論文ID: CJ-23-0577
論文ID: CJ-23-0577
Background: Diabetes increases the risk of heart failure (HF). 3-Hydroxyisobutyric acid (3-HIB) is a muscle-derived metabolite reflecting systemic insulin resistance. In this study, we investigated the prognostic impact of 3-HIB in patients with chronic HF.
Methods and Results: The KUNIUMI Registry chronic cohort is a community-based cohort study of chronic HF in Awaji Island, Japan. We analyzed the association between serum 3-HIB concentrations and adverse cardiovascular (CV) events in 784 patients from this cohort. Serum 3-HIB concentrations were significantly higher in patients with than without diabetes (P=0.0229) and were positively correlated with several metabolic parameters. According to Kaplan-Meier analysis, rates of CV death and HF hospitalization at 2 years were significantly higher among HF patients without diabetes in the high 3-HIB group (3-HIB concentrations above the median; i.e., >11.30 μmol/L) than in the low 3-HIB group (log-rank P=0.0151 and P=0.0344, respectively). Multivariable Cox proportional hazard models adjusted for established risk factors for HF revealed high 3-HIB as an independent predictor of CV death (hazard ratio [HR] 1.82; 95% confidence interval [CI] 1.16–2.85; P=0.009) and HF hospitalization (HR 1.72; 95% CI 1.17–2.53, P=0.006) in HF patients without diabetes, whereas no such trend was seen in subjects with diabetes.
Conclusions: In a community cohort, circulating 3-HIB concentrations were associated with prognosis in chronic HF patients without diabetes.
Heart failure (HF) is a growing public health problem worldwide due to the severe burden of HF morbidity and mortality.1–3 A rapid increase in the number of HF patients is expected in developed countries over the next 10 to 20 years.3,4 Similarly, there was a 30% increase in the prevalence of diabetes globally between 2005 and 2015, and this increase is expected to continue over time.5 HF and diabetes are associated with considerable morbidity and mortality and worsen patient prognosis and quality of life synergistically, whereas it is well documented that diabetes independently increases the risk of developing HF.6–9 Although multiple mechanisms, such as myocardial ischemia, oxidative stress, inflammation, and lipotoxicity, are related to HF pathophysiology in patients with diabetes, impaired cardiac energy metabolism due to insulin resistance is one of the notable causal factors of HF.10–12
3-Hydroxyisobutyric acid (3-HIB) is a valine-derived metabolite produced in skeletal muscle that reflects systemic insulin resistance.13–15 Circulating 3-HIB concentrations are significantly increased in individuals with type 2 diabetes (T2D) or obesity and are positively correlated with blood glucose, body mass index (BMI), insulin C-peptide, and homeostasis model assessment 2 of insulin resistance (HOMA2-IR).15 Interestingly, it has also been reported that elevated plasma 3-HIB concentrations are a predictor of the incidence of T2D.14 However, the relationship between 3-HIB and HF is totally unknown, and there is no evidence to verify the impact of serum 3-HIB concentrations on the prognosis of HF patients.
To investigate this issue, we measured serum 3-HIB concentrations in patients with chronic HF from the Kobe University HF registry in Awaji Medical Center (KUNIUMI Registry) cohort, which is a community-based prospective observational study of chronic HF in Awaji Island, Japan.4 The findings of this study could contribute to the development of a novel biomarker to predict adverse outcomes of chronic HF.
As described previously,4 the KUNIUMI Registry cohort, a single-center prospective observational study, enrolled a total of 1,646 chronic HF patients, corresponding to approximately 1.3% of the residents in Awaji Island, from March 2019 to March 2021. The inclusion criteria and staging categories of HF followed those in the “Chronic Heart Failure Analysis and Registry in the Tohoku District 2 (CHART-2)” study,16 which was one of the largest HF cohort studies in Japan. Among the 1,646 patients in the KUNIUMI Registry, serum 3-HIB concentrations were measured in 799 consecutive patients registered from March 2019 to January 2020; these patients were monitored for 2 years for cardiovascular (CV) death and HF hospitalization as primary outcomes. Fifteen patients were excluded from the study because of missing BMI, HbA1c, and B-type natriuretic peptide (BNP) data. When analyzing the association between prognosis and 3-HIB concentrations, patients were divided into 2 groups: those with and without diabetes (Figure 1). Diabetes was defined by fasting glucose >126 mg/dL and/or 2-h blood glucose ≥200 mg/dL during the oral glucose tolerance test and/or HbA1c >6.5% (NGSP), according to the clinical guidelines of the Japan Diabetes Society. Diabetes was also defined as treatment with antidiabetic medications.
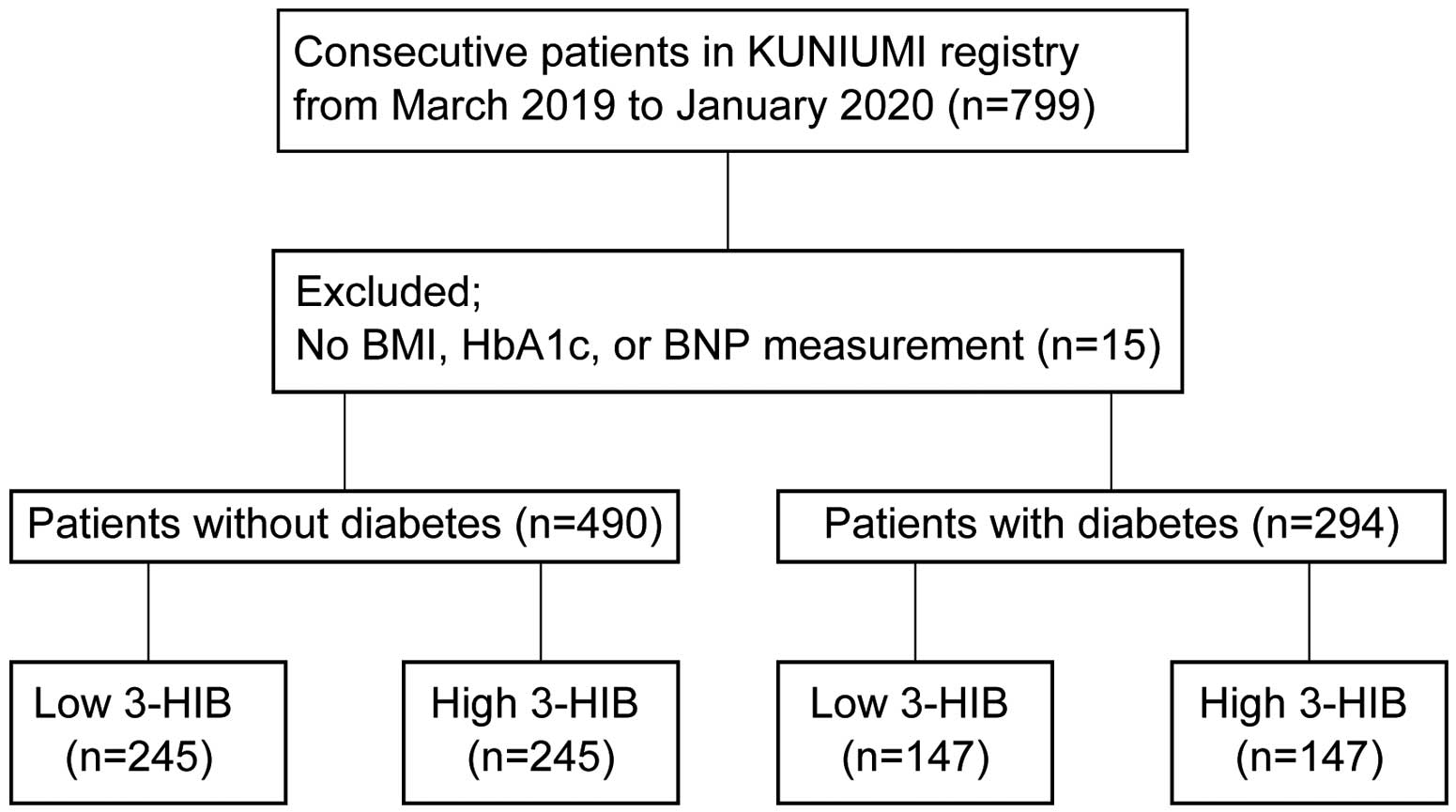
Flow chart of patient enrollment. 3-HIB, 3-hydroxyisobutyric acid; BMI, body mass index; BNP, B-type natriuretic peptide.
This study was conducted in accordance with the principles outlined in the 1975 Declaration of Helsinki. The study was approved by the Ethics Review Board of Awaji Medical Center and Kobe University and registered with the Japan Registry of Clinical Trials (jRCT1050200024). Written informed consent was obtained from all patients.
Measurement of 3-HIB by Gas Chromatography-Mass SpectrometryWhole blood was collected from patients in the morning after an overnight fast. Serum was immediately separated by centrifugation (3,000 rpm, 4℃ for 10 min) and frozen at −80℃ until analysis.
3-HIB concentrations were measured by gas chromatography-mass spectrometry (GC-MS; GCMS-QP2010 Ultra, Shimadzu, Japan). Metabolites were extracted from serum as described previously.17 Briefly, 50 μL serum was quenched with cold methanol. After 30 min incubation at 37℃, the samples were centrifuged, and the aqueous phase was collected and lyophilized. Dried polar metabolites were derivatized and subjected to GC-MS analysis. Data were acquired with selected ion monitoring and calibrated using the peak height of sinapic acid (internal standard).
Statistical AnalysesContinuous variables are expressed as the mean±SD or median with interquartile range (IQR). Baseline characteristics were compared between the non-diabetic and diabetic groups. Continuous variables were compared by using Student’s t-test or the Mann-Whitney U test, as appropriate. Categorical variables were tested by using the χ2 test. Correlations were assessed by using Spearman’s correlation test. The event-free rate of the primary outcomes was evaluated using Kaplan-Meier analysis, and the significance of differences between groups was compared by the log-rank test. The associations of 3-HIB concentrations and established risk factors for HF with primary outcomes were assessed using the Cox proportional hazards model for both univariable and multivariable analysis. Variables with P<0.10 in the univariable analysis were enrolled in multivariable analysis. A two-tailed P<0.05 was considered statistically significant. All statistical analyses were performed using Stata version 17.0 software (StataCorp, College Station, TX, USA).
In all, 784 chronic HF patients were enrolled in this study (Table 1). The mean age was 75.9±11.0 years, and 32.5% were female. Among the patients, there were 294 and 490 with and without diabetes, respectively. Regarding medical history, patients with diabetes had significantly higher rates of hypertension, dyslipidemia, and coronary artery disease than patients without diabetes, whereas the prevalence of atrial fibrillation was higher in patients without than with diabetes. There were no significant differences in left ventricular ejection fraction (LVEF) or prior hospitalization due to HF between the 2 groups (Table 1). The median serum 3-HIB concentration in all 784 participants was 11.49 μmol/L (IQR 9.06–14.97 μmol/L). The distribution was skewed to the left (Figure 2A).
Baseline Characteristics in All Patients and According to Diabetes Status
| Total | Diabetes | No diabetes | P value | |
|---|---|---|---|---|
| No. patients | 784 | 294 | 490 | |
| Age (years) | 75.9±11.0 | 74.0±9.9 | 77.1±11.4 | <0.0001 |
| Female sex (%) | 32.5 | 28.2 | 35.1 | 0.0473 |
| BMI (kg/m2) | 22.9±4.1 | 23.7±4.2 | 22.4±4.0 | <0.0001 |
| NYHA class (%) | ||||
| Class I | 52.0 | 57.5 | 48.8 | 0.0182 |
| Class II | 37.9 | 34.7 | 39.8 | 0.1542 |
| Class III/IV | 10.1 | 7.8 | 11.4 | 0.1047 |
| Medical history (%) | ||||
| Prior hospitalization due to HF | 36.1 | 32.7 | 38.2 | 0.1207 |
| Hypertension | 75.0 | 79.9 | 72.0 | 0.0138 |
| Dyslipidemia | 49.9 | 61.6 | 42.9 | <0.0001 |
| Atrial fibrillation | 31.6 | 24.8 | 35.7 | 0.0015 |
| Coronary intervention | 55.4 | 69.4 | 46.9 | <0.0001 |
| Cancer | 16.3 | 15.3 | 16.9 | 0.5509 |
| Event | ||||
| HF hospitalization | 178 (22.7) | 61 (20.7) | 117 (23.8) | 0.3112 |
| CV death | 121 (15.4) | 37 (12.5) | 84 (17.1) | 0.0872 |
| LVEF (%) | 53.5±12.3 | 53.5±12.3 | 53.5±12.4 | 0.9766 |
| Laboratory data | ||||
| Hemoglobin (mg/dL) | 12.5±2.1 | 12.7±2.2 | 12.5±2.0 | 0.1416 |
| eGFR (mL/min/1.73 m2) | 51.4±23.5 | 50.6±26.6 | 51.8±21.4 | 0.5665 |
| Albumin (mg/dL) | 3.7±0.5 | 3.7±0.6 | 3.7±0.5 | 0.1701 |
| Total bilirubin (mg/dL) | 0.6±0.3 | 0.6±0.3 | 0.7±0.3 | 0.0054 |
| C-reactive protein (mg/dL) | 0.7±1.9 | 0.9±2.4 | 0.6±1.4 | 0.2848 |
| Total cholesterol (mg/dL) | 162.7±36.8 | 158.7±34.9 | 165.2±37.7 | 0.0179 |
| Triglycerides (mg/dL) | 128.3±76.9 | 138.9±76.0 | 122.0±76.7 | 0.0002 |
| HDL cholesterol (mg/dL) | 51.2±13.9 | 48.6±12.7 | 52.8±14.3 | <0.0001 |
| Glucose (mg/dL) | 125.7±43.7 | 150.0±54.6 | 111.1±26.4 | <0.0001 |
| HbA1c (%) | 6.2±0.8 | 6.8±0.9 | 5.9±0.5 | <0.0001 |
| BNP (pg/mL) | 119.0 [46.5–274.7] | 90.4 [40.0–249.1] | 131.1 [52.4–288.8] | 0.0057 |
| Medications (%) | ||||
| Loop diuretic | 40.1 | 35.4 | 43.9 | 0.0192 |
| MRA | 24.2 | 19.0 | 27.3 | 0.0088 |
| β-blocker | 65.8 | 68.7 | 64.1 | 0.1871 |
| ACEI/ARB | 68.2 | 70.4 | 66.9 | 0.3135 |
| Statin | 52.6 | 65.6 | 44.7 | <0.0001 |
| SGLT-2i | 9.4 | 23.1 | 1.2 | <0.0001 |
| Insulin | 7.7 | 20.4 | 0.0 | <0.0001 |
Unless indicated otherwise, data are given as the mean±SD, median [interquartile range], or n (%). ACEI, angiotensin-converting enzyme inhibitors; ARB, angiotensin II receptor blocker; BMI, body mass index; BNP, B-type natriuretic peptide; CV, cardiovascular; eGFR, estimated glomerular filtration rate; HDL, high-density lipoprotein; HF, heart failure; LVEF, left ventricular ejection fraction; MRA, mineralocorticoid receptor antagonists; NYHA, New York Heart Association; SGLT2i, sodium-glucose cotransporter 2 inhibitor.
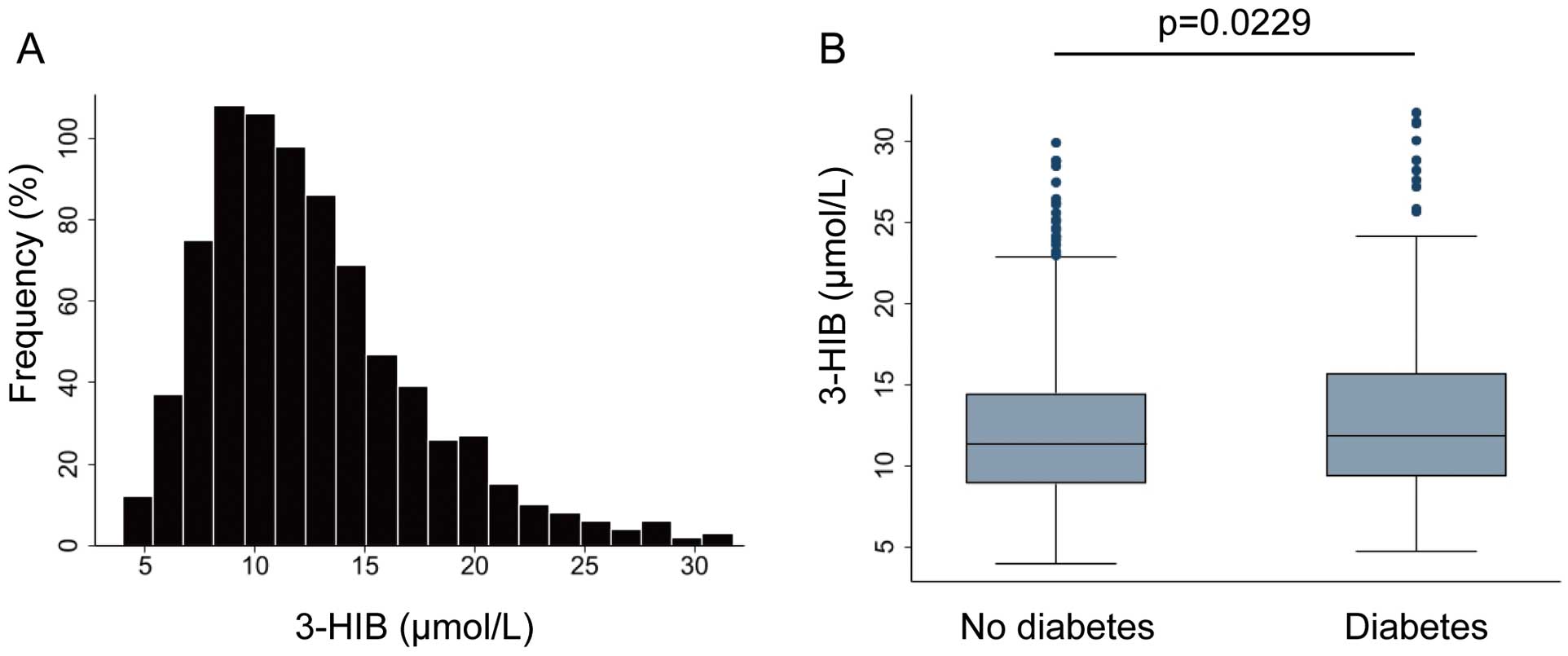
(A) Distribution of serum 3-hydroxyisobutyric acid (3-HIB) concentrations in all patients included in the study (n=784). (B) Serum 3-HIB concentrations in patients without and with diabetes (n=490 and n=294, respectively). The boxes show the interquartile range, with the median value indicated by the horizontal line; whiskers show the range. Significance was calculated using the Mann-Whitney U test.
Association Between 3-HIB and Diabetes
3-HIB concentrations were significantly higher in patients with than without diabetes patients (median 11.84 (IQR 9.37–15.67) vs. 11.30 (IQR 8.88–14.45) μmol/L, respectively; P=0.0229, Figure 2B). In addition, 3-HIB was positively correlated with diabetes-associated parameters, including HbA1c (Spearman’s R=0.1200, P=0.0008), fasting blood glucose (Spearman’s R=0.1413, P=0.0001), BMI (Spearman’s R=0.1523, P<0.0001), and triglycerides (TGs; Spearman’s R=0.1263, P=0.0004; Supplementary Figure A–D). Although there was no correlation between 3-HIB and either BNP or LVEF, there was a slight negative correlation between 3-HIB and estimated glomerular filtration rate (Supplementary Figure E–G).
Primary OutcomesTo investigate the prognostic impact of 3-HIB on HF incidence, we divided the 784 patients into 2 groups according to diabetes status (294 and 490 individuals with and without diabetes, respectively) and examined 2-year rates of CV death and HF hospitalization in the 2 groups.
In the non-diabetic group, CV death at 2 years after registration occurred in 84 patients. Of these 84 patients, 52 and 32 were in the high 3-HIB group (3-HIB concentrations above the median value; i.e., >11.30 μmol/L) and low 3-HIB group (below the median value; i.e., ≤11.30 μmol/L), respectively. Regarding HF hospitalization, there were 117 cases over the 2-year period after registration, with 68 and 49 patients in the high and low 3-HIB groups, respectively. The baseline characteristics of the high and low 3-HIB groups are presented in Supplementary Table 1. Kaplan-Meier analysis showed that the incidence rates of 2-year CV death and HF hospitalization were significantly higher in the high 3-HIB group than in the low 3-HIB group (log-rank P=0.0151 and P=0.0344, respectively; Figure 3A,B).
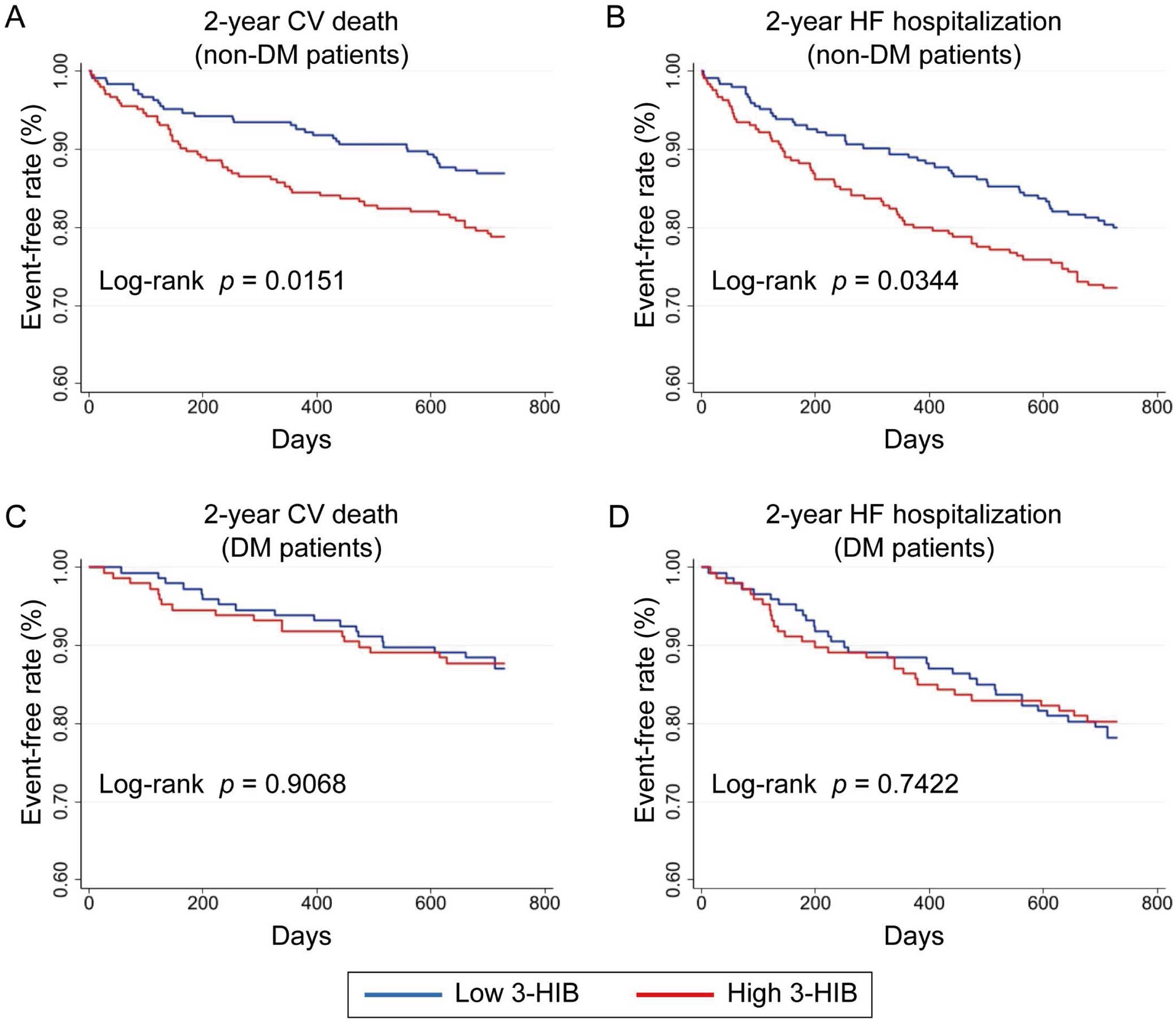
Kaplan-Meier analysis for event-free rates of primary outcomes over 2 years after registration. Patients were divided into 2 groups based on the median 3-hydroxyisobutyric acid (3-HIB) concentration, namely a high 3-HIB group (3-HIB >11.30 μmol/L) and low 3-HIB group (3-HIB ≤11.30 μmol/L). (A,B) Cardiovascular (CV) death (A) and heart failure (HF) hospitalization (B) in patients without diabetes (n=490). (C,D) CV death (C) and HF hospitalization (D) in patients with diabetes (DM; n=294).
In the diabetic group, after dividing patients into 2 groups according to the median 3-HIB concentration (11.84 μmol/L; Supplementary Table 2), Kaplan-Meier analysis did not reveal a significant difference in the rate of either 2-year CV death or HF hospitalization between the high and low 3-HIB groups (log-rank P=0.9068 and P=0.7422, respectively; Figure 3C,D).
Next, we used univariable and multivariable Cox proportional hazard models to determine the prognostic factors associated with CV death and HF hospitalization in the non-diabetic group. As indicated in Table 2, high 3-HIB was a significant predictor of 2-year CV death (hazard ratio [HR] 1.71; 95% confidence interval [CI] 1.10–2.66; P=0.016) and HF hospitalization (HR 1.48; 95% CI 1.02–2.14; P=0.036) in univariable Cox analysis. According to multivariable Cox analysis, after adjusting for established risk factors for HF, high 3-HIB was independently associated with the incidence of 2-year CV death (HR 1.82; 95% CI 1.16–2.85; P=0.009) and HF hospitalization (HR 1.72; 95% CI 1.17–2.53; P=0.006; Table 2). In contrast, a similar trend was not found in the diabetic group. In addition, we analyzed the impact of mineralocorticoid receptor antagonists (MRA) and sodium–glucose cotransporter 2 inhibitors (SGLT2i) on HF hospitalization because their rate of use was significantly higher in the diabetic group (Table 1). However, MRA and SGLT-2i did not affect the HF prognosis in patients (data not shown).
Cox Proportional Hazard Models for Cardiovascular Death and HF Hospitalization in Patients Without Diabetes
| Variables | Univariable | Multivariable | ||||
|---|---|---|---|---|---|---|
| HR | 95% CI | P value | HR | 95% CI | P value | |
| CV death | ||||||
| 3-HIB > median | 1.71 | 1.10–2.66 | 0.016 | 1.82 | 1.16–2.85 | 0.009 |
| Age | 1.11 | 1.08–1.15 | <0.001 | 1.07 | 1.04–1.10 | <0.001 |
| Sex | 0.91 | 0.58–1.42 | 0.691 | – | – | – |
| BMI | 0.82 | 0.77–0.88 | <0.001 | 0.91 | 0.84–0.98 | 0.014 |
| Hemoglobin | 0.65 | 0.58–0.72 | <0.001 | 0.75 | 0.65–0.86 | <0.001 |
| HbA1c | 0.69 | 0.42–1.13 | 0.150 | – | – | – |
| Hypertension | 0.57 | 0.36–0.88 | 0.012 | 0.50 | 0.32–0.80 | 0.004 |
| Dyslipidemia | 0.38 | 0.23–0.62 | <0.001 | 0.78 | 0.46–1.31 | 0.358 |
| Log[BNP] | 2.06 | 1.70–2.49 | <0.001 | 1.45 | 1.17–1.81 | 0.001 |
| LVEF | 0.98 | 0.97–1.00 | 0.128 | – | – | – |
| eGFR | 0.97 | 0.96–0.98 | <0.001 | 0.99 | 0.98–1.00 | 0.367 |
| HF hospitalization | ||||||
| 3-HIB > median | 1.48 | 1.02–2.14 | 0.036 | 1.72 | 1.17–2.53 | 0.006 |
| Age | 1.11 | 1.09–1.14 | <0.001 | 1.07 | 1.04–1.10 | <0.001 |
| Sex | 0.87 | 0.59–1.26 | 0.470 | – | – | – |
| BMI | 0.85 | 0.81–0.90 | <0.001 | 0.94 | 0.88–1.00 | 0.050 |
| Hemoglobin | 0.67 | 0.61–0.73 | <0.001 | 0.75 | 0.67–0.85 | <0.001 |
| HbA1c | 0.92 | 0.62–1.37 | 0.704 | – | – | – |
| Hypertension | 0.65 | 0.45–0.96 | 0.030 | 0.59 | 0.39–0.89 | 0.012 |
| Dyslipidemia | 0.46 | 0.31–0.70 | <0.001 | 0.96 | 0.63–1.48 | 0.878 |
| Log[BNP] | 2.18 | 1.85–2.58 | <0.001 | 1.45 | 1.19–1.77 | <0.001 |
| LVEF | 0.98 | 0.96–0.99 | 0.009 | 0.97 | 0.96–0.99 | 0.003 |
| eGFR | 0.97 | 0.96–0.97 | <0.001 | 0.99 | 0.98–1.00 | 0.154 |
The multivariate model was adjusted for variables with P<0.10 in the univariable analysis. 3-Hydroxyisobutyric acid (3-HIB) > median indicates serum concentrations >11.30 μmol/L. CI, confidence interval; HR, hazard ratio. Other abbreviations as in Table 1.
Over past decades, numerous studies have reported that HF is accompanied by impaired cardiac metabolism.18–20 Changes in several circulating metabolites, such as ketone bodies, branched-chain amino acids (BCAAs; leucine, isoleucine, and valine), and acylcarnitines, occur in HF patients.21–24 In line with these findings, the present study revealed that 3-HIB, an intermediate of the BCAA valine, is elevated in HF patients with diabetes and is positively correlated with diabetes-related parameters. Importantly, circulating 3-HIB concentrations are associated with adverse events in HF patients, especially those who do not have diabetes.
Previous studies reported that 3-HIB is a marker of insulin resistance in individuals with T2D and obesity.14,15 Nilsen et al demonstrated that serum 3-HIB is positively correlated with HOMA2-IR, BMI, and insulin C-peptide, and significantly decreased 1 year after bariatric surgery.15 Consistent with these findings, the diabetic patients in the present study had higher serum concentrations of 3-HIB, and 3-HIB showed a mildly positive correlation with fasting blood glucose, HbA1c, BMI, and TGs. Notably, high 3-HIB significantly increased the risk of CV death and HF hospitalization in HF patients without diabetes. After adjusting for established risk factors, 3-HIB was independently associated with adverse outcomes in HF patients without diabetes. Given that 3-HIB is a credible marker for insulin resistance, our findings seem to be reasonable because numerous studies have indicated that there is a strong relationship between insulin resistance and the incidence of HF and its prognosis.25–27 In particular, Vardeny et al, who prospectively analyzed 12,606 participants without diabetes, found that insulin resistance, defined by HOMA-IR, was significantly associated with the incidence of HF.26 That study suggests the effectiveness of assessing insulin resistance to evaluate the future risk for HF even in non-diabetic patients. Although previous studies evaluated insulin resistance using various methods, such as HOMA-IR, euglycemic insulin clamp, and intravenous glucose tolerance tests, we are the first to identify the utility of 3-HIB measurement in the estimation of HF patient prognosis. Conversely, we also found that 3-HIB does not work as a predictor of HF incidence in HF patients with diabetes. This may be due to the influence of antidiabetic medications or differences in the severity or duration of diabetes among individual patients.
Insulin resistance induced by T2D and obesity is well known to affect BCAA metabolism in several organs, including skeletal muscle, heart, liver, and adipose tissue.28,29 According to Newgard et al, BCAA levels are elevated in plasma samples from obese compared with lean individuals.28 Furthermore, the BCAA valine, of which 3-HIB is a metabolite, is 20% higher in obese than lean individuals.28 Although there are no large-scale observational studies analyzing the links between the prognosis and pathophysiology of HF and circulating BCAA concentrations in HF patients, Tobias et al reported that BCAAs are positively associated with coronary events, including myocardial infarction and revascularization, in women.30 Considering that high circulating BCAA concentrations are a hallmark of T2D or CV disease, our data, which indicate the effectiveness of the BCAA valine-derived 3-HIB as the prognostic marker for HF, are likely to be compatible with the prior findings.
Interestingly, it has been reported that 3-HIB itself functions as a signaling metabolite to induce insulin resistance. Jang et al demonstrated that skeletal muscle-derived 3-HIB upregulates trans-endothelial fatty acid transport in a paracrine manner, followed by increased fatty acid uptake in the muscle, which causes insulin resistance in mice.31 Moreover, 3-HIB is known to be released from muscle as well as adipocytes.15,32 Both white and brown adipocytes increase BCAA catabolism and release 3-HIB during differentiation.15 The addition of 3-HIB to both subtypes of adipocytes modulates fatty acid and glucose uptake, mitochondrial oxygen consumption, and reactive oxygen species generation, suggesting that 3-HIB plays a fundamental role in adipocyte function.15 In the present study, the main sources of circulating 3-HIB were not elucidated; circulating 3-HIB may not only reflect insulin resistance in HF patients, but also induce cardiac dysfunction and subsequent HF through the impairment of glucose oxidation in the myocardium.
The present study has several limitations. First, the KUNIUMI Registry chronic cohort was a single-center prospective observational study, which may have introduced selection bias in the study population. However, our data gathered in Awaji Medical Center are likely to have low bias in the population because it is the only hospital on Awaji Island where cardiologists are available full time. Second, blood insulin concentrations were not measured in this study, which did not allow us to calculate HOMA-IR and analyze the correlation between 3-HIB and HOMA-IR. Third, we did not measure the serum concentrations of BCAAs. Therefore, we were not able to evaluate how 3-HIB is related to BCAA concentrations in HF patients. Further studies are needed to address these issues.
To the best of our knowledge, we are the first to demonstrate the association between circulating 3-HIB concentrations and the incidence of chronic HF in patients. 3-HIB could be a prognostic biomarker for CV events in HF patients without diabetes. This novel metabolite may be useful for the early detection of HF development or deterioration associated with insulin resistance.
The authors thank Emiko Yoshida, Shiho Fukushima, Haruka Okada, Michiko Iwasaki, Yukimi Kato, Hitomi Kodani, Mami Motomura, and Yoko Fujimoto for providing technical assistance and collecting and managing the data.
This work was supported by the Osaka Gas Group Welfare Foundation, Japan; Hyogo Science and Technology Association; and a Grant-in-Aid for Scientific Research (C) (22K08205) and a Grant-in-Aid for Young Scientists (20K17081) from the Ministry of Education, Culture, Sports, Science and Technology of Japan.
The Division of Evidence-based Laboratory Medicine, Kobe University Graduate School of Medicine was established by an endowment fund from Sysmex Corporation, Japan. K.H. is a member of Circulation Journal’s Editorial Team.
This study was approved by the Ethics Review Board of Awaji Medical Center (Approval no. 21-20) and Kobe University (Approval no. B210236) and is registered with the Japan Registry of Clinical Trials (jRCT1050200024).
The deidentified participant data will not be shared.
Please find supplementary file(s);
https://doi.org/10.1253/circj.CJ-23-0577