Abstract
Background: Polypharmacy was reported to be associated with major bleeding in various populations. However, there are no data on polypharmacy and its association with bleeding in patients undergoing percutaneous coronary intervention (PCI).
Methods and Results: Among 12,291 patients in the CREDO-Kyoto PCI Registry Cohort-3, we evaluated the number of medications at discharge and compared major bleeding, defined as Bleeding Academic Research Consortium Type 3 or 5 bleeding, across tertiles (T1–3) of the number of medications. The median number of medications was 6, and 88.0% of patients were on ≥5 medications. The cumulative 5-year incidence of major bleeding increased incrementally with increasing number of medications (T1 [≤5 medications] 12.5%, T2 [6–7] 16.5%, and T3 [≥8] 20.4%; log-rank P<0.001). After adjusting for confounders, the risks for major bleeding of T2 (hazard ratio [HR] 1.21; 95% confidence interval [CI] 1.08–1.36; P=0.001) and T3 (HR 1.27; 95% CI 1.12–1.45; P<0.001) relative to T1 remained significant. The adjusted risks of T2 and T3 relative to T1 were not significant for a composite of myocardial infarction or ischemic stroke (HR 0.95 [95% CI 0.83–1.09; P=0.47] and HR 1.06 [95% CI 0.91–1.23; P=0.48], respectively).
Conclusions: In a real-world population of patients undergoing PCI, approximately 90% were on ≥5 medications. Increasing number of medications was associated with a higher adjusted risk for major bleeding, but not ischemic events.
Multiple medications are common in contemporary evidence-based practice, and the use of multiple medications, called polypharmacy, is an increasingly prevalent problem in rapidly aging societies.1 Polypharmacy has various negative impacts for patients, such as drug-drug interactions, drug-disease interactions, errors in prescribing and taking medications, decreased adherence, and lower quality of life, even though previous randomized controlled trials and observational studies have demonstrated beneficial effects for individual medications in individual diseases.2–4 It has been reported that polypharmacy is associated with bleeding in patients with atrial fibrillation or venous thromboembolism taking oral anticoagulants.5–9 Patients undergoing percutaneous coronary intervention (PCI) are often exposed to polypharmacy because they need to take dual antiplatelet therapy (DAPT) with P2Y12
inhibitors and aspirin in addition to medications for hypertension, diabetes, dyslipidemia, and heart failure. It is known that clopidogrel, which is widely used as a P2Y12
inhibitor after PCI, has several drug-drug interactions,10 which could increase bleeding risk in patients with polypharmacy after PCI. However, there are scarce data on the effects of polypharmacy on long-term bleeding in patients undergoing PCI. Therefore, we sought to evaluate the effect of polypharmacy on long-term major bleeding using a large observational database of patients undergoing PCI in Japan.
Methods
Study Design
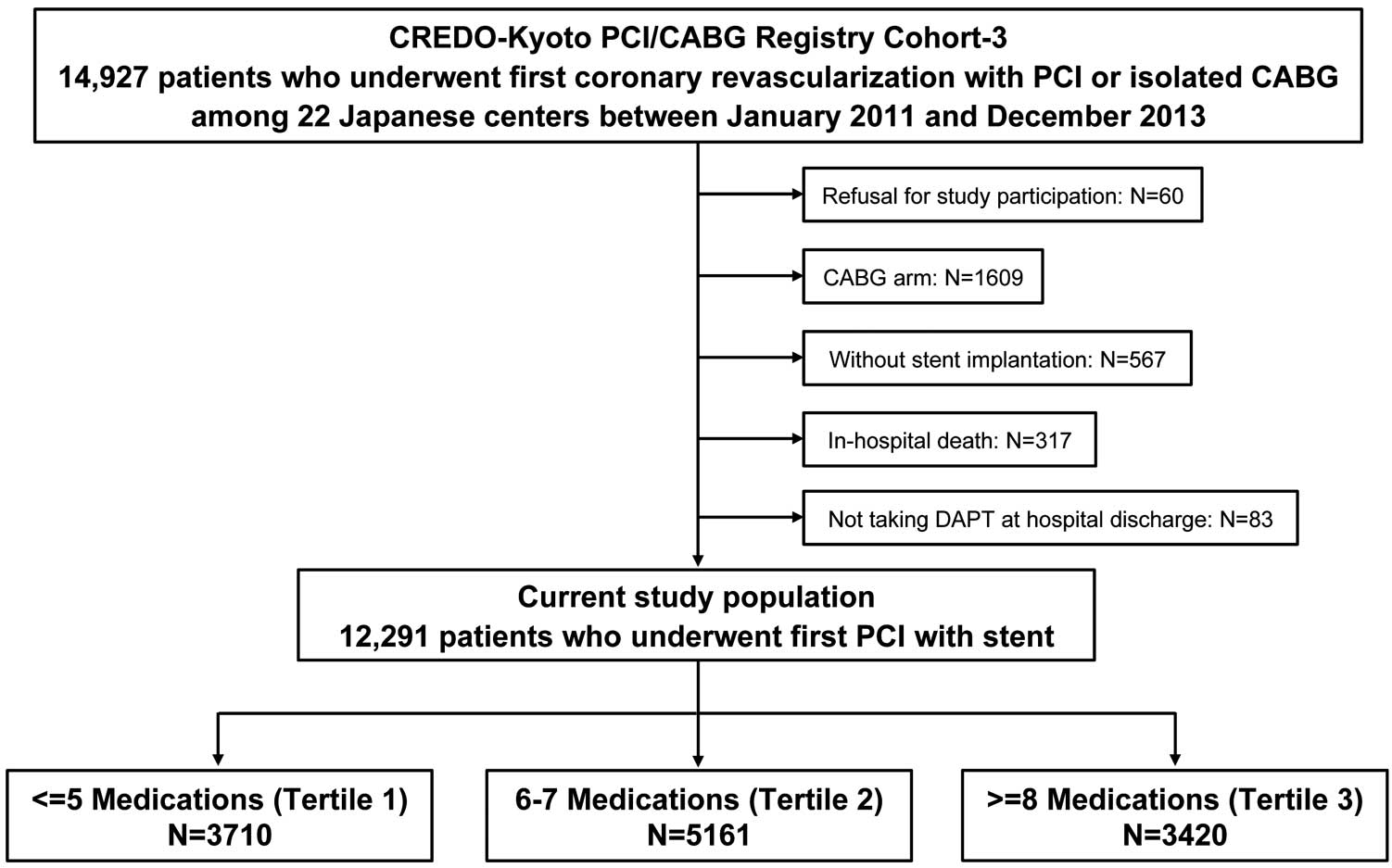
The CREDO-Kyoto PCI/CABG (Coronary Revascularization Demonstrating Outcome Study in Kyoto Percutaneous Coronary Intervention/Coronary Artery Bypass Grafting) Registry Cohort-3 is a physician-initiated, non-company-sponsored, multicenter registry enrolling consecutive patients who underwent first coronary revascularization with PCI or isolated coronary artery bypass grafting (CABG) without combined non-coronary surgery at 22 Japanese centers between January 2011 and December 2013 (Supplementary Appendix). The design of the registry and patient enrollment have been described previously.11,12 We previously reported the status of polypharmacy and its association with mortality in this registry.13 In the present study, we evaluated the effect of polypharmacy on major bleeding in the same population. Among the 14,927 patients enrolled in the CREDO-Kyoto PCI/CABG Registry Cohort-3, 60 who refused to participate in the study were excluded, as were a further 1,609 who underwent CABG, 567 without stent implantation, 317 who died during the index hospitalization for PCI, and 83 who did not take DAPT; therefore, the present study population consisted of 12,291 patients who had a coronary stent implanted and were discharged alive taking DAPT. The study population was divided into 3 groups according to tertiles for the number of medications at discharge from the index hospitalization (Tertile [T] 1: ≤5 medications; T2: 6–7 medications; and T3: ≥8 medications; Figure 1).
The relevant ethics committees in all participating centers approved the study protocol. Because of the retrospective enrollment to this study, the need for written informed consents from patients was waived; however, we excluded those patients who refused study participation when contacted for follow-up. This strategy is concordant with the guidelines of the Japanese Ministry of Health, Labour and Welfare.
Medications
We evaluated oral medications at the time of discharge from the index hospitalization, including those medications that were prescribed outside the cardiology department and/or outside the participating centers. We did not collect data on sleep medications, psychotropic medications, herbal medications, injections, topical medications, eye medications, and medications that were not taken routinely. We categorized the medications by drug classes according to the Anatomical Therapeutic Chemical classification system.14
Outcome Measures
The primary bleeding outcome measure was major bleeding, defined as Bleeding Academic Research Consortium (BARC) Type 3 or 5 bleeding.15 Major bleeding was classified into the following categories without overlap according to the location and cause of bleeding: gastrointestinal, access site, intracranial, and others. The primary ischemic outcome measure was a composite of myocardial infarction or ischemic stroke. Myocardial infarction was adjudicated according to the Academic Research Consortium (ARC) definition.16 Stroke was defined as stroke with neurological symptoms lasting >24 h. Secondary outcome measures and their definitions are described in the Supplementary Appendix. Persistent discontinuation of DAPT was defined as withdrawal of P2Y12
inhibitor or aspirin for at least 2 months.11,12
Data Collection
Clinical, angiographic, and procedural data and medications were collected from hospital charts or hospital databases according to the prespecified definitions by experienced clinical research coordinators belonging to an independent clinical research organization (Research Institute for Production Development, Kyoto, Japan; Supplementary Appendix). Follow-up data were collected from hospital charts and/or obtained by contacting patients, their relatives or referring physicians between January 2018 and December 2019. Follow-up was regarded as completed if we obtained follow-up data beyond July 1, 2017. All outcome measures were adjudicated by the clinical event committee (Supplementary Appendix).
Statistical Analysis
Categorical variables are presented as numbers and percentages, and were compared with the Chi-squared test. Continuous variables are expressed as the mean±SD or as the median with interquartile range (IQR). Continuous variables were compared with analysis of variance or the Kruskal-Wallis test based on their distribution. Cumulative incidences were estimated by the Kaplan-Meier method, and the significance of differences was assessed with the log-rank test. We used Cox proportional hazard models to estimate hazard ratios (HRs) and their 95% confidence intervals (CIs) of T2 and T3 relative to T1 for the outcome measures, adjusting the 28 clinically relevant clinical and procedural factors listed in Table 1 in accordance with our previous study.13 In the Cox proportional hazard model, we developed dummy code variables for T2 and T3 with T1 as the reference. As a sensitivity analysis, we took into consideration the competing risk of all-cause death for the primary bleeding and ischemic outcome measures using the Fine-Gray method. In addition, we performed the main analysis after excluding patients who were prescribed antithrombotic medications other than DAPT, such as warfarin, direct oral anticoagulants (DOAC), cilostazol, and other antiplatelet medications, as a sensitivity analysis.
Table 1. Baseline Characteristics According to Tertiles of the Number of Medications
| |
No. medications |
P value |
| ≤5 (T1) |
6–7 (T2) |
≥8 (T3) |
| No. patients |
3,710 |
5,161 |
3,420 |
|
| Clinical characteristics at index PCI |
| Age (years) |
68.8±10.8 |
69.0±11.3 |
70.3±10.9 |
<0.001 |
| Age ≥80 yearsA |
602 (16.2) |
934 (18.1) |
716 (20.9) |
<0.001 |
| Male sexA |
2,812 (75.8) |
3,822 (74.1) |
2,392 (69.9) |
<0.001 |
| BMI (kg/m2) |
23.4±3.4 |
23.9±3.6 |
24.2±3.9 |
<0.001 |
| BMI <25.0A |
2,634 (71.0) |
3,406 (66.0) |
2,167 (63.4) |
<0.001 |
| Acute coronary syndromeA |
1,152 (31.1) |
2,366 (45.8) |
1,357 (39.7) |
<0.001 |
| Acute myocardial infarction |
1,082 (29.2) |
2,283 (44.2) |
1,314 (38.4) |
<0.001 |
| Unstable angina |
70 (1.9) |
83 (1.6) |
43 (1.3) |
0.11 |
| HTA |
2,551 (68.8) |
4,468 (86.6) |
3,142 (91.9) |
<0.001 |
| DiabetesA |
797 (21.5) |
1,776 (34.4) |
2,111 (61.7) |
<0.001 |
| On insulin therapy |
215 (5.8) |
360 (7.0) |
426 (12.5) |
<0.001 |
| Current smokerA |
1,016 (27.4) |
1,518 (29.4) |
895 (26.2) |
0.003 |
| HFA |
486 (13.1) |
974 (18.9) |
1,200 (35.1) |
<0.001 |
| Multivessel coronary diseaseA |
1,807 (48.7) |
2,905 (56.3) |
2,167 (63.4) |
<0.001 |
| LVEF (%) |
62.1±10.8 |
59.4±12.1 |
55.1±14.4 |
<0.001 |
| LVEF ≤40% |
131 (4.1) |
328 (7.2) |
521 (17.1) |
<0.001 |
| Mitral regurgitation Grade ≥3/4 |
155 (4.8) |
321 (6.9) |
331 (10.8) |
<0.001 |
| Prior myocardial infarctionA |
321 (8.7) |
526 (10.2) |
516 (15.1) |
<0.001 |
| Prior strokeA |
364 (9.8) |
614 (11.9) |
562 (16.4) |
<0.001 |
| Peripheral vascular diseaseA |
306 (8.2) |
416 (8.1) |
391 (11.4) |
<0.001 |
| eGFR <30 mL/min/1.73 m2 or hemodialysis |
221 (6.0) |
383 (7.4) |
412 (12.0) |
<0.001 |
| eGFR <30 mL/min/1.73 m2, without hemodialysisA |
83 (2.2) |
178 (3.4) |
211 (6.2) |
<0.001 |
| HemodialysisA |
138 (3.7) |
205 (4.0) |
201 (5.9) |
<0.001 |
| Atrial fibrillationA |
158 (4.3) |
407 (7.9) |
550 (16.1) |
<0.001 |
| Hemoglobin <11.0 g/dLA |
346 (9.3) |
505 (9.8) |
564 (16.5) |
<0.001 |
| Platelets <100×109/LA |
52 (1.4) |
85 (1.6) |
79 (2.3) |
0.01 |
| Chronic obstructive pulmonary diseaseA |
161 (4.3) |
190 (3.7) |
136 (4.0) |
0.29 |
| Liver cirrhosisA |
99 (2.7) |
129 (2.5) |
85 (2.5) |
0.85 |
| Malignancy |
522 (14.1) |
613 (11.9) |
409 (12.0) |
0.004 |
| Active malignancyA |
102 (2.7) |
84 (1.6) |
48 (1.4) |
<0.001 |
| Severe frailtyA,B |
111 (3.0) |
181 (3.5) |
177 (5.2) |
<0.001 |
| ARC-HBR |
1,414 (38.1) |
2,235 (43.3) |
2,108 (61.6) |
<0.001 |
| Procedural characteristics of index PCI |
| No. target lesions |
1.41±0.72 |
1.50±0.79 |
1.59±0.86 |
<0.001 |
| Target of proximal LADA |
2,197 (59.2) |
3,219 (62.4) |
2,194 (64.2) |
<0.001 |
| Target of unprotected LMCAA |
125 (3.4) |
212 (4.1) |
184 (5.4) |
<0.001 |
| Target of chronic total occlusionA |
305 (8.2) |
476 (9.2) |
408 (11.9) |
<0.001 |
| Target of bifurcationA |
1,416 (38.2) |
2,106 (40.8) |
1,496 (43.7) |
<0.001 |
| Bifurcation with 2 stentsA |
140 (3.8) |
206 (4.0) |
164 (4.8) |
0.07 |
| Emergency procedure |
1,117 (30.1) |
2,322 (45.0) |
1,318 (38.5) |
<0.001 |
| Total no. stents |
1 [1–2] |
1 [1–2] |
2 [1–3] |
<0.001 |
| Total stent length (mm) |
28 [18–46] |
28 [18–53] |
33 [20–56] |
<0.001 |
| Total stent length >28 mmA |
1,595 (43.0) |
2,552 (49.5) |
1,891 (55.3) |
<0.001 |
| Minimum stent size (mm) |
3.0 [2.5–3.0] |
2.75 [2.5–3.0] |
2.5 [2.5–3.0] |
<0.001 |
| Minimum stent size <3.0 mmA |
1,847 (49.8) |
2,660 (51.5) |
1,974 (57.7) |
<0.001 |
| DES use |
3,078 (83.0) |
4,136 (80.1) |
2,872 (84.0) |
<0.001 |
| New-generation DES use |
3,032 (81.7) |
4,069 (78.8) |
2,836 (82.9) |
<0.001 |
| IVUS or OCT use |
2,863 (77.2) |
3,814 (73.9) |
2,601 (76.1) |
0.001 |
| IVUS use |
2,818 (76.0) |
3,783 (73.3) |
2,573 (75.2) |
0.01 |
| OCT use |
91 (2.5) |
116 (2.2) |
71 (2.1) |
0.56 |
| Staged PCI |
618 (16.7) |
1,158 (22.4) |
832 (24.3) |
<0.001 |
| Access site |
|
|
|
<0.001 |
| Radial |
1,779 (48.0) |
1,892 (36.7) |
1,161 (34.0) |
|
| Femoral |
1,554 (41.9) |
2,825 (54.8) |
1,927 (56.4) |
|
| Brachial |
375 (10.1) |
440 (8.5) |
330 (9.7) |
|
Continuous variables are expressed as the mean±SD or median [interquartile range]. Categorical variables are expressed as n (%). Values were missing for left ventricular ejection fraction (LVEF) in 1,478 patients, for mitral regurgitation in 1,342 patients, and for access site in 9 patients. ARisk-adjusting variables selected for the Cox proportional hazard models. BSevere frailty was regarded as present when the inability to perform usual activities of daily living was documented in the hospital charts. ARC-HBR, Academic Research Consortium for High Bleeding Risk; BMI, body mass index; DES, drug-eluting stent; eGFR, estimated glomerular filtration rate; HF, heart failure; HT, hypertension; IVUS, intravascular ultrasound; LAD, left anterior descending coronary artery; LMCA, left main coronary artery; LVEF, left ventricular ejection fraction; OCT, optical coherence tomography; PCI, percutaneous coronary intervention: T, tertile.
All P values were 2-tailed and P<0.05 was considered statistically significant. All analyses were performed using R version 4.1.2 (R Foundation for Statistical Computing, Vienna, Austria).
Results
Details of Medications
Among the study population, the median number of medications was 6 (IQR 5–8), and 10,821 (88.0%) patients had ≥5 medications (Figure 2). Regarding specific types of medications, the median number of antithrombotic medications was 2, the median number of lipid-modifying medications was 1, the median number of medications for hypertension and/or heart failure was 2, and the median number of medications for peptic ulcer was 1 (Table 2). Most of the medications were those related to cardiovascular disease, diabetes, and prevention of gastrointestinal bleeding after prescription of DAPT in patients receiving PCI. The median number of antithrombotic medications, lipid-modifying medications, and medications to prevent gastrointestinal bleeding did not differ across tertiles of the total number of medications, whereas the median number of medications for hypertension and/or heart failure, as well as medications for diabetes, increased with the increasing total number of medications (Table 2). Among the study population, 96.4% of patients took clopidogrel and 2.7% of patients took ticlopidine as P2Y12
inhibitors. In terms of antithrombotic medications other than DAPT with P2Y12
inhibitors and aspirin, the prescription rates of warfarin, DOAC, cilostazol, and other antiplatelet medications were 8.8%, 1.3%, 2.4%, and 3.1%, respectively.

Table 2. Details of Medications at Discharge From the Index Hospitalization in the Entire Study Population and in Tertiles of the Number of Medications Separately
Medication
class |
Median [IQR] no. medications |
Medication |
Entire study
population
(n=12,291) |
T1
(n=3,710) |
T2
(n=5,161) |
T3
(n=3,420) |
Entire
study
population |
T1 |
T2 |
T3 |
Antithrombotic
medications |
2 [2–2] |
2 [2–2] |
2 [2–2] |
2 [2–3] |
P2Y12 inhibitor |
12,291 (100) |
3,710 (100) |
5,161 (100) |
3,420 (100) |
| Ticlopidine |
331 (2.7) |
99 (2.7) |
126 (2.4) |
106 (3.1) |
| Clopidogrel |
11,848 (96.4) |
3,572 (96.3) |
4,979 (96.5) |
3,297 (96.4) |
| Unknown |
112 (0.9) |
39 (1.1) |
56 (1.1) |
17 (0.5) |
| Aspirin |
12,291 (100) |
3,710 (100) |
5,161 (100) |
3,420 (100) |
| Cilostazol |
290 (2.4) |
37 (1.0) |
107 (2.1) |
146 (4.3) |
Other antiplatelet
medications |
387 (3.1) |
48 (1.3) |
127 (2.5) |
212 (6.2) |
| Beraprost |
78 (0.6) |
8 (0.2) |
25 (0.5) |
45 (1.3) |
Limaprost
alfadex |
204 (1.7) |
27 (0.7) |
66 (1.3) |
111 (3.2) |
| Sarpogrelate |
76 (0.6) |
11 (0.3) |
22 (0.4) |
43 (1.3) |
| Dipyridamole |
39 (0.3) |
3 (0.1) |
16 (0.3) |
20 (0.6) |
| Warfarin |
1,077 (8.8) |
61 (1.6) |
354 (6.9) |
662 (19.4) |
| DOAC |
156 (1.3) |
13 (0.4) |
46 (0.9) |
97 (2.8) |
| Dabigatran |
111 (0.9) |
9 (0.2) |
33 (0.6) |
69 (2.0) |
| Apixaban |
2 (0.02) |
0 (0) |
0 (0) |
2 (0.1) |
| Rivaroxaban |
43 (0.3) |
4 (0.1) |
13 (0.3) |
26 (0.8) |
Lipid-modifying
medications |
1 [1–1] |
1 [0–1] |
1 [1–1] |
1 [1–1] |
Statins |
9,761 (79.4) |
2,520 (67.9) |
4,275 (82.8) |
2,966 (86.7) |
| Ezetimibe |
285 (2.3) |
38 (1.0) |
119 (2.3) |
128 (3.7) |
| Fibrates |
136 (1.1) |
29 (0.8) |
48 (0.9) |
59 (1.7) |
Polyunsaturated
fatty acids |
521 (4.2) |
50 (1.3) |
211 (4.1) |
260 (7.6) |
Bile acid
sequestrants |
4 (0.03) |
1 (0.03) |
0 (0) |
3 (0.1) |
Nicotinic acid and
derivatives |
156 (1.3) |
18 (0.5) |
59 (1.1) |
79 (2.3) |
Other
lipid-modifying
medications |
19 (0.2) |
4 (0.1) |
5 (0.1) |
10 (0.3) |
Medications for
HT and/or HF |
2 [1–3] |
1 [0–1] |
2 [1–2] |
3 [2–4] |
β-blockers |
4,879 (39.7) |
584 (15.7) |
2,226 (43.1) |
2,069 (60.5) |
Angiotensin-
converting
enzyme inhibitors |
2,936 (23.9) |
484 (13.0) |
1,427 (27.6) |
1,025 (30.0) |
Angiotensin II
receptor
blockers |
5,251 (42.7) |
872 (23.5) |
2,425 (47.0) |
1,954 (57.1) |
Mineralocorticoid
receptor
antagonists |
1,363 (11.1) |
46 (1.2) |
365 (7.1) |
952 (27.8) |
| Renin inhibitors |
32 (0.3) |
0 (0) |
5 (0.1) |
27 (0.8) |
Calcium channel
blockers |
4,938 (40.2) |
891 (24.0) |
2,144 (41.5) |
1,903 (55.6) |
α-Adrenergic
receptor
antagonists |
300 (2.4) |
8 (0.2) |
76 (1.5) |
216 (6.3) |
| Loop diuretics |
1,960 (15.9) |
96 (2.6) |
530 (10.3) |
1,334 (39.0) |
| Thiazides |
542 (4.4) |
24 (0.6) |
176 (3.4) |
342 (10.0) |
Vasopressin
antagonists |
14 (0.1) |
0 (0) |
1 (0.02) |
13 (0.4) |
| Digitalis |
171 (1.4) |
7 (0.2) |
33 (0.6) |
131 (3.8) |
| Cardiac stimulants |
32 (0.3) |
0 (0) |
6 (0.1) |
26 (0.8) |
Other medications
for HT and/or HF |
12 (0.1) |
1 (0.03) |
3 (0.1) |
8 (0.2) |
Medications for
angina |
0 [0–1] |
0 [0–0] |
0 [0–1] |
0 [0–1] |
Nitrates |
2,435 (19.8) |
354 (9.5) |
1,129 (21.9) |
952 (27.8) |
| Nicorandil |
2,035 (16.6) |
264 (7.1) |
868 (16.8) |
903 (26.4) |
Other medications
for angina |
27 (0.2) |
2 (0.1) |
10 (0.2) |
15 (0.4) |
Medications for
cardiac
arrhythmia |
0 [0–0] |
0 [0–0] |
0 [0–0] |
0 [0–0] |
Class Ia |
51 (0.4) |
6 (0.2) |
24 (0.5) |
21 (0.6) |
| Class Ib |
104 (0.8) |
6 (0.2) |
30 (0.6) |
68 (2.0) |
| Class Ic |
87 (0.7) |
11 (0.3) |
31 (0.6) |
45 (1.3) |
| Class III |
138 (1.1) |
9 (0.2) |
30 (0.6) |
99 (2.9) |
Medications for
diabetes |
0 [0–0] |
0 [0–0] |
0 [0–0] |
1 [0–2] |
Biguanides |
555 (4.5) |
26 (0.7) |
150 (2.9) |
379 (11.1) |
| Sulfonylureas |
1,252 (10.2) |
71 (1.9) |
344 (6.7) |
837 (24.5) |
| Thiazolidinediones |
318 (2.6) |
17 (0.5) |
97 (1.9) |
204 (6.0) |
| DPP-4 inhibitors |
1,682 (13.7) |
108 (2.9) |
544 (10.5) |
1,030 (30.1) |
α-Glucosidase
inhibitors |
811 (6.6) |
57 (1.5) |
200 (3.9) |
554 (16.2) |
| Glinides |
217 (1.8) |
15 (0.4) |
62 (1.2) |
140 (4.1) |
Other antidiabetic
medications |
89 (0.7) |
5 (0.1) |
21 (0.4) |
63 (1.8) |
Medications to
prevent
gastrointestinal
bleeding |
1 [1–1] |
1 [0–1] |
1 [1–1] |
1 [1–1] |
Proton pump
inhibitors |
8,124 (66.1) |
1,980 (53.4) |
3,601 (69.8) |
2,543 (74.4) |
Histamine H2
receptor blockers |
1,576 (12.8) |
357 (9.6) |
702 (13.6) |
517 (15.1) |
Other medications
for peptic ulcer |
22 (0.2) |
2 (0.1) |
10 (0.2) |
10 (0.3) |
Medications for
BPH and/or
pollakiuria |
0 [0–0] |
0 [0–0] |
0 [0–0] |
0 [0–0] |
α-Adrenergic
receptor
antagonists |
7 (0.1) |
1 (0.03) |
1 (0.02) |
5 (0.1) |
Testosterone-5α-
reductase
inhibitors |
1 (0.01) |
0 (0) |
0 (0) |
1 (0.03) |
Urinary
antispasmodics |
6 (0.05) |
0 (0) |
2 (0.04) |
4 (0.1) |
Medications for
asthma |
0 [0–0] |
0 [0–0] |
0 [0–0] |
0 [0–0] |
Leukotriene
receptor
antagonists |
2 (0.02) |
0 (0) |
1 (0.02) |
1 (0.03) |
| Theophylline |
2 (0.02) |
0 (0) |
0 (0) |
2 (0.1) |
| Others |
0 [0–0] |
0 [0–0] |
0 [0–0] |
0 [0–0] |
Antigout
medications |
19 (0.2) |
1 (0.03) |
8 (0.2) |
10 (0.3) |
Antidementia
medications |
6 (0.05) |
1 (0.03) |
2 (0.04) |
3 (0.1) |
| Corticosteroids |
9 (0.1) |
1 (0.03) |
5 (0.1) |
3 (0.1) |
| NSAIDs |
1 (0.01) |
0 (0) |
1 (0.02) |
0 (0) |
| Antihistamines |
5 (0.04) |
0 (0) |
1 (0.02) |
4 (0.1) |
Oral
antihypotensive
medications |
28 (0.2) |
8 (0.2) |
13 (0.3) |
7 (0.2) |
Gastroprokinetic
medications |
8 (0.1) |
2 (0.1) |
3 (0.1) |
3 (0.1) |
| Probiotics |
1 (0.01) |
0 (0) |
1 (0.02) |
0 (0) |
Ursodeoxycholic
acid |
3 (0.02) |
0 (0) |
2 (0.04) |
1 (0.03) |
| Expectorants |
4 (0.03) |
1 (0.03) |
2 (0.04) |
1 (0.03) |
| Thiamazole |
1 (0.01) |
0 (0) |
1 (0.02) |
0 (0) |
| Muscle relaxants |
2 (0.02) |
0 (0) |
2 (0.04) |
0 (0) |
| Ergot alkaloids |
40 (0.3) |
4 (0.1) |
14 (0.3) |
22 (0.6) |
| Kallidinogenase |
46 (0.4) |
3 (0.1) |
15 (0.3) |
28 (0.8) |
Vitamin D and
analogs |
411 (3.3) |
54 (1.5) |
149 (2.9) |
208 (6.1) |
| Vitamin K |
1 (0.01) |
1 (0.03) |
0 (0) |
0 (0) |
Lanthanum
carbonate |
1 (0.01) |
0 (0) |
0 (0) |
1 (0.03) |
| Calcium carbonate |
3 (0.02) |
0 (0) |
1 (0.02) |
2 (0.1) |
| Cinacalcet |
6 (0.05) |
1 (0) |
4 (0.1) |
1 (0.03) |
| Folic acid |
1 (0.01) |
0 (0) |
1 (0.02) |
0 (0) |
| Pantothenic acid |
2 (0.02) |
0 (0) |
0 (0) |
2 (0.1) |
| Sodium valproate |
1 (0.01) |
0 (0) |
0 (0) |
1 (0.03) |
| Pregabalin |
4 (0.03) |
0 (0) |
1 (0.02) |
3 (0.1) |
| Ambenonium |
1 (0.01) |
1 (0.03) |
0 (0) |
0 (0) |
Unless indicated otherwise, data are given as n (%). BPH, benign prostatic hyperplasia; DOAC, direct oral anticoagulant; DPP-4, dipeptidyl peptidase-4; NSAIDs, non-steroidal anti-inflammatory drugs. Other abbreviations as in Table 1.
Patients taking more medications, compared with those taking fewer medications, were older, more often women, and more often had comorbidities such as hypertension, diabetes, heart failure, systolic dysfunction, prior myocardial infarction, prior stroke, peripheral vascular disease, chronic kidney disease, atrial fibrillation, anemia, thrombocytopenia, and malignancy (Table 1). The prevalence of ARC high bleeding risk was higher in patients taking more medications than in those taking fewer medications (T1: 38.1%; T2: 43.3%; and T3: 61.6%). Regarding procedural characteristics, patients taking more medications, compared with those taking fewer medications, had a greater number of target lesions and more complex coronary anatomy, as indicated by the greater number of targets of the left main coronary artery, chronic total occlusion, and bifurcation (Table 1). The femoral approach was more often used in patients taking more medications than in those taking fewer medications.
DAPT Discontinuation
The cumulative incidence of persistent discontinuation of DAPT was approximately 30% at 1 year, 62% at 3 years, and 74% at 5 years, and did not differ across the tertiles of the number of medications (Figure 3).
Clinical Outcomes
The median follow-up duration was 6.0 years (IQR 5.1–6.9 years), and complete 1-, 3-, and 5-year clinical follow-up information was obtained for 97.6%, 94.8%, and 82.6% of patients, respectively. Complete 1-, 3-, and 5-year clinical follow-up rates did not differ across the tertiles of the number of medications (T1: 97.8%, 95.6%, and 83.3%, respectively; T2: 97.7%, 94.9%, and 82.4%, respectively; T3: 97.1%, 94.0%, and 81.9%, respectively).
The cumulative 5-year incidence of the primary bleeding outcome measure increased incrementally with increasing number of medications (T1, T2, and T3: 12.5%, 16.5%, and 20.4%, respectively; log-rank P<0.001; Figure 4A). After adjusting for confounders, the risks of T2 and T3 relative to T1 remained significant for the primary bleeding outcome measure (HR 1.21 [95% CI 1.08–1.36; P=0.001] and HR 1.27 [95% CI 1.12–1.45; P<0.001], respectively; Table 3). The adjusted risks of T2 and T3 relative to T1 were significant for access site and intracranial bleeding, but not for gastrointestinal bleeding (Table 3). The adjusted risk of T3 relative to T1 was significant for BARC Type 5 bleeding and hemorrhagic stroke (Table 3).

Table 3. Clinical Outcomes
| |
N of
medication |
N of
patients
with event |
Cumulative
5-year
incidence |
Crude HR
(95% CI) |
P value |
Adjusted HR
(95% CI) |
P value |
The primary bleeding
outcome measure:
BARC type 3 or 5
bleeding |
T1: ≤5 |
481 |
12.5% |
Ref. |
|
Ref. |
|
| T2: 6–7 |
877 |
16.5% |
1.35 (1.21–1.51) |
<0.001 |
1.21 (1.08–1.36) |
0.001 |
| T3: ≥8 |
706 |
20.4% |
1.72 (1.53–1.94) |
<0.001 |
1.27 (1.12–1.45) |
<0.001 |
Gastrointestinal
bleeding |
T1: ≤5 |
173 |
4.8% |
Ref. |
|
Ref. |
|
| T2: 6–7 |
271 |
5.7% |
1.17 (0.96–1.41) |
0.11 |
1.03 (0.85–1.26) |
0.75 |
| T3: ≥8 |
192 |
6.0% |
1.31 (1.07–1.61) |
0.01 |
0.95 (0.75–1.19) |
0.64 |
| Access site bleeding |
T1: ≤5 |
54 |
1.5% |
Ref. |
|
Ref. |
|
| T2: 6–7 |
150 |
2.9% |
2.02 (1.48–2.75) |
<0.001 |
1.60 (1.16–2.21) |
0.004 |
| T3: ≥8 |
129 |
3.9% |
2.64 (1.92–3.63) |
<0.001 |
1.80 (1.27–2.56) |
0.001 |
| Intracranial bleeding |
T1: ≤5 |
65 |
1.7% |
Ref. |
|
Ref. |
|
| T2: 6–7 |
130 |
2.5% |
1.50 (1.12–2.02) |
0.01 |
1.39 (1.02–1.89) |
0.04 |
| T3: ≥8 |
104 |
3.4% |
1.95 (1.43–2.66) |
<0.001 |
1.43 (1.01–2.03) |
0.046 |
Surgery-related
bleeding |
T1: ≤5 |
94 |
2.6% |
Ref. |
|
Ref. |
|
| T2: 6–7 |
152 |
3.0% |
1.21 (0.94–1.56) |
0.15 |
1.17 (0.89–1.53) |
0.25 |
| T3: ≥8 |
116 |
3.8% |
1.48 (1.13–1.95) |
0.01 |
1.17 (0.86–1.58) |
0.32 |
| BARC type 5 bleeding |
T1: ≤5 |
18 |
0.5% |
Ref. |
|
Ref. |
|
| T2: 6–7 |
37 |
0.7% |
1.49 (0.85–2.62) |
0.16 |
1.48 (0.83–2.66) |
0.19 |
| T3: ≥8 |
43 |
1.3% |
2.74 (1.58–4.74) |
<0.001 |
2.23 (1.20–4.14) |
0.01 |
| Hemorrhagic stroke |
T1: ≤5 |
47 |
1.1% |
Ref. |
|
Ref. |
|
| T2: 6–7 |
90 |
1.5% |
1.40 (0.98–1.99) |
0.06 |
1.32 (0.92–1.91) |
0.13 |
| T3: ≥8 |
84 |
2.4% |
2.07 (1.45–2.96) |
<0.001 |
1.72 (1.15–2.56) |
0.01 |
The primary ischemic
outcome measure:
Myocardial infarction
or ischemic stroke |
T1: ≤5 |
368 |
9.2% |
Ref. |
|
Ref. |
|
| T2: 6–7 |
553 |
9.8% |
1.09 (0.96–1.24) |
0.20 |
0.95 (0.83–1.09) |
0.47 |
| T3: ≥8 |
466 |
13.2% |
1.47 (1.28–1.68) |
<0.001 |
1.06 (0.91–1.23) |
0.48 |
| Myocardial infarction |
T1: ≤5 |
234 |
6.1% |
Ref. |
|
Ref. |
|
| T2: 6–7 |
325 |
5.8% |
1.00 (0.85–1.19) |
0.96 |
0.95 (0.80–1.13) |
0.58 |
| T3: ≥8 |
283 |
7.9% |
1.38 (1.16–1.64) |
<0.001 |
1.17 (0.96–1.42) |
0.12 |
| Ischemic stroke |
T1: ≤5 |
144 |
3.4% |
Ref. |
|
Ref. |
|
| T2: 6–7 |
253 |
4.5% |
1.28 (1.04–1.57) |
0.02 |
0.99 (0.80–1.23) |
0.96 |
| T3: ≥8 |
210 |
6.1% |
1.69 (1.37–2.09) |
<0.001 |
0.98 (0.78–1.24) |
0.88 |
| Definite stent thrombosis |
T1: ≤5 |
22 |
0.5% |
Ref. |
|
Ref. |
|
| T2: 6–7 |
49 |
0.9% |
1.62 (0.98–2.67) |
0.06 |
1.41 (0.84–2.37) |
0.19 |
| T3: ≥8 |
34 |
1.0% |
1.75 (1.02–2.99) |
0.04 |
1.70 (0.94–3.06) |
0.08 |
Target vessel
revascularization |
T1: ≤5 |
591 |
16.0% |
Ref. |
|
Ref. |
|
| T2: 6–7 |
937 |
17.8% |
1.17 (1.06–1.30) |
0.002 |
1.05 (0.94–1.17) |
0.37 |
| T3: ≥8 |
699 |
20.8% |
1.38 (1.23–1.54) |
<0.001 |
1.08 (0.96–1.23) |
0.20 |
Any coronary
revascularization |
T1: ≤5 |
909 |
24.8% |
Ref. |
|
Ref. |
|
| T2: 6–7 |
1,358 |
26.0% |
1.11 (1.02–1.21) |
0.01 |
1.02 (0.94–1.12) |
0.63 |
| T3: ≥8 |
1,014 |
30.2% |
1.32 (1.20–1.44) |
<0.001 |
1.08 (0.98–1.20) |
0.13 |
Death, myocardial
infarction, or ischemic
stroke |
T1: ≤5 |
856 |
20.0% |
Ref. |
|
Ref. |
|
| T2: 6–7 |
1,227 |
20.4% |
1.04 (0.96–1.14) |
0.33 |
0.93 (0.85–1.02) |
0.10 |
| T3: ≥8 |
1,057 |
27.0% |
1.46 (1.33–1.60) |
<0.001 |
0.98 (0.89–1.09) |
0.71 |
Number of patients with event was counted until the end of follow-up. Cumulative 5-year incidence was estimated by the Kaplan-Meier method. HRs with 95% CIs were estimated throughout the entire follow-up period by the Cox proportional hazard models, and were expressed relative to T1. BARC, Bleeding Academic Research Consortium; CI, confidence interval; HR, hazard ratio.
The cumulative 5-year incidence of the primary ischemic outcome measure was higher in T3 than in T1 and T2 (T1, T2, and T3: 9.2%, 9.8%, and 13.2%, respectively; log-rank P<0.001; Figure 4B). However, after adjusting for confounders, the risks of T2 and T3 relative to T1 were no longer significant for the primary ischemic outcome measure (HR 0.95 [95% CI 0.83–1.09; P=0.47] and HR 1.06 [95% CI 0.91–1.23; P=0.48], respectively; Table 3). The adjusted risks of T2 and T3 relative to T1 were not significant for myocardial infarction, ischemic stroke, definite stent thrombosis, target vessel revascularization, and any coronary revascularization (Table 3).
In the sensitivity analysis considering the competing risk of all-cause death, the results were fully consistent with those of the main analysis (Supplementary Table 1). In the sensitivity analysis after excluding 1,804 patients who were prescribed antithrombotic medications other than DAPT, the results were fully consistent with those of the main analysis (Supplementary Table 2).
Discussion
The main findings of this real-world study evaluating polypharmacy and long-term bleeding in patients undergoing PCI were as follows: (1) the median number of medications at discharge from index PCI hospitalization was 6, and 88.0% of patients were on ≥5 medications; (2) the main medications were antiplatelet medications, lipid-modifying medications, medications for hypertension and/or heart failure, and medications to prevent gastrointestinal bleeding; (3) polypharmacy was mainly related to the increasing number of medications for hypertension, heart failure, and diabetes; and (4) the crude incidence of the primary bleeding and ischemic outcome measures increased incrementally with increasing number of medications, whereas the adjusted effect of polypharmacy was significant for the primary bleeding outcome measure and not for the primary ischemic outcome measure.
To the best of our knowledge, this study is the first to report the association between polypharmacy and major bleeding in patients undergoing PCI. In consistent with previous studies conducted in patients with atrial fibrillation or venous thromboembolism,5–9 polypharmacy was associated with higher crude incidences of bleeding and ischemic events in the present study. This may be primarily explained by a higher prevalence of advanced age, comorbidities, frailty, and coronary anatomical complexity in patients with polypharmacy. After adjusting for these variables, the risk of polypharmacy was significant for bleeding events, but not ischemic events, in the present study. In patients with atrial fibrillation taking oral anticoagulants enrolled in the ROCKET-AF (Rivaroxaban Once Daily Oral Direct Factor Xa Inhibition Compared with Vitamin K Antagonism for Prevention of Stroke and Embolism Trial in Atrial Fibrillation) trial, polypharmacy was associated with a higher risk of bleeding, but not stroke.5 These comparable results from the present study and the ROCKET-AF trial may indicate that polypharmacy is an independent risk factor for major bleeding in patients taking antithrombotic medications. The high adjusted risk of polypharmacy for major bleeding, but not ischemic events, in the present study would imply the importance of preventing bleeding event rather than ischemic events in patients with polypharmacy after PCI. The strategy of reducing the number of medications to reduce major bleeding should be evaluated in future.
The mechanisms underlying the higher risk with polypharmacy for major bleeding after PCI are not clear, but they may be multifactorial. One of the main reasons for the increased risk would be that patients with polypharmacy more often are taking antithrombotic medications other than DAPT, such as oral anticoagulants, cilostazol, and other antiplatelet medications. However, the adjusted risk of polypharmacy for major bleeding remained significant in the sensitivity analysis after excluding patients who were prescribed antithrombotic medications other than DAPT in the present study. Several mechanisms could be suggested to explain the excess risk of polypharmacy due to non-antithrombotic medications for major bleeding in this study. First, errors in prescribing and taking DAPT may be one reason for the increased risk of major bleeding. It has been reported that polypharmacy is associated with an increased risk of medication errors.17 Second, polypharmacy due to drugs that increase the falls risk may be related to the higher risk of major traumatic bleeding. In the present study, polypharmacy was primarily caused by multiple prescription of antihypertensive or antidiabetic medications and diuretics, which are well known as drugs that increase the falls risk.18–21 Previous studies have reported that polypharmacy is associated with an increased risk of falls.18–21 A higher adjusted risk of polypharmacy for intracranial bleeding in the present study may support this mechanism. Third, drug-drug interactions related to P2Y12
inhibitors may be associated with high bleeding risk in patients with polypharmacy. More than 95% of patients in this study were prescribed clopidogrel as a P2Y12
inhibitor. It is known that concomitant administration of selective serotonin reuptake inhibitors (SSRI) or serotonin-norepinephrine reuptake inhibitors (SNRI) with clopidogrel may increase the risk of bleeding,10 although we could not evaluate this drug-drug interaction in the present study due to lack of data on psychotropic medications. Fourth, medications that have adverse effects on the gastrointestinal tract, such as corticosteroids or non-steroidal anti-inflammatory drugs (NSAIDs), on top of DAPT, including aspirin, may be related to the higher risk of major bleeding. However, the prescription rates of corticosteroid and NSAIDs were very low, and the risk of polypharmacy for gastrointestinal bleeding was not significant in the present study. The neutral effect of polypharmacy for gastrointestinal bleeding may be caused by a higher prescription of proton pump inhibitors in patients with polypharmacy, suggesting that proton pump inhibitors for patients with high gastrointestinal bleeding risk would be important even if this may increase the number of medications. Fifth, the higher risk of polypharmacy for access site bleeding may be associated with a higher prevalence of the use of a femoral approach, which may be primarily due to more complex coronary anatomy and comorbidities in patients with polypharmacy. A meta-analysis showed that the radial approach, compared with the femoral approach, reduced major bleeding regardless of coronary anatomical complexity and comorbidities.22 The radial approach is a preferable strategy, especially in patients with polypharmacy. Further studies would be needed to unravel the mechanisms underlying the higher risk of polypharmacy for major bleeding in patients after PCI to help establish appropriate strategies for preventing bleeding in patients with polypharmacy.
There were several important limitations in this study. First, and most importantly, we could not assess the appropriateness of drug prescriptions. The essence of the problem with polypharmacy is inappropriate prescription, and not the total number of medications per se. Second, we did not collect data on some important medications, such as sleep medications, psychotropic medications, and herbal medications. Sleep and psychotropic medications were reported to be associated with an increased risk of falls.18–21 In addition, the medications for non-cardiovascular disease may be underreported, because the prescription rates of these medications were very low in the present study. Therefore, the number of medications in this study may be underestimated. Furthermore, drug-drug interactions between clopidogrel and SSRI/SNRI on major bleeding could not be evaluated in this study. Third, we did not collect data on follow-up drug information and non-compliance, except for antiplatelet therapy. Therefore, we could not evaluate the effect of the discontinuation of any medications in this study. However, most of the prescribed medications were used for a chronic disease, and substantial changes to medications were unlikely during follow-up, except for DAPT, which should be switched to antiplatelet monotherapy after the recommended duration in patients undergoing PCI. Finally, the clinical practice and the usage patterns of medications in this study, which was conducted almost 10 years ago, would be different from those in contemporary clinical practice. For example, the duration of DAPT after PCI was relatively long and the prescription rate of DOAC was low in the present study. Moreover, there are an increasing number of cardiovascular drug classes that have demonstrated efficacy in improving clinical outcomes in randomized controlled trials. The use of statins, ezetimibe, sodium–glucose cotransporter-2 inhibitors, glucagon-like peptide-1 receptor agonists, angiotensin receptor–neprilysin inhibitors, colchicine, and proton pump inhibitors would undoubtedly lead to polypharmacy. We are not certain whether the use of these evidence-based medications would lead to increased bleeding, other than the use of antithrombotic medications. Further discussion is warranted on how to implement evidence-based medications in individual patients with multiple comorbidities in real-world clinical practice.
In conclusion, in a real-world population of patients undergoing PCI, approximately 90% of patients were on ≥5 medications. An increasing number of medications was associated with a higher crude incidence of bleeding and ischemic events, whereas adjusted risk was significant for bleeding events, and not for ischemic events.
Acknowledgments
The authors appreciate the support and collaboration of the coinvestigators participating in the CREDO-Kyoto PCI/CABG Registry Cohort-3. The authors are indebted to the clinical research coordinators for data collection.
Sources of Funding
This work was supported by an educational grant from the Research Institute for Production Development (Kyoto, Japan).
Disclosures
T.M. reports lecturer fees from Bayer, Daiichi Sankyo, Japan Lifeline, Kyocera, Mitsubishi Tanabe, Novartis, and Toray; manuscript fees from Bristol-Myers Squibb and Kowa; and having served on advisory boards for Asahi Kasei, Boston Scientific, Bristol-Myers Squibb, and Sanofi. H. Shiomi reports personal fees from Abbott Vascular, Boston Scientific, and Daiichi Sankyo. Y.F. reports honoraria from Ono Pharmaceutical, Novartis, Daiichi Sankyo, Bayer, Otsuka Pharmaceutical, Kowa, Takeda, Sumitomo Dainippon Pharma, Pfizer, Bristol-Myers Squibb, and Sanofi. Y.N. reports research grants from Abbott Vascular and Boston Scientific, and honoraria from Abbott Vascular, Bayer, Boston Scientific, and Daiichi Sankyo. T.K. reports personal fees from Abbott Vascular, Abiomed, Astellas, Astellas Amgen Biopharma, AstraZeneca, Bayer, Boston Scientific, Boehringer Ingelheim, Bristol-Myers Squibb, Chugai Pharmaceutical, Edwards Lifescience, Eisai, Daiichi Sankyo, Interscience, Japan Society for the Promotion of Science, Kowa, Kowa Pharmaceutical, Lifescience, Medical Review, MSD, MSD Life Science Foundation, Mitsubishi Tanabe Pharma, Novartis Pharma, Ono Pharmaceutical, OrbusNeich, Otsuka Pharmaceutical, Pharmaceuticals and Medical Devices Agency, Philips, Public Health Research Foundation, Sanofi, Sumitomo Dainippon Pharma, Takeda Pharmaceutical, Terumo, Toray, and Tsumura. The remaining authors have nothing to disclose.
IRB Information
This study was approved by Kyoto University Certified Review Board and Ethics Committee (E2400).
Data Availability
The deidentified participant data will not be shared.
Supplementary Files
Please find supplementary file(s);
https://doi.org/10.1253/circj.CJ-23-0558
References
- 1.
Hovstadius B, Hovstadius K, Astrand B, Petersson G. Increasing polypharmacy: An individual-based study of the Swedish population 2005–2008. BMC Clin Pharmacol 2010; 10: 16.
- 2.
Leendertse AJ, Egberts AC, Stoker LJ, van den Bemt PM; HARM Study Group. Frequency of and risk factors for preventable medication-related hospital admissions in the Netherlands. Arch Intern Med 2008; 168: 1890–1896.
- 3.
Fincke BG, Miller DR, Spiro A 3rd. The interaction of patient perception of overmedication with drug compliance and side effects. J Gen Intern Med 1998; 13: 182–185.
- 4.
Inouye SK, Charpentier PA. Precipitating factors for delirium in hospitalized elderly persons: Predictive model and interrelationship with baseline vulnerability. JAMA 1996; 275: 852–857.
- 5.
Piccini JP, Hellkamp AS, Washam JB, Becker RC, Breithardt G, Berkowitz SD, et al. Polypharmacy and the efficacy and safety of rivaroxaban versus warfarin in the prevention of stroke in patients with nonvalvular atrial fibrillation. Circulation 2016; 133: 352–360.
- 6.
Jaspers Focks J, Brouwer MA, Wojdyla DM, Thomas L, Lopes RD, Washam JB, et al. Polypharmacy and effects of apixaban versus warfarin in patients with atrial fibrillation: Post hoc analysis of the ARISTOTLE trial. BMJ 2016; 353: i2868.
- 7.
Bistervels IM, Bavalia R, Gebel M, Lensing AWA, Middeldorp S, Prins MH, et al. Effect of polypharmacy on bleeding with rivaroxaban versus vitamin K antagonist for treatment of venous thromboembolism. J Thromb Haemost 2022; 20: 1376–1384.
- 8.
Leiss W, Méan M, Limacher A, Righini M, Jaeger K, Beer HJ, et al. Polypharmacy is associated with an increased risk of bleeding in elderly patients with venous thromboembolism. J Gen Intern Med 2015; 30: 17–24.
- 9.
Honda T, Abe K, Oda M, Harada F, Maruyama K, Aoyagi H, et al. Gastrointestinal bleeding during direct oral anticoagulant therapy in patients with nonvalvular atrial fibrillation and risk of polypharmacy. J Clin Pharmacol 2022; 62: 1548–1556.
- 10.
US Food and Drug Administration (FDA). Drugs@FDA: FDA-approved drugs. https://www.accessdata.fda.gov/scripts/cder/daf/index.cfm?event=overview.process&ApplNo=020839 (accessed December 29, 2022).
- 11.
Matsumura-Nakano Y, Shiomi H, Morimoto T, Yamaji K, Ehara N, Sakamoto H, et al. Comparison of outcomes of percutaneous coronary intervention versus coronary artery bypass grafting among patients with three-vessel coronary artery disease in the new-generation drug-eluting stents era (from CREDO-Kyoto PCI/CABG Registry Cohort-3). Am J Cardiol 2021; 145: 25–36.
- 12.
Yamamoto K, Shiomi H, Morimoto T, Kadota K, Tada T, Takeji Y, et al. percutaneous coronary intervention versus coronary artery bypass grafting among patients with unprotected left main coronary artery disease in the new-generation drug-eluting stents era (from the CREDO-Kyoto PCI/CABG Registry Cohort-3). Am J Cardiol 2021; 145: 47–57.
- 13.
Yamamoto K, Natsuaki M, Morimoto T, Shiomi H, Ozasa N, Sakamoto H, et al. Effect of polypharmacy on long-term mortality after percutaneous coronary intervention. Am J Cardiol 2021; 159: 19–29.
- 14.
World Health Organization Collaborating Centre for Drug Statistics Methodology. ATC/DDD Index 2023. 2023. https://www.whocc.no/atc_ddd_index/ (accessed August 31, 2023).
- 15.
Mehran R, Rao SV, Bhatt DL, Gibson CM, Caixeta A, Eikelboom J, et al. Standardized bleeding definitions for cardiovascular clinical trials: A consensus report from the Bleeding Academic Research Consortium. Circulation 2011; 123: 2736–2747.
- 16.
Cutlip DE, Windecker S, Mehran R, Boam A, Cohen DJ, van Es GA, et al. Clinical end points in coronary stent trials: A case for standardized definitions. Circulation 2007; 115: 2344–2351.
- 17.
Morimoto T, Sakuma M, Matsui K, Kuramoto N, Toshiro J, Murakami J, et al. Incidence of adverse drug events and medication errors in Japan: The JADE study. J Gen Intern Med 2011; 26: 148–153.
- 18.
Seppala LJ, van der Velde N, Masud T, Blain H, Petrovic M, van der Cammen TJ, et al. EuGMS Task and Finish Group on Fall-Risk-Increasing Drugs (FRIDs): Position on knowledge dissemination, management, and future research. Eur Geriatr Med 2019; 10: 275–283.
- 19.
de Vries M, Seppala LJ, Daams JG, van de Glind EMM, Masud T, van der Velde N, et al. Fall-risk-increasing drugs: A systematic review and meta-analysis: I. Cardiovascular drugs. J Am Med Dir Assoc 2018; 19: 371.e1–371.e9.
- 20.
Richardson K, Bennett K, Kenny RA. Polypharmacy including falls risk-increasing medications and subsequent falls in community-dwelling middle-aged and older adults. Age Ageing 2015; 44: 90–96.
- 21.
Kojima T, Akishita M, Nakamura T, Nomura K, Ogawa S, Iijima K, et al. Polypharmacy as a risk for fall occurrence in geriatric outpatients. Geriatr Gerontol Int 2012; 12: 425–430.
- 22.
Gargiulo G, Giacoppo D, Jolly SS, Cairns J, Le May M, Bernat I, et al. Effects on mortality and major bleeding of radial versus femoral artery access for coronary angiography or percutaneous coronary intervention: Meta-analysis of individual patient data from 7 multicenter randomized clinical trials. Circulation 2022; 146: 1329–1343.





