined as a history of a transient ischemic attack, ischemic stroke, cerebral hemorrhage, or subarachnoid hemorrhage. PAD was defined as the presence of >50% stenosis in the peripheral arteries, including the kidneys, iliac, and femoral arteries, symptoms of intermittent claudication, an ankle-brachial index ≤0.9, and a history of toe amputation, bypass surgery, or endovascular treatment.

This study was conducted in accordance with the ethical guidelines for medical research on humans outlined in the Declaration of Helsinki. The research protocol was approved by the Institutional Review Board of the National Cerebral and Cardiovascular Center (M27-019-12) and the local ethics committees or local institutional review board at each study site. Due to the observational nature of the registry, informed consent was not obtained. However, the study details were made available on a website and at the study sites to inform the subjects about the study design and timeline, providing them with the opportunity to decline participation in this registry (opt-out). Furthermore, the research secretariat ensured compliance with opt-out procedures at each study site. This study was registered with the Japanese University Hospital Medical Information Network (UMIN) Clinical Trials Registry (UMIN000019479).
Study Endpoints
The primary endpoint of the present study was all-cause death within 1 year. Secondary endpoints were major adverse cardiovascular events (MACE), defined as a composite of a cardiovascular death, non-fatal myocardial infarction, and non-fatal stroke, and major bleeding, defined as Type 3 or 5 bleeding based on the Bleeding Academic Research Consortium (BARC) criteria.8 The definitions of these clinical events have been described in detail elsewhere.5 The investigators, clinical research coordinators, and local data managers at each study site registered the data using the JAMIR registration system. A follow-up study of patients was conducted 1 year after the onset of AMI based on the medical information available at each study site. A letter requesting follow-up was sent to the patients whose medical information was not available at the study sites after 1 year due to hospital transfer or other reasons. When the letter was sent, appropriate informed consent was obtained.5
Statistical Analysis
Continuous variables are presented as the median with interquartile range (IQR) and categorical variables are presented as the number and percentage of patients. Patient background and treatment were compared among the 3 groups. In particular, the thrombotic and bleeding risks of patients were compared among the 3 groups by referring to the CREDO-Kyoto thrombotic and bleeding risk scores.9 For comparisons among the 3 groups, the Kruskal-Wallis test was used for continuous variables and the Chi-squared test or Fisher’s exact test was used for categorical variables, followed by post hoc tests with a Bonferroni correction. The cumulative incidence of the primary and secondary endpoints was evaluated using the Kaplan-Meier method, and differences were assessed using log-rank tests. Multivariate Cox proportional hazard models were used to estimate hazard ratios (HRs) and 95% confidence intervals (CIs) of PolyVD on the primary and secondary endpoints by a forced entry method. Confounding factors in the multivariate model included fundamental background variables, well-established prognostic factors for patients with AMI,10–12 and the prognostic factors reported in previous studies within this registry.13,14 Finally, age, male sex, body mass index, hypertension, diabetes, dyslipidemia, current smoking, prior myocardial infarction, prior CABG, atrial fibrillation, left main trunk culprit, extracorporeal membrane oxygenation, Killip Class IV, estimated glomerular filtration rate <60 mL/min/1.73 m2, systolic blood pressure <90 mmHg, and left ventricular ejection fraction <40% were included in the models as confounding factors.
All analyses were performed using SPSS Statistics 19.0 (SPSS Inc., Chicago, IL, USA), and statistical significance was considered at P<0.05 in a two-tailed test.
Results
Baseline Characteristics of the Study Patients
The characteristics of patients in each of the 3 groups are presented in Table 1; 11.2% (n=383) and 1.4% (n=48) patients were in the 2- and 3-bed groups, respectively. Patients in the AMI-only (1-bed) group (n=2,980) were younger and had a lower prevalence of a Killip IV and multivessel disease compared with the other 2 groups. Body mass index, the prevalence of current smoking and STEMI, left ventricular ejection fraction, estimated glomerular filtration rate, and hemoglobin, low density lipoprotein cholesterol (LDL-C), and triglyceride levels decreased from the AMI-only group to the 2-bed and then 3-bed group. The prevalence of hypertension, diabetes, prior myocardial infarction, prior PCI, prior CABG, malignancy, atrial fibrillation, left main trunk culprit, and the use of an intra-aortic balloon pump increased from the AMI-only group to the 2-bed group and then to the 3-bed group. Radial access was more frequent in the AMI-only group, with the rate of radial access decreasing from the 2- to the 3-bed group. The 3-bed group had a lower rate of a primary PCI and higher rate of extracorporeal membrane oxygenation than the other 2 groups. DES were used in >80% of patients overall, with the highest percentage (89.5%) observed in the AMI-only group.
Table 1.
Background Characteristics
| |
AMI-only
(n=2,980) |
PolyVD 2-bed
(n=383) |
PolyVD 3-bed
(n=48) |
P value |
| Age (years) |
68.0 [58.0–77.0] |
75.0 [67.0–82.0]* |
74.5 [69.0–81.0]† |
<0.001 |
| Age ≥75 years |
959 (32.2) |
203 (53.0)* |
24 (50.0)† |
<0.001 |
| Male sex |
2,276 (76.4) |
301 (78.6) |
37 (77.1)† |
0.63 |
| Body mass index (kg/m2) |
23.6 [21.4–26.0] |
22.9 [20.9–25.3]* |
22.0 [19.6–24.9] |
<0.001 |
| HT |
2,114 (70.9) |
313 (81.7)* |
42 (87.5)† |
<0.001 |
| DLP |
2,041 (68.5) |
251 (65.5) |
35 (72.9) |
0.39 |
| DM |
991 (33.3) |
167 (43.6)* |
29 (60.4)† |
<0.001 |
| Current smoking |
1,238 (41.5) |
120 (31.3)* |
8 (16.7)† |
<0.001 |
| Prior myocardial infarction |
243 (8.2) |
71 (18.5)* |
13 (27.1)† |
<0.001 |
| PAD |
0 (0) |
96 (25.1)* |
48 (100)†,‡ |
<0.001 |
| Prior stroke |
0 (0) |
287 (74.9)* |
48 (100)†,‡ |
<0.001 |
| Prior PCI |
294 (9.9) |
83 (21.7)* |
16 (33.3)† |
<0.001 |
| Prior CABG |
58 (1.9) |
20 (5.2)* |
7 (14.6)† |
<0.001 |
| Malignancy |
227 (7.6) |
56 (14.6) |
9 (18.8)† |
<0.001 |
| Atrial fibrillation |
171 (5.7) |
51 (13.3)* |
7 (14.6)† |
<0.001 |
| STEMI |
2,329 (78.2) |
269 (70.2)* |
28 (58.3)† |
<0.001 |
| Killip Class IV |
251 (8.4) |
52 (13.6)* |
6 (12.5) |
0.003 |
| SBP (mmHg) |
141.0 [119.0–161.0] |
134.0 [113.0–155.0]* |
143.0 [114.5–162.5] |
0.003 |
| SBP <90 mmHg |
166 (5.8) |
42 (11.4)* |
5 (10.4) |
<0.001 |
| Heart rate (beats/min) |
77.0 [65.0–90.0] |
80.0 [63.0–95.0] |
84.5 [75.0–100.8]† |
0.002 |
| LVEF (%) |
52.9 [45.0–60.0] |
50.0 [40.8–60.0]* |
46.5 [37.5–59.3] |
0.007 |
| LVEF <40% |
298 (12.7) |
57 (19.2)* |
11 (32.4)† |
<0.001 |
| Puncture site |
| Radial |
1,920 (66.2) |
199 (54.7)* |
22 (52.4) |
<0.001 |
| Femoral |
927 (32.0) |
154 (42.3)* |
17 (40.5) |
<0.001 |
| Brachial |
54 (1.9) |
11 (3.0) |
3 (7.1) |
0.022 |
| Culprit lesion |
| LMT |
60 (2.0) |
18 (4.7)* |
3 (6.3) |
0.001 |
| LAD |
1,399 (46.9) |
161 (42.0) |
20 (41.7) |
0.16 |
| LCX |
448 (15.0) |
60 (15.7) |
5 (10.4) |
0.63 |
| RCA |
1,048 (35.2) |
135 (35.2) |
14 (29.2) |
0.69 |
| Multivessel disease |
1,210 (41.7) |
189 (51.9)* |
21 (50.0) |
0.001 |
| Thrombolysis |
15 (0.5) |
3 (0.8) |
1 (2.4) |
0.23 |
| Primary PCI |
2,715 (93.6) |
334 (91.8) |
33 (78.6)†,‡ |
<0.001 |
| Stent use |
2,494 (91.9) |
290 (86.8)* |
28 (84.8) |
0.004 |
| DES use |
2,430 (89.5) |
278 (83.2)* |
28 (84.8) |
0.002 |
| Concomitant PCI for non-culprit lesion |
154 (5.3) |
24 (6.6) |
3 (7.1) |
0.53 |
| IABP |
394 (13.2) |
63 (16.4) |
12 (25.0) |
0.017 |
| ECMO |
60 (2.0) |
9 (2.3) |
3 (6.3) |
0.12 |
| CABG during hospitalization |
79 (2.7) |
10 (2.6) |
4 (8.3) |
0.056 |
| Hb (mg/dL) |
14.1 [12.7–15.4] |
13.1 [11.6–14.5]* |
12.7 [10.8–14.3]† |
<0.001 |
| Creatinine (mg/dL) |
0.9 [0.7–1.1] |
1.0 [0.8–1.3]* |
1.2 [0.8–2.4]†,‡ |
<0.001 |
| eGFR (mL/min/1.73 m2) |
66.4 [52.2–80.8] |
55.9 [37.7–71.2]* |
47.3 [21.6–63.4]†,‡ |
<0.001 |
| eGFR <60 mL/min/1.73 m2 |
1,122 (37.7) |
219 (57.2)* |
34 (70.8)† |
<0.001 |
| HDL-C (mg/dL) |
46.0 [38.0–55.0] |
44.0 [38.0–53.0] |
46.0 [38.0–52.0] |
0.11 |
| LDL-C (mg/dL) |
119.0 [96.0–143.0] |
103.0 [78.0–128.5]* |
96.0 [72.0–115.0]† |
<0.001 |
| Triglycerides (mg/dL) |
107.0 [72.0–162.0] |
96.0 [62.0–135.5]* |
85.0 [60.5–111.5] |
<0.001 |
| Peak CK (IU/L) |
1,567.0
[591.0–3,378.0] |
1,232.0
[374.0–2,918.0]* |
831.5
[258.0–2,081.5]† |
<0.001 |
| Medications used during hospitalization |
| Aspirin |
2,918 (97.9) |
359 (93.7)* |
43 (89.6)† |
<0.001 |
| Clopidogrel |
452 (15.2) |
127 (33.2)* |
25 (52.1)†,‡ |
<0.001 |
| Prasugrel |
2,491 (83.6) |
242 (63.2)* |
16 (33.3)†,‡ |
<0.001 |
| Cilostazol |
20 (0.7) |
6 (1.6) |
6 (12.5)†,‡ |
<0.001 |
| Oral anticoagulants |
374 (12.6) |
82 (21.4)* |
12 (25.0)† |
<0.001 |
| β-blockers |
1,929 (64.7) |
225 (58.7) |
25 (52.1) |
0.017 |
| RAS inhibitors |
2,270 (76.2) |
269 (70.2)* |
29 (60.4)† |
0.002 |
| Statins |
2,676 (89.8) |
313 (81.7)* |
38 (79.2) |
<0.001 |
| Proton pump inhibitors |
2,714 (91.1) |
337 (88.0) |
41 (85.4) |
0.068 |
| DAPT duration |
|
* |
† |
<0.001 |
| <6 months |
671 (27.2) |
100 (41.8) |
11 (68.8) |
|
| 6–12 months |
1,045 (42.3) |
77 (32.2) |
3 (18.8) |
|
| >12 months |
753 (30.5) |
62 (25.9) |
2 (12.5) |
|
Unless indicated otherwise, data are expressed as n (%) or median [interquartile range]. P values were obtained by comparing the 3 groups using the Kruskal-Wallis test for continuous variables and the Chi-squared test or Fisher’s exact test for categorical variables. *P<0.0167, AMI-only vs. PolyVD in 2-bed; †P<0.0167, AMI-only vs. PolyVD in 3-bed; ‡P<0.0167, PolyVD 2-bed vs. PolyVD 3-bed. 2-bed, 2 vascular beds; 3-bed, 3 vascular beds; AMI, acute myocardial infarction; CABG, coronary artery bypass graft surgery; CK, creatine kinase; DAPT, dual antiplatelet therapy; DES, drug-eluting stent; DLP, dyslipidemia; DM, diabetes; ECMO, extracorporeal membrane oxygenation; eGFR, estimated glomerular filtration rate; Hb, hemoglobin; HDL-C, high density lipoprotein cholesterol; HT, hypertension; IABP, intra-aortic balloon pump; LAD, left anterior descending artery; LCX, left circumflex artery; LDL-C, low density lipoprotein cholesterol; LMT, left main trunk; LVEF, left ventricular ejection fraction; PAD, peripheral artery disease; PCI, percutaneous coronary intervention; PolyVD, polyvascular disease; RAS, renin-angiotensin system; RCA, right coronary artery; SBP, systolic blood pressure; STEMI, ST-segment elevation myocardial infarction.
Regarding medications used during hospitalization, the rate of prescriptions for clopidogrel, cilostazol, and oral anticoagulants increased significantly from the AMI-only group to the 2-bed group to the 3-bed group. The rate of prescriptions for aspirin, prasugrel, β-blockers, renin-angiotensin system (RAS) inhibitors, and statins decreased from the AMI-only group to the 2-bed and to the 3-bed group.
The duration of the DAPT became progressively shorter from the AMI-only group to the 2-bed group to the 3-bed group. Furthermore, 68.8% and 41.8% of patients in the 3- and 2-bed groups, respectively, continued the DAPT for <6 months.
Thrombotic and Bleeding Risks and Clinical Outcomes
The thrombotic and bleeding risks of patients, according to CREDO-Kyoto thrombotic and bleeding risk scores, are presented in Table 2. Both the thrombotic and bleeding risk scores increased from the AMI-only group to the 2-bed group to the 3-bed group.
Table 2.
Background Characteristics of Patients Determined to Be at High Thrombotic and Bleeding Risk According to CREDO‐Kyoto
| |
AMI-only
(n=2,980) |
PolyVD 2-bed
(n=383) |
PolyVD 3-bed
(n=48) |
P value |
| A. CREDO-Kyoto thrombotic risk scores |
| Severe CKD |
209 (7.0) |
67 (17.5)* |
16 (33.3)†,‡ |
<0.001 |
| Atrial fibrillation |
171 (5.7) |
51 (13.3)* |
7 (14.6)† |
<0.001 |
| Peripheral vascular diseaseA |
0 (0) |
96 (25.1)* |
48 (100)†,‡ |
<0.001 |
| Anemia (Hb <11 g/dL) |
270 (9.1) |
69 (18.0)* |
12 (25.0)† |
<0.001 |
| Age ≥75 years |
959 (32.2) |
203 (53.0)* |
24 (50.0)† |
<0.001 |
| History of heart failure |
655 (22) |
120 (31.3)* |
20 (41.7)† |
<0.001 |
| DM |
991 (33.3) |
167 (43.6)* |
29 (60.4)† |
<0.001 |
| CTO |
N/A |
N/A |
N/A |
– |
| Total score without CTO data |
1.0 [0.0–2.0] |
4.0 [3.0–5.0]* |
5.0 [3.0–6.0]†,‡ |
<0.001 |
| B. CREDO-Kyoto bleeding risk scores |
| Low platelet count (<100,000/μL) |
N/A |
N/A |
N/A |
– |
| Severe CKD |
209 (7.0) |
67 (17.5)* |
16 (33.3)†,‡ |
<0.001 |
| Peripheral vascular disease |
0 (0) |
383 (100.0)* |
48 (100.0)† |
<0.001 |
| History of heart failure |
655 (22.0) |
120 (31.3)* |
20 (41.7)† |
<0.001 |
| Prior myocardial infarction |
243 (8.2) |
71 (18.5)* |
13 (27.1)† |
<0.001 |
| Malignancy |
227 (7.6) |
56 (14.6)* |
9 (18.8)† |
<0.001 |
| Atrial fibrillation |
171 (5.7) |
51 (13.3)* |
7 (14.6)† |
<0.001 |
| Total score without low platelet data |
0.0 [0.0–2.0] |
3.0 [2.0–4.0]* |
4.0 [2.0–5.0]† |
<0.001 |
Unless indicated otherwise, data are expressed as the n (%) or median [interquartile range]. P values were obtained by comparing the 3 groups using the Kruskal-Wallis test for continuous variables and the chi-square test or Fisher’s exact test for categorical variables. *P<0.0167, AMI-only vs. PolyVD in 2-bed; †P<0.0167, AMI-only vs. PolyVD in 3-bed; ‡P<0.0167, PolyVD 2-bed vs. PolyVD 3-bed. APeripheral vascular disease was defined as PAD in the present study. CKD, chronic kidney disease; CTO, chronic total occlusion; N/A, not applicable. Other abbreviations as in Table 1.
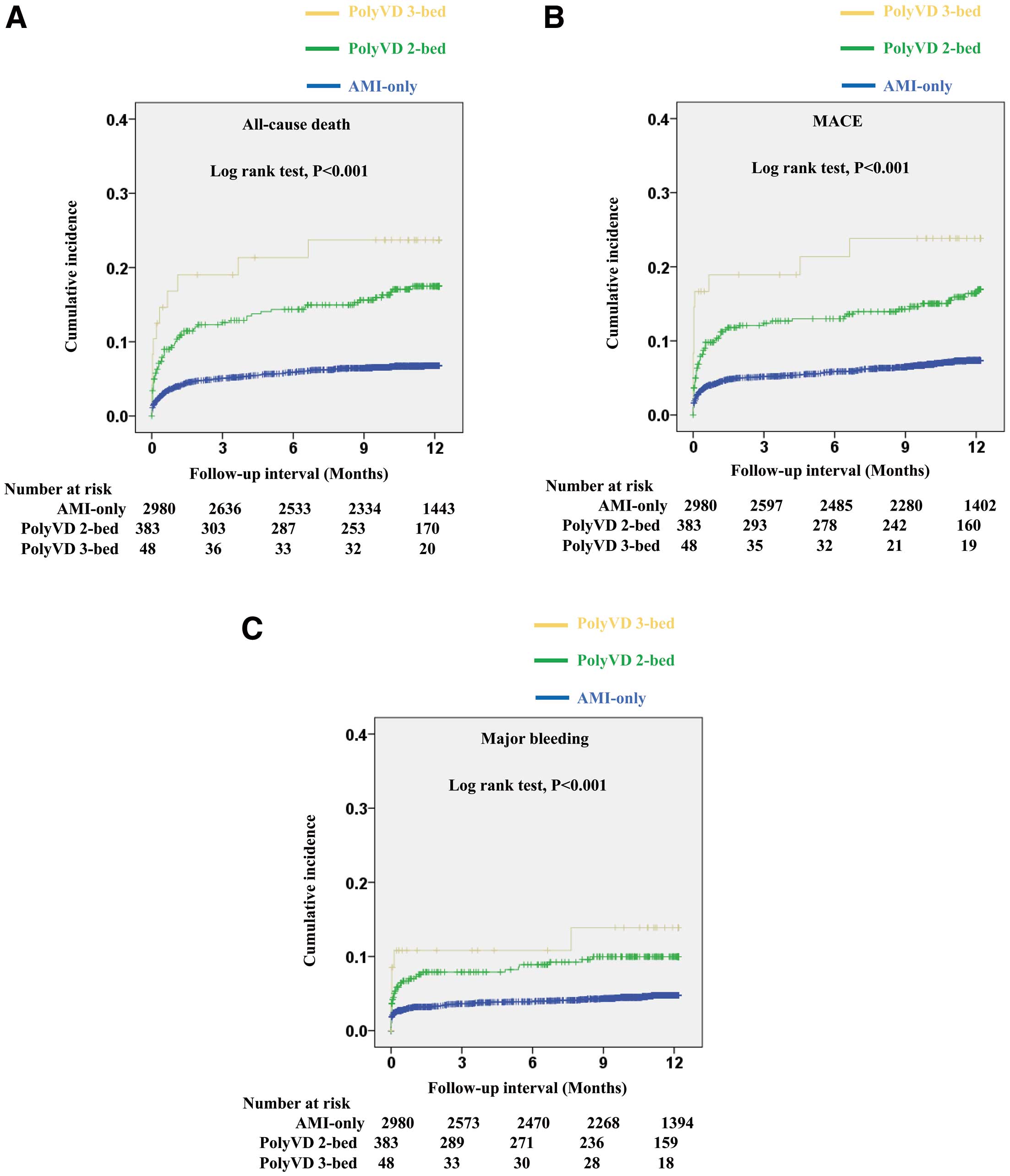
Figure 2 shows Kaplan-Meier curves for all-cause death, MACE, and major bleeding in the 3 groups. According to the number of diseased beds, the cumulative incidence at 1 year increased significantly for all-cause death (6.8%, 17.5%, and 23.7% in the 1- [AMI only], 2-, and 3-bed groups, respectively; log-rank P<0.001), MACE (7.4%, 16.4%, and 23.8% in the 1-, 2-, and 3-bed groups, respectively; log-rank P<0.001), and major bleeding (4.8%, 10.0%, and 13.9% in the 1-, 2-, and 3-bed groups, respectively; log-rank P<0.001).
In the multivariate Cox proportional hazards analysis, PolyVD was independently associated with a higher all-cause death (HR 2.21; 95% CI 1.48–3.29; P<0.001), MACE (HR 2.07; 95% CI 1.40–3.07; P<0.001), and major bleeding (HR 1.68; 95% CI 1.04–2.71; P=0.035; Table 3).
Table 3.
Adjusted Hazard Ratios for All-Cause Death, MACE, and Major Bleeding
| |
All-cause death |
MACE |
Major bleeding |
| OR (95% CI) |
P value |
OR (95% CI) |
P value |
OR (95% CI) |
P value |
| PolyVD |
2.21 (1.48–3.29) |
<0.001 |
2.07 (1.40–3.07) |
<0.001 |
1.68 (1.04–2.71) |
0.035 |
| Age |
1.06 (1.04–1.08) |
<0.001 |
1.03 (1.01–1.04) |
0.003 |
1.05 (1.03–1.07) |
<0.001 |
| Male sex |
0.96 (0.63–1.47) |
0.855 |
1.19 (0.79–1.81) |
0.408 |
0.62 (0.40–0.96) |
0.034 |
| Body mass index |
1.02 (0.97–1.07) |
0.452 |
1.00 (0.96–1.05) |
0.878 |
0.98 (0.92–1.03) |
0.427 |
| HT |
0.95 (0.63–1.43) |
0.802 |
0.71 (0.49–1.03) |
0.072 |
1.17 (0.72–1.90) |
0.529 |
| DM |
1.10 (0.76–1.59) |
0.611 |
1.08 (0.76–1.54) |
0.676 |
1.01 (0.67–1.54) |
0.96 |
| DLP |
0.66 (0.46–0.95) |
0.025 |
0.68 (0.48–0.97) |
0.032 |
0.86 (0.57–1.31) |
0.488 |
| Current smoking |
0.90 (0.58–1.40) |
0.654 |
0.75 (0.50–1.11) |
0.152 |
0.89 (0.54–1.47) |
0.65 |
| Prior myocardial infarction |
0.82 (0.47–1.41) |
0.467 |
0.88 (0.50–1.53) |
0.647 |
0.79 (0.40–1.57) |
0.509 |
| Prior CABG |
2.05 (0.96–4.39) |
0.064 |
1.46 (0.62–3.44) |
0.381 |
1.10 (0.34–3.56) |
0.877 |
| Atrial fibrillation |
1.33 (0.81–2.17) |
0.257 |
1.75 (1.11–2.75) |
0.017 |
1.66 (0.96–2.86) |
0.07 |
| LMT culprit |
1.49 (0.75–2.94) |
0.255 |
1.39 (0.68–2.84) |
0.36 |
1.00 (0.38–2.63) |
0.994 |
| ECMO |
6.70 (3.67–12.24) |
<0.001 |
5.55 (2.89–10.65) |
<0.001 |
8.75 (4.15–18.46) |
<0.001 |
| Killip Class IV |
2.46 (1.42–4.26) |
0.001 |
2.34 (1.33–4.10) |
0.003 |
2.57 (1.31–5.02) |
0.006 |
| eGFR <60 mL/min/1.73 m2 |
1.17 (0.77–1.76) |
0.461 |
1.14 (0.78–1.65) |
0.503 |
1.17 (0.75–1.82) |
0.495 |
| SBP <90 mmHg |
1.04 (0.57–1.89) |
0.894 |
0.99 (0.54–1.83) |
0.983 |
0.69 (0.32–1.52) |
0.358 |
| LVEF <40% |
4.35 (2.91–6.51) |
<0.001 |
2.24 (1.49–3.38) |
<0.001 |
2.12 (1.30–3.46) |
0.003 |
CI, confidence interval; MACE, major adverse cardiovascular events. Other abbreviations as in Table 1.
Clinical outcomes at 1 year are presented in Supplementary Table 1. The incidence of all-cause death, cardiovascular death, non-cardiovascular death, stroke, BARC Type 3 or 5 bleeding, BARC Type 5 bleeding, and blood transfusions due to bleeding events increased from the AMI-only group to the 2-bed group to the 3-bed group. In contrast, the prevalence of myocardial infarction, stent thrombosis, and intracranial bleeding was comparable among the 3 groups.
Lipid Profile at 1 Year
The patient lipid profile at 1 year is presented in Table 4. There were no significant differences in high-density lipoprotein cholesterol and triglyceride levels among the 3 groups, whereas LDL-C levels were significantly lower in the 3-bed group than in the other 2 groups. In the AMI-only group, the ratio of LDL-C at 1 year to that at admission was the lowest, and the all-cause death rate was also low (Supplementary Table 2). Conversely, in the 3-bed group, although LDL-C levels at 1 year were the lowest compared with the other 2 groups, the rate of all-cause death was the highest.
Table 4.
Lipid Profile at 1 Year
| |
AMI-only
(n=2,980) |
PolyVD 2-bed
(n=383) |
PolyVD 3-bed
(n=48) |
P value |
| HDL-C (mg/dL) (n=2,252) |
48.0 (40.0–57.0) |
47.0 (38.0–53.0) |
47.0 (36.3–54.8) |
0.10 |
| n=2,009 |
n=219 |
n=24 |
|
| LDL-C (mg/dL) (n=2,287) |
80.0 (67.0–95.0) |
81.0 (66.0–99.0) |
60.0 (52.0–83.0)†,‡ |
0.022 |
| n=2,045 |
n=219 |
n=23 |
|
| Triglycerides (mg/dL) (n=2,276) |
123.0 (87.0–183.0) |
124.0 (85.0–166.0) |
124.0 (73.0–209.0) |
0.57 |
| n=2,026 |
n=227 |
n=23 |
|
Unless indicated otherwise, data are expressed as median [interquartile range] with number of applicable patients. P values were obtained by comparing the 3 groups using the Kruskal-Wallis test for continuous variables and the Chi-squared test or Fisher’s exact test for categorical variables. *P<0.0167, AMI-only vs. PolyVD in 2-bed; †P<0.0167, AMI-only vs. PolyVD in 3-bed; ‡P<0.0167, PolyVD 2-bed vs. PolyVD 3-bed. Abbreviations as in Table 1.
Discussion
The major findings in this study are that: (1) the prevalence of 2- and 3-bed PolyVD in patients with AMI was 11.2% and 1.4%, respectively; (2) the cumulative incidence of all-cause death, MACE, and major bleeding increased significantly in accordance with the number of PolyVD beds, but LDL-C levels were significantly lower in the 3-bed group than in the other 2 groups; and (3) PolyVD was independently associated with the 1-year all-cause death, MACE, and major bleeding.
Prevalence and Impact of PolyVD Presenting With AMI
Prevalence, patient background, treatment, and in-hospital mortality among patients with and without PolyVD who presented with an AMI from observational studies are summarized in Table 5.15–19 The prevalence of PolyVD in patients with AMI is reported to range from 5.6% to 16.6%, with the prevalence of 3-bed PolyVD ranging from 0.4% to 1.9%,15–19 which is consistent with our findings. In the JAMIR study from Japan, where the population is rapidly aging,1 patients with and without PolyVD are older than reported in the other studies.15–19 In the JAMIR study, the prevalence of STEMI and the rate of PCI were higher than in the other studies, but the trend remained consistent, with a lower rate of both in patients with PolyVD than without PolyVD.15–19 Despite an increase in the rates of PCI and OMT, and similar to previous observational studies, the in-hospital mortality rate was still high in the JAMIR study.15–19 One possible explanation for this could be that the JAMIR study included the highest proportion of elderly patients and a higher frequency of Killip class IV patients, which are well-known worse prognostic factors in Japan,20,21 compared to the GRACE study, indicating a more severe patient profile. Regarding mid-term outcomes (defined as 6 months in the present study), the MASCARA and Gulf RACE-2 studies demonstrated mid-term mortalities that were double those of in-hospital mortalities. However, in the JAMIR study, mid-term mortality was less than double the in-hospital mortality. This suggests that even with a less favorable patient profile, the use of contemporary treatment approaches may have contributed to the more favorable mid-term outcome.
Table 5.
Observational Studies of PolyVD With AMI
| Studies |
Groups |
Patients
(%) |
Age
(years) |
Coronary risk factors |
STEMI
(%) |
Killip
Class IV |
PCI
(%) |
Medication during hospitalization |
In-hospital
mortality (%) |
Mid-term
mortality
(%) |
HT/DLP/DM/smoking
(%) |
β-blockers
(%) |
RAS inhibitors
(%) |
Statins
(%) |
Aspirin
(%) |
| GRACE15 |
AMI-only |
84.4 |
64.0 |
58/46/22/59 |
37 |
0.5 |
40.0 |
* |
* |
* |
* |
4.5 |
6M: 3.9 |
| 1999–2005 |
AMI+PAD |
7.6 |
71.0 |
72/58/38/69 |
26.0 |
0.7 |
31.0 |
* |
* |
* |
* |
7.2 |
6M: 8.8 |
| n=32,735 |
AMI+stroke |
6.2 |
73.0 |
78/52/24/53 |
29 |
0.3 |
28.0 |
* |
* |
* |
* |
8.9 |
6M: 9.3 |
| |
3-bed |
1.8 |
73.0 |
82/65/42/68 |
23.0 |
0.50 |
26.0 |
* |
* |
* |
* |
9.2 |
6M: 12.0 |
| ALLIANCE16 |
AMI-only |
87.0 |
65.0 |
48/43/19/59 |
24.0 |
N/A |
62.0 |
72 |
56 |
65 |
85 |
5.7 |
N/A |
| 2000–2005 |
AMI + PAD |
8.0 |
|
|
|
|
|
|
|
|
|
9.8 |
N/A |
| n=8,904 |
AMI+stroke |
4.0 |
|
|
|
|
|
|
|
|
|
14.3 |
N/A |
| |
3-bed |
1.0 |
|
|
|
|
|
|
|
|
|
13 |
N/A |
| |
PolyVD |
13.0 |
72.0 |
66/47/34/63 |
32.0 |
N/A |
48.0 |
64 |
61 |
60 |
82 |
11.6 |
N/A |
| MASCARA19 |
AMI-only |
83.4 |
66.9 |
58.3/46.4/28.1/28.3 |
41.5 |
7.0† |
42.6 |
71.3 |
50.9/6.0 |
71.6 |
84.1 |
4.8 |
6M: 10.8 |
| 2004–2005 |
AMI+PAD |
8.9 |
70.3 |
71.9/57.0/49.4/21.6 |
24.6 |
13.1† |
34.0 |
58.1 |
47.6/6.2 |
63.3 |
75.4 |
9.1 |
6M: 24.5 |
| n=6,745 |
AMI+stroke |
5.8 |
73.5 |
76.3/50.5/42.6/15.8 |
29.6 |
12.6† |
38.8 |
58.7 |
51.0/7.4 |
65.6 |
77.3 |
9.2 |
6M: 22.4 |
| |
3-bed |
1.9 |
72.5 |
69.5/53.4/51.9/20.6 |
23.7 |
16.4† |
30.5 |
48.1 |
42.0/9.2 |
64.9 |
51 |
16 |
6M: 29.8 |
| Gulf RACE-218 |
AMI-only |
94.4 |
56.0 |
45.3/36.0/38.0/54.0 |
47.0 |
|
22.0 |
75 |
ACEi: 71.0 |
95.0 |
98.5 |
4.2 |
1Y: 11.2 |
| 2008–2009 |
AMI+PAD |
1.4 |
|
|
|
|
|
|
|
|
|
12 |
|
| n=7,689 |
AMI+stroke |
3.7 |
|
|
|
|
|
|
|
|
|
7 |
|
| |
3-bed |
0.4 |
|
|
|
|
|
|
|
|
|
15 |
|
| |
PolyVD |
5.6 |
62.0 |
78.0/55.0/67.0/45.0 |
31.0 |
N/A |
12.4 |
68 |
ACEi: 69.0 |
92.5 |
96 |
9.1 |
1Y: 24.6 |
| JAMIR (present study) |
AMI-only |
87.4 |
68.0 |
70.9/68.5/33.3/41.5 |
78.2 |
8.4 |
93.6 |
64.7 |
76.2 |
89.8 |
97.9 |
4.5 |
1Y: 6.4 |
| 2015–2017 |
2-bed |
11.2 |
75.0 |
81.7/65.5/43.6/31.3 |
70.2 |
13.6 |
91.8 |
58.7 |
70.2 |
81.7 |
93.7 |
11.2 |
1Y: 16.2 |
| n=3,411 |
3-bed |
1.4 |
74.5 |
87.5/72.9/60.4/16.7 |
58.3 |
12.5 |
78.6 |
52.1 |
60.4 |
79.2 |
89.6 |
18.8 |
1Y: 22.9 |
*There were no significant differences in the use of evidence-based therapies or appropriateness levels across the groups. However, the use of appropriate therapies was suboptimal in all 4 groups, with only a 50% utilization of all indicated therapies in each group. †Killip Class III or IV. ‡ACEi/angiotensin II receptor antagonists. 1Y, 1-year mortality; 2-bed, polyvascular disease in 2 vascular beds; 3-bed, polyvascular disease in 3 vascular beds; 6M, 6-month mortality; ACEi, angiotensin-converting enzyme inhibitors; JAMIR, Japan Acute Myocardial Infarction Registry. Other abbreviations as in Table 1.
Association Between PolyVD and Thrombotic and Bleeding Risks and Events in AMI Patients
The CREDO-Kyoto risk scores identify patients at high risk of thrombotic and bleeding events after PCI.9 In the present study, patients with PolyVD had higher CREDO-Kyoto thrombotic risk scores (Table 2A), which may have been associated with the higher incidence of MACE than in the AMI-only group.
In the MASCARA study, PolyVD was reported to increase major bleeding according to the number of vascular beds, whereas in the Gulf RACE-2 study, PolyVD was reported to be unrelated to major bleeding.18,19 Previous Japanese observational studies reported that PAD and prior stroke were independently associated with major bleeding.9,22 In the present study, patients with PolyVD had higher CREDO-Kyoto bleeding risk scores (Table 2B) than those in the AMI-only group. This may be associated with the higher incidence of major bleeding in the PolyVD group than in the AMI-only group in the present study. We consider that differences in background factors, such as a higher age, higher frequency of renal failure, and higher rate of PCI procedures in the present study than in the Gulf RACE-2 study, may have contributed to the observed differences in bleeding events. Recently, concerns regarding an elevated risk of bleeding during antithrombotic therapy have been further raised due to the growing number of elderly patients with multiple comorbidities, such as PolyVD.5 The subsequent debate over post-PCI antithrombotic therapy has shifted from simply reducing the thrombotic risk to safely minimizing the bleeding risk.23 Due to the significant impact of bleeding on clinical outcomes, current guidelines on antithrombotic therapy prioritize the stratification of patients to identify those at high bleeding risk in determining post-PCI antithrombotic therapy.23
Treatment of PolyVD With Coronary Artery Disease
In the present study, there was a higher rate of DES use and a higher rate of OMT, including β-blockers, RAS inhibitors, and statins, compared with previous studies.24,25 In the CREDO-Kyoto Registry Cohort-2, which enrolled patients with coronary artery disease between 2005 and 2007, ticlopidine was the most commonly used thienopyridine (76.1%), whereas in the present study of patients with AMI, prasugrel was the most commonly used drug (80.6%).24 Conversely, it is important to note that as the number of PolyVD beds increased, the use of prasugrel decreased and the use of clopidogrel increased. The Trial to Assess Improvement in Therapeutic Outcomes by Optimizing Platelet Inhibition with Prasugrel–Thrombolysis in Myocardial Infarction (TRITON–TIMI) 38 trial found that prasugrel therapy reduced the incidence of ischemic events in patients with acute coronary syndrome, but also increased the incidence of major bleeding compared with clopidogrel therapy.26 Furthermore, clopidogrel has already been shown to have a superior effect on cardiovascular death compared with aspirin in the treatment of PAD.27 That may have influenced the use of antithrombotic drugs in the JAMIR study. Similar to previous studies, the JAMIR study showed that the treatment strategy in the PolyVD group was less aggressive, with a lower rate of PCI and suboptimal medical therapy (Table 5). An increased risk is often accompanied by heightened vulnerability. Although patients in the PolyVD 3-bed group had a poor prognosis, their LDL-C levels at 1 year were lower than those in the other 2 groups, and there were certain discrepancies between the clinical outcomes and LDL-C levels. It is possible that the individuals patients in the PolyVD 3-bed group already had advanced atherosclerotic changes, potentially making aggressive lipid-lowering therapy less effective. However, caution should be exercised in interpreting the results because 22.9% of patients in the PolyVD 3-bed group died within 1 year. Moreover, it is worth considering that the patients whose lipid profiles were measured after 1 year may specifically have represented those whose condition was relatively well managed (Supplementary Table 2). That is, it remains uncertain whether this group consisted of patients with optimal lipid management or whether the lipid-lowering therapy was ineffective in the PolyVD 3-bed group. Further investigations are required to understand the impact of aggressive lipid-lowering therapy on prognosis for patients in the PolyVD 3-bed group.
Clinical Implications
Table 1 presents data on DAPT duration in the 3 groups. Specifically, approximately 70% of patients in the 3-bed group and approximately 40% of those in the 2-bed group continued DAPT for <6 months. During the period when the JAMIR study was conducted, Japanese Circulation Society guidelines recommended 6- to 12-month DAPT with aspirin (81–162 mg/day) in combination with either clopidogrel (75 mg/day) or prasugrel (3.75 mg/day) following stent implantation.28 In addition, for patients who undergo DES implantation and are at high risk of bleeding, the guidelines suggested a DAPT duration <3 months.23 In the real-world practice of the JAMIR, clinicians prioritized bleeding risk; however, that approach did not result in a significant reduction in the incidence of BARC Type 3 or 5 bleeding. Instead, it led to a 2- to 3-fold increase in bleeding rates compared with the AMI-only group. Those findings indicate that the management of ‘appropriate’ antithrombotic therapy is likely more challenging in this patient population. Therefore, we must carefully consider the implications of these results for future research in this area.
Study Limitations
Our study had several limitations. First, in the JAMIR database, a prior stroke was defined as a history of a transient ischemic attack, ischemic stroke, cerebral hemorrhage, or subarachnoid hemorrhage. However, that content was unable to be specifically analyzed focusing on ischemic strokes. Second, baseline characteristics differed markedly among the 3 groups and, due to the observational nature of this study, residual or unmeasured confounding factors were likely to persist. Third, because we excluded patients who did not have a return of spontaneous circulation upon admission after an out-of-hospital cardiopulmonary arrest, those with a greater number of cardiovascular risk factors were more likely to be excluded from the study.
Conclusions
The JAMIR study demonstrated that AMI patients with PolyVD had both thrombotic and bleeding risk factors and that PolyVD was significantly associated with worse clinical outcomes, including thrombotic and bleeding events. These outcomes persisted despite major advancements in the contemporary era of PCI and the use of more potent antithrombotic and lipid-lowering therapies.
Acknowledgments
The authors thank all the investigators, clinical research coordinators, and data managers involved in the JAMIR study for their contributions. The members and institutions involved in the JAMIR study group are listed in the Appendix. The authors also thank Mr. John Martin (Company: John Martin Inc., Title: President, City: Maple Grove, State: Minnesota, Country: USA) for help with English language editing.
Sources of Funding
This work was planned by the Japan Cardiovascular Research Foundation and was funded by Daiichi Sankyo Co., Ltd.
Disclosures
S.Y. reports remuneration for lectures from Takeda, Daiichi Sankyo, and Bristol-Myers Squibb and trust research/joint research funds from Takeda and Daiichi Sankyo. M. Takayama reports lecture fees from Daiichi Sankyo. H.O. reports lecture fees and research grants from AstraZeneca, Bayer, Boehringer Ingelheim, Daiichi Sankyo, Pfizer, and Sanofi. Y.S., H.O., K.K., and S.Y. are members of Circulation Journal’s Editorial Team. The remaining authors have no conflicts of interest to declare.
IRB Information
This study was conducted in accordance with the Declaration of Helsinki. The research protocol was approved by the Institutional Review Board of the National Cerebral and Cardiovascular Center (M27-019-12) and local ethics committees or the local institutional review board at each study site.
Data Availability
Data will not be shared so as not to compromise the privacy of the research participants.
Appendix
Members of the JAMIR study group and participating institutions are listed below:
Hokkaido Medical Center: Takashi Takenaka; Hirosaki University: Hirofumi Tomita, Hiroaki Yokoyama; Iwate Medical University: Tomonori Ito, Masaru Ishida, Yorihiko Koeda; Yamagata University: Masafumi Watanabe, Tetsu Watanabe, Taku Toshima; Tohoku University: Hiroaki Shimokawa, Yasuhiko Sakata, Jun Takahashi, Kiyotaka Hao; Sakakibara Heart Institute: Tetsuya Sumiyoshi, Masamori Takayama; Yokohama City University Hospital: Kazuo Kimura, Masami Kosuge, Toshiaki Ebina; Showa University Fujigaoka Hospital: Hiroshi Suzuki, Atsuo Maeda; Mie University Hospital: Masaaki Ito, Tairo Kurita, Jun Masuda; Matsuzaka Central General Hospital: Takashi Tanigawa; Ehime University Hospital: Jitsuo Higaki, Kazuhisa Nishimura; Oita University: Naohiko Takahashi, Hidefumi Akioka, Kyoko Kawano; Nagasaki University: Koji Maemura, Yuji Koide; Kumamoto University: Sunao Kojima, Kenichi Tsujita; National Cerebral and Cardiovascular Center: Hisao Ogawa, Satoshi Yasuda, Yasuhide Asaumi, Kensaku Nishihira, Yoshihiro Miyamoto, Misa Takegami, Satoshi Honda; Nagasaki Harbor Medical Center: Hiroshi Nakajima; Isahaya General Hospital: Kenji Yamaguchi; Sapporo City General Hospital: Takao Makino; Sapporo Cardiovascular Clinic: Daitarou Kannno; Teine Keijinkai Hospital: Yasuhiro Omoto; Hokkaido Cardiology Hospital: Daisuke Otta; Sapporo Kosei General Hospital: Toshiya Sato; Nippon Medical School Musashi Kosugi Hospital: Naoki Sato, Arifumi Kikuchi, Michiko Sone, Koji Takagi; Ayase Heart Hospital: Imun Tei; Tokyo Metropolitan Hiroo General Hospital: Takashi Shibui, Sho Nagamine; Nippon Medical School Hospital: Wataru Shimuzu, Takeshi Yamamoto; Tokyo Saiseikai Central Hospital: Toshiyuki Takahashi; Tokyo Medical Center: Yukihiko Momiyama; St. Luke’s International Hospital: Atsushi Mizuno; Edogawa Hospital: Hiroshi Ohira; Kyorin University Hospital: Hideaki Yoshino, Youhei Shigeta; Nihon University Itabashi Hospital: Atsushi Hirayama, Yasuo Okumura, Daisuke Fukamachi, Tadateru Takayama; Toho University Omori Medical Center: Hiroki Niikura, Hiroki Takenaka; Mitsui Memorial Hospital: Shuzo Tanimoto, Kazuyuki Yahagi; Tokyo Metropolitan Tama Medical Center: Hiroyuki Tanaka; Disaster Medical Center: Yasuhiro Sato, Masakazu Ohno; Musashino Red Cross Hospital: Takamichi Miyamoto, Nobuhiro Hara; NTT Medical Center: Mikio Kishi; Oume Municipal General Hospital: Sigeo Shimizu, Ken Kurihara; Oghikubo Hospital: Yasuhiro Ishii; Teikyo University Hospital: Ken Kozuma, Yusuke Watanabe; Fraternity Memorial Hospital: Yasuhiro Takahashi; Jikei University Hospital: Michihiro Yoshimura, Satoshi Morimoto; Tokyo Women’s Medical University Hospital: Nobuhisa Hagiwara, Yuichiro Minami; Tokyo Medical University Hospital: Jun Yamashita; Osaki Citizen Hospital: Kaoru Iwabuchi, Takeshi Yamauchi; Sendai Open Hospital: Atsushi Kato, Shigeto Namiuchi; Sendai Medical Center: Tsuyoshi Shinozaki; Kesennuma City Hospital: Kazunori Ogata, Ryuji Tsuburaya
Supplementary Files
Please find supplementary file(s);
https://doi.org/10.1253/circj.CJ-23-0477
References
- 1.
Yasuda S, Miyamoto Y, Ogawa H. Current status of cardiovascular medicine in the aging society of Japan. Circulation 2018; 138: 965–967.
- 2.
Gutierrez JA, Aday AW, Patel MR, Jones WS. Polyvascular disease: Reappraisal of the current clinical landscape. Circ Cardiovasc Interv 2019; 12: e007385.
- 3.
McDermott MM, Mehta S, Ahn H, Greenland P. Atherosclerotic risk factors are less intensively treated in patients with peripheral arterial disease than in patients with coronary artery disease. J Gen Intern Med 1997; 12: 209–215.
- 4.
Cotter G, Cannon CP, McCabe CH, Michowitz Y, Kaluski E, Charlesworth A, et al. Prior peripheral arterial disease and cerebrovascular disease are independent predictors of adverse outcome in patients with acute coronary syndromes: Are we doing enough?: Results from the Orbofiban in Patients with Unstable Coronary Syndromes-Thrombolysis In Myocardial Infarction (OPUS-TIMI) 16 study. Am Heart J 2003; 145: 622–627.
- 5.
Honda S, Nishihira K, Kojima S, Takegami M, Asaumi Y, Suzuki M, et al. Rationale, design, and baseline characteristics of the prospective Japan Acute Myocardial Infarction Registry (JAMIR). Cardiovasc Drugs Ther 2019; 33: 97–103.
- 6.
Tunstall-Pedoe H, Kuulasmaa K, Amouyel P, Arveiler D, Rajakangas AM, Pajak A. Myocardial infarction and coronary deaths in the World Health Organization MONICA Project: Registration procedures, event rates, and case-fatality rates in 38 populations from 21 countries in four continents. Circulation 1994; 90: 583–612.
- 7.
Thygesen K, Alpert JS, Jaffe AS, Simoons ML, Chaitman BR, White HD, et al. Third universal definition of myocardial infarction. Circulation 2012; 126: 2020–2035.
- 8.
Mehran R, Rao SV, Bhatt DL, Gibson CM, Caixeta A, Eikelboom J, et al. Standardized bleeding definitions for cardiovascular clinical trials: A consensus report from the Bleeding Academic Research Consortium. Circulation 2011; 123: 2736–2747.
- 9.
Natsuaki M, Morimoto T, Yamaji K, Watanabe H, Yoshikawa Y, Shiomi H, et al. Prediction of thrombotic and bleeding events after percutaneous coronary intervention: CREDO-Kyoto thrombotic and bleeding risk scores. J Am Heart Assoc 2018; 7: e008708, doi:10.1161/JAHA.118.008708.
- 10.
Fox KA, Dabbous OH, Goldberg RJ, Pieper KS, Eagle KA, Van de Werf F, et al. Prediction of risk of death and myocardial infarction in the six months after presentation with acute coronary syndrome: Prospective multinational observational study (GRACE). BMJ 2006; 333: 1091.
- 11.
Antman EM, Cohen M, Bernink PJ, McCabe CH, Horacek T, Papuchis G, et al. The TIMI risk score for unstable angina/non-ST elevation MI: A method for prognostication and therapeutic decision making. JAMA 2000; 284: 835–842.
- 12.
Nishihira K, Kojima S, Takegami M, Honda S, Nakao YM, Takahashi J, et al. Clinical characteristics and in-hospital mortality according to left main and non-left main culprit lesions: Report from the Japan Acute Myocardial Infarction Registry (JAMIR). Circ Rep 2019; 1: 601–609.
- 13.
Yokoyama H, Tomita H, Honda S, Nishihira K, Kojima S, Takegami M, et al. Effect of low body mass index on the clinical outcomes of Japanese patients with acute myocardial infarction: Results from the prospective Japan Acute Myocardial Infarction Registry (JAMIR). Circ J 2022; 86: 632–639.
- 14.
Nishihira K, Honda S, Takegami M, Kojima S, Asaumi Y, Suzuki M, et al. Impact of bleeding on mortality in patients with acute myocardial infarction complicated by cardiogenic shock. Eur Heart J Acute Cardiovasc Care 2021; 10: 388–396.
- 15.
Mukherjee D, Eagle KA, Kline-Rogers E, Feldman LJ, Juliard JM, Agnelli G, et al. Impact of prior peripheral arterial disease and stroke on outcomes of acute coronary syndromes and effect of evidence-based therapies (from the Global Registry of Acute Coronary Events). Am J Cardiol 2007; 100: 1–6.
- 16.
Meizels A, Zeitoun DM, Bataille V, Cambou JP, Collet JP, Cottin Y, et al. Impact of polyvascular disease on baseline characteristics, management and mortality in acute myocardial infarction: The Alliance project. Arch Cardiovasc Dis 2010; 103: 207–214.
- 17.
Jeremias A, Gruberg L, Patel J, Connors G, Brown DL. Effect of peripheral arterial disease on in-hospital outcomes after primary percutaneous coronary intervention for acute myocardial infarction. Am J Cardiol 2010; 105: 1268–1271.
- 18.
Al Thani H, El-Menyar A, Alhabib KF, Al-Motarreb A, Hersi A, Alfaleh H, et al. Polyvascular disease in patients presenting with acute coronary syndrome: Its predictors and outcomes. ScientificWorldJournal 2012; 2012: 284851.
- 19.
Ferreira-González I, Permanyer Miralda G, Heras M, Ribera A, Marsal JR, Cascant P, et al. Prognosis and management of patients with acute coronary syndrome and polyvascular disease. Rev Esp Cardiol 2009; 62: 1012–1021.
- 20.
Moriwaki K, Kurita T, Hirota Y, Ito H, Ishise T, Fujimoto N, et al. Prognostic impact of prehospital simple risk index in patients with ST-elevation myocardial infarction. Circ J 2023; 87: 629–639.
- 21.
Arai R, Fukamachi D, Migita S, Miyagawa M, Ohgaku A, Koyama Y, et al. Prognostic significance of a combination of cardiogenic shock and the critical culprit lesion location in ST-elevation myocardial infarctions. Int Heart J 2022; 30: 191–201.
- 22.
Nakamura M, Kozuma K, Kitazono T, Iizuka T, Sekine T, Shiosakai K, et al. Prasugrel for Japanese Patients With Ischemic Heart Disease in Long-Term Clinical Practice (PRASFIT-Practice II): A 3-month interim analysis of a postmarketing observational study. Circ J 2019; 83: 637–646.
- 23.
Nakamura M, Kimura K, Kimura T, Ishihara M, Otsuka F, Kozuma K, et al. JCS 2020 guideline focused update on antithrombotic therapy in patients with coronary artery disease. Circ J 2020; 84: 831–865.
- 24.
Morikami Y, Natsuaki M, Morimoto T, Ono K, Nakagawa Y, Furukawa Y, et al. Impact of polyvascular disease on clinical outcomes in patients undergoing coronary revascularization: An observation from the CREDO-Kyoto Registry Cohort-2. Atherosclerosis 2013; 228: 426–431.
- 25.
Miura T, Soga Y, Doijiri T, Aihara H, Yokoi H, Iwabuchi M, et al. Prevalence and clinical outcome of polyvascular atherosclerotic disease in patients undergoing coronary intervention. Circ J 2013; 77: 89–95.
- 26.
Wiviott SD, Braunwald E, McCabe CH, Montalescot G, Ruzyllo W, Gottlieb S, et al. Prasugrel versus clopidogrel in patients with acute coronary syndromes. N Engl J Med 2007; 357: 2001–2015.
- 27.
CAPRIE Steering Committee. A randomised, blinded, trial of clopidogrel versus aspirin in patients at risk of ischaemic events (CAPRIE). Lancet 1996; 348: 1329–1339.
- 28.
Kimura K, Kimura T, Ishihara M, Nakagawa Y, Nakao K, Miyauchi K, et al. JCS 2018 guideline on diagnosis and treatment of acute coronary syndrome. Circ J 2019; 83: 1085–1196.