論文ID: CJ-23-0579
論文ID: CJ-23-0579
Despite advancements in treatments for heart failure and lethal arrhythmias, achieving satisfactory life prognoses remains a challenge. A fresh perspective on the pathogenesis of heart disease is imperative to improve these prognoses. Our research has highlighted the role of cardiac macrophages in inhibiting the onset of heart failure and sudden cardiac death. We have recently unveiled a collaborative mechanism involving immune cells, brain neural networks, and the kidneys, which work in concert to combat cardiovascular diseases. This intricate organ network, orchestrated by the brain neural network and immune system, is pivotal in maintaining whole-body homeostasis. Disruptions in this harmonious interplay can precipitate various conditions, including heart failure and multiple organ failure, underscoring the significance of technological advancements in analytical methods and the advent of artificial intelligence. Recent strides in circulatory organ research have facilitated concurrent high-level analysis of the neural network and cardiovascular system. This review encapsulates these cutting-edge reports, evaluates the progress of research anchored in the fundamental concept that system failure of the cardiovascular organ precipitates cardiovascular disease, and offers valuable insights to guide future research.
Various treatments have been developed for heart failure or lethal arrhythmias; however, a satisfactory life prognosis has not yet been achieved.1 To improve the life prognoses of patients with heart disease, a new perspective is needed in the study of the pathogenesis of heart disease.
We have shown that cardiac macrophages suppress the onset of heart failure and sudden cardiac death (Figure).2–4 Furthermore, we have recently identified the mechanism by which immune system cells, including macrophages, and the brain neural network work protectively against cardiovascular diseases.2,5 In this way, the organ network mediated by the brain neural network and immune system network works to maintain the homeostasis of the entire body, and various diseases, such as heart failure and multiple organ failure, have been reported to occur when this collaboration breaks down. This is greatly related to technological innovation in terms of analytical methods and the emergence of artificial intelligence.
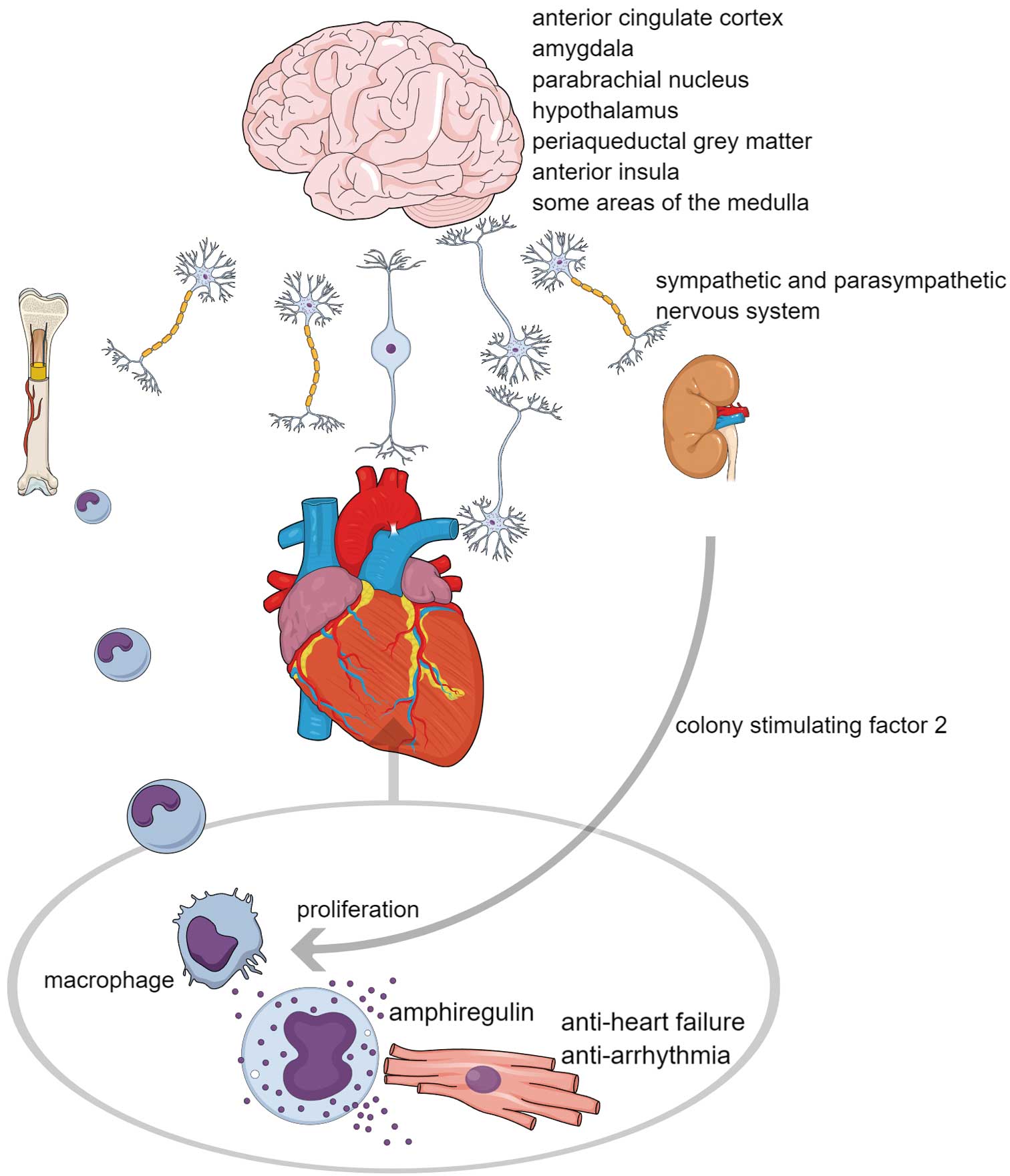
Neural pathway control of heart homeostasis. The brain senses signals from internal organs and external stressors and processes these signals, as well as emotions, within various brain regions. There have been reports of specific areas related to tachycardia and bradycardia. Ultimately, heart homeostasis is maintained through multiorgan interplay mediated by the sympathetic and parasympathetic nervous systems. For example, cardiac macrophages interact with cardiomyocytes through the secretion of amphiregulin, suppressing heart failure and arrhythmias. These macrophages originate from the bone marrow, which the sympathetic nervous system controls. Signals reach the brain during cardiac stress via the vagal afferent pathway and sensory neurons through dorsal root ganglia. Subsequently, the brain releases colony-stimulating factor 2 from the kidneys through the sympathetic nervous system. This action promotes the proliferation of cardiac macrophages, ensuring a proper stress response.
In the field of circulatory organ research, recent studies have analyzed the neural network and cardiovascular system simultaneously at a high level. This article summarizes such recent reports, determines the progress of research based on the basic concept that system failure of the cardiovascular organ as a system leads to the onset of cardiovascular disease, and seeks to inform future research.
In a recent report about brain and cardiac structure, Zhao et al used imaging and genetic data from tens of thousands of participants in the UK Biobank and BioBank Japan to determine correlations between the structure and function of both the heart and brain.6 These authors found links between specific features of cardiac imaging and neuropsychiatric disorders and identified the shared genetic influences on both the brain and the heart.6
That study deepens our understanding of heart–brain links and their genetic bases. Furthermore, Zhao et al observed that magnetic resonance imaging measurements of the 2 organs were associated with each other, and that this was independent of a wide variety of body measures, shared risk factors, and imaging confounders.6 The authors also uncovered genetic colocalizations and correlations between heart structure and function and brain clinical endpoints, suggesting that adverse heart metrics may have implications for brain abnormalities and the risk of brain diseases.6
A few thought-provoking questions and insights have been gleaned from that article.
• The interplay of the heart and brain: The study highlights the intricate relationship between the heart and brain in terms of their anatomical structures and physiological functions. How might this understanding influence the way we approach cardiovascular and neurological health in the future?
• Genetic influences: Shared genetic influences on both the brain and heart suggest a deeper biological connection between these 2 organs. What are the implications of these findings for genetic research and personalized medicine?
• Implications for disease treatment and prevention: The study of Zhao et al suggests that adverse heart metrics may have implications for brain abnormalities and increased risk of brain disease.6 How might this influence strategies for disease prevention and treatment, particularly for conditions that affect both the heart and brain?
In addition to that observational study, the direct influence of cardiac diseases on brain diseases, such as sleep disorders, has been studied. Ziegler et al found that in both humans and mice, sleep disruption in cardiac disease is driven by the loss of neurons that normally project from the superior cervical ganglia into the pineal gland, which secretes melatonin.7 The authors discovered that heart disease triggers the infiltration of macrophages into the superior cervical ganglia, where they orchestrate neuronal cell death. The depletion of macrophages or the inhibition of their activation attenuated these defects in a mouse model of heart disease,7 suggesting an actionable target for future therapies. Furthermore, the study provides insights into the mechanism by which diurnal rhythmicity in cardiac disease is disturbed and suggests a potential target for therapeutic intervention.
The autonomic nerve is the main nerve controlling the heart from the brain in a centrifugal manner. The relationship between the nucleus controlling the autonomic nervous system and other parts of the brain has been studied for a long time. Farkas et al examined projections from the periaqueductal gray (PAG) to the parabrachial nucleus in rats.8 In that study, pseudoviruses were used as transneuronal tracers to identify the PAG sites involved in sympathetic regulation. The researchers also used the plant lectin Phaseolus vulgaris leuco-agglutinin (PHA-L) for anterograde axonal tracing to identify centrifugal projections from specific PAG layers to sympathetic preganglionic neurons (SPNs) and sympathetic premotor brainstem areas in the medulla.8 It was then found that the PAG projects to the hypothalamus, cerebral cortex, and sympathetic premotor neurons in the medulla. Centrifugal projections from the lateral and ventrolateral layers of the PAG were found to be similar, but inputs to different sympathetic precursor cell groups were not uniform. The distributions that arose from the lateral PAG column and the ventrolateral PAG column were similar.8
The study concluded that the PAG plays an important role in autonomic function, particularly sympathetic function.8 These findings provide valuable insights into the neural circuits involved in the fight-or-flight response and are thought to be useful in understanding and treating various neurological and cardiovascular diseases.
Next, we introduce a study on how the brain senses blood pressure, which is the most important parameter of circulatory organs, and controls it. A report by Min et al discusses the role of pressure receptor neurons that sense blood pressure.9 These neurons transmit inputs directly to the brainstem and, when activated, act on the neural pathway, reducing the output of the sympathetic nerves, increasing the output of the parasympathetic nerves, and ultimately lowering heart rate and blood pressure.9 Piezo proteins function as mechanosensitive ion channels important for neuronal sensation of blood pressure and the baroreceptor reflex. These channels are usually essentially gated by force and are essential for normal touch, proprioception, and airway stretch sensation function.
The study of Min et al also mentions the use of optogenetic experiments and electrical nerve stimulation to understand the function of pressure receptor neurons. Physiological measurements were performed in anesthetized mice, and the baroreceptor reflex was induced via the intravenous administration of phenylephrine.9
Following this, a study has been conducted on the impact of artificial stimulation of the brain on the cardiovascular system. Pereira et al investigated the impact of deep brain stimulation (DBS) on heart rate variability (HRV) in patients with chronic pain. In that study, the adaptive effects obtained in the autonomic regulation of the cardiovascular system during the implementation of DBS for chronic pain were investigated. The authors’ goal was to evaluate whether objective changes in HRV correlate with the degree of subjective analgesia and whether such changes differ with dorsal and ventral PAG stimulation.
The study of Pereira et al targeted patients with chronic neuropathic pain who received DBS in the PAG region. Using diffusion tensor imaging, which allows for the examination of macroscopic axonal organization in the brain by fitting diffusion tensors to determine the fractional anisotropy of neuronal tissue, the authors evaluated whether the anatomical connections of the dorsal PAG and ventral PAG differed.
It was shown that changes in HRV, especially high-frequency power and the low-frequency/high-frequency power ratio, correlate well with pain relief, and hence analgesic effects. The authors concluded that these findings advance research on objective markers of chronic pain and enable the selection of patients most likely to respond to PAG DBS.
In summary, that study provides valuable insights into the impact of DBS on HRV in patients with chronic pain and offers a new approach to studying objective pain indicators and patient selection for PAG DBS.
Recent studies of organ interplay at the single-cell level have made it possible to classify and analyze new functions of afferent nerves from the heart to the brain, enabling the acquisition of knowledge at a new level. The paper by Veerakumar et al focuses on the molecular definition of the parasympathetic nerves that control the heart.10 In that study, single-cell RNA sequencing (scRNA-seq) was used to profile the nerves governing the heart from the mouse brainstem. These nerves were labeled using the fluorescent retrograde neuronal tracer cholera toxin B. The labeled cells, called AmbCardiac neurons, identified over 500 different genes expressed between them and the AmbLaryngeal neurons that govern the pharynx. Genes selectively expressed in AmbCardiac neurons include genes coding for transcription and splicing regulatory factors and genes regulating synaptic connectivity, electrophysiological characteristics, input signaling, and disease progression processes.10 In addition, a series of AmbCardiac-specific genes expressed in other brainstem parasympathetic nuclei were identified.10 These genes are not expressed in adjacent somatic brain nerve nuclei, indicating that they are markers of visceral motor neurons.
It is suggested that changes occur in the heart and its upstream regulator, the nervous system, in the presence of cardiovascular diseases.11 But what governs the nerves of heart disease patients at a more upstream level? This is a major issue to be clarified.
The autonomic nervous system regulates heart function by balancing the actions of sympathetic and parasympathetic inputs to the heart. It has been reported that intrinsic cardiac neural circuits fine-tune the control of the heart by integrating these autonomic nervous system signals, and that sensory feedback loops regulate autonomic neurotransmission in response to external stimuli.12 Furthermore, the heart can adapt to constantly changing situations, from simple changes in posture to marathons, through these interconnected nervous systems.12 However, cardiac reflexes that maintain homeostasis in a healthy state are impaired in many pathologies and are often characterized by increased sympathetic neurotransmission and decreased parasympathetic neurotransmission.12
Studies on cardiovascular diseases have revealed that remodeling of cardiac neural circuits occurs at several functional and anatomical levels. Central neural circuits change to overactivate sympathetic pathways, and parasympathetic circuits show decreased activity after this change. Peripheral sensory nerves also become overactive during disease, increasing the risk of poor cardiac prognoses in patients.
Injury or disease changes the types of neurotransmitters and neuropeptides released from cardiac autonomic nerves, causing local neural hyperinnervation (increased nerve density) or denervation (decreased nerve density) in cardiac tissue. Although the mechanisms of neural remodeling are not fully understood, it is likely that neurotrophins and inflammatory cytokines are involved. This emphasizes the need for further research to understand how inflammation induced by disease or injury leads to the breakdown of neural cardiac control, identify new targets for intervention, and possibly improve treatment strategies to improve health outcomes.
In clinical research, the association between sympathetic abnormalities and cardiovascular diseases is well known in Takotsubo cardiomyopathy. Radfar et al examined the stress-related neurobiological activity of Takotsubo syndrome (TTS), which is often triggered by acute stress, and its relationship with subsequent onset risk and onset timing.13
In that study, 104 people (median age 67.5 years; 72% female; 86% malignant tumors) who underwent clinical 18F-fluorodeoxyglucose positron emission tomography/computed tomography (18F-FDG PET/CT) imaging were studied.13 Of these 104 people, 41 later developed TTS, and 63 were matched controls. The researchers measured amygdala activity (AmygA), a marker of stress-related neurobiological activity, using a validated method. The amygdala is part of the cerebral limbic system of the brain and is involved in the perception of emotions and the onset of stress. It was shown that people who developed TTS had higher baseline AmygA than those who did not, even after adjusting for TTS risk factors.13 Furthermore, AmygA was found to be associated with the risk of subsequent TTS, even after adjusting for risk factors. Among the subset that developed TTS, those with the highest AmygA developed TTS approximately 2 years earlier after imaging diagnosis than those with lower AmygA.13
That study concluded that in a retrospective population with a high proportion of malignant tumors, an increase in AmygA was associated with an increased risk of TTS.13 This heightened neurobiological activity exists several years before the onset of TTS and may influence the timing of its onset. Therefore, increased stress-related neural activity may be a therapeutic target for reducing stress-related diseases, including TTS.
Several reports have been made regarding the amygdala, which is related to diseases associated with the sympathetic nervous system mentioned in the previous section, and its connection to the cardiovascular system. In a study investigating the role of the medial amygdala in hemodynamic control mediated by GABAA receptors in anesthetized rats, bicuculline and muscimol were injected into the anterior medial amygdala of anesthetized rats and bilateral adrenalectomized rats.14 The researchers monitored blood pressure (BP) and heart rate (HR), measured plasma catecholamine concentrations, and evaluated the role of the anterior medial basal amygdala in controlling these parameters. When the GABAA receptor antagonist bicuculline was injected into the anterior medial basal amygdala, BP and HR increased significantly.14 This response was suppressed when the GABA agonist muscimol was administered afterwards. The researchers also discovered that the cardiovascular response to bicuculline was not suppressed by adrenalectomy.14
There have been reports on the relationship between the amygdala and heart function in humans. For example, Fiechter et al investigated the relationship between amygdala activity and heart function in women with known or suspected coronary artery disease (CAD).15 In that study, 302 patients with known or suspected CAD (88 women, 214 men) were targeted. The researchers measured resting amygdala activity using 18F-FDG PET/CT images. It was shown that in women, a decrease in left-ventricular function and myocardial perfusion abnormalities were associated with increased amygdala activity; this association was not observed in men.15 Therefore, the authors suggested that the neural stress response to cardiovascular disease differs greatly between men and women, and that a stronger response may be observed in women.15
Fiechter et al suggested that such differences in neural stress responses may contribute to the worsening of the outcomes observed in women with cardiovascular disease.15 They also proposed that the imbalance in the burden of mental stress may be at least one cause of such poor prognoses. The study concluded that neurocellular imaging plays a clinical role in determining the phenotype of patients with a future risk of heart events.15 That study provides valuable insights into the relationship between amygdala activity and heart function in female CAD patients, suggesting a potential association between mental stress and the occurrence of cardiovascular disease in women.
There have been reports on which bodily phenomena occur when the amygdala is stimulated. One paper explored the impact of the microstimulation of the amygdala on the cardiovascular response in rats.16 In that study, microstimulation of the central nucleus of the amygdala (CeA) resulted in a slow pressor response and tachycardia, whereas microstimulation of the area around the CeA, including the basolateral amygdala, caused a phasic decrease in arterial pressure but no change in heart rate.16
The researchers also found that the gain of the baroreceptor reflex does not change with microstimulation of the CeA, and that GABAergic inhibition of the amygdala complex induces a baroreceptor response and tachycardia.16 Interestingly, bilateral amygdala lesions cause chronic arterial pressure elevation.16
In summary, that study provides valuable insights into the bidirectional cardiovascular responses induced by microstimulation of the amygdala in rats, providing a deeper understanding of the role of the amygdala in the regulation of the cardiovascular system.
Finally, other researchers have observed changes in glial cells in the amygdala in the pathology of heart failure in rats. Althammer et al examined changes in microglia and astrocytes in the central amygdala and paraventricular nucleus (PVN) of the hypothalamus in rats with heart failure.17 The study used 3-dimensional morphometric analysis to examine these changes. The researchers collected samples from the PVN and CeA of rats and performed RNA extraction and cDNA synthesis. They then analyzed gene expression in these samples. The paper discusses the role of microglia, which are highly dynamic surveillants of brain parenchyma, in neuroinflammation.17 It also elaborates on the role of astrocytes in regulating neuroinflammation.17 The authors conducted an in-depth analysis of the diversity within the microglial community. They also examined the impact of heart failure on the nervous system, citing activation of oxytocin neurons in the PVN after myocardial infarction (MI) in rats and determining the existence of an enhanced angiotensin-mediated excitation of renal sympathetic nerve activity within the PVN in rats with heart failure.17 In addition, the authors discussed the interaction between neurons and microglia in mental health disorders and mentioned that stress-induced elevation of glucocorticoids increases microglia proliferation through N-methyl-D-aspartate (NMDA) receptor activation.17 The localization of tumor necrosis factor-α and interleukin-1α immunoreactivities in striatal neurons after surgical injury to the hippocampus was also discussed.17 In conclusion, that paper provides valuable insights into the structural changes in microglia and astrocytes in the central amygdala and hypothalamic PVN of heart failure rats. It highlights the complex interplay between neuroinflammation, heart failure, and mental health disorders.
Koba et al discussed the role of the PVN of the hypothalamus and its impact on the sympathetic nervous system.18 The PVN is part of the brain and plays a crucial role in regulating the body’s response to stress, including the “fight-or-flight” response. Koba et al suggested that the role of the PVN in heart failure is mediated by oxidative stress in the brain and can be normalized by exercise training.18
Koba et al also discussed the role of glutamate, a neurotransmitter, in sympathetic nerve activation in heart failure, suggesting that the balance between angiotensin AT1 and AT2 receptors in the ventromedial medulla could be a mechanism for excessive sympathetic activity in heart failure.18 The authors also discussed the role of nitric oxide in the PVN and its impact on renal nerve discharge in heart failure rats, suggesting that a decrease in endogenous GABA-mediated inhibition in the PVN for renal nerve discharge is a factor in heart failure.18
In conclusion, this paper reported on an in-depth evaluation of the role of the PVN and the sympathetic nervous system in heart failure. The findings suggest that changes in the balance of neurotransmitters and receptors in the PVN may play a significant role in heart failure.
Zhu et al examined changes in the intrinsic cardiac nervous system after chronic MI.19 Using a mouse model of chronic MI, the researchers combined optical mapping, immunohistochemistry, and computational image processing to study structural and functional changes in the hearts of mice, finding that chronic MI significantly remodels the structure and function of the myocardium and the distribution of sympathetic nerves.19
That study revealed that the infarct border zone (BZ) is characterized by high myocardial fiber anisotropy and slow activation times.19 In addition, in the infarct BZ, there was a significant increase in small nerve fibers and a decrease in medium nerve fibers compared with sham hearts.19 Interestingly, the prevalence of small nerve fibers was also reduced in the left ventricular base of MI hearts.19
Zhu et al also found that these local changes in nerves have functional effects on impulse propagation in the myocardium.19 In sham hearts, sympathetic nerve stimulation uniformly prolonged the action potential duration measured to 80% repolarization (APD80) throughout the heart. In contrast, in MI hearts, there was a significant regional difference in APD80 prolongation, with more APD80 prolongation in the right ventricle than in the left ventricular base or infarct BZ.19
That study concluded that chronic MI induces structural and functional remodeling of the intrinsic cardiac nervous system, which may be involved in the etiology of arrhythmias after MI.19 These findings provide insights into the complex interactions between the heart and nervous system in chronic MI.
Salavatian et al investigated the role of cardiac sensory neurons in MI.20 The researchers combined techniques such as optical mapping, immunohistochemistry, and computational image processing to study structural and functional changes in the heart with MI. The authors discovered that a considerable proportion of neurons in both normal and infarcted animals were responsive when chemicals such as capsaicin, bradykinin, and veratridine were applied.20 In normal animals, these chemicals induced responses in 13% of neurons, whereas aortic occlusion, electrical muscle stimulation (EMS), and inferior vena cava (IVC) occlusion significantly altered the firing rate in 34%, 23%, and 23% of neurons, respectively. In normal animals, ventricular pacing showed the maximum response (52%).20 In chronic infarct animals, most neurons (60%) were active when chemicals were applied to the epicardium, followed by ventricular pacing (43%) and aortic occlusion (37%). IVC occlusion activated 33% of cardiac-related neurons, and EMS affected the activity of 26% of neurons.20 When comparing the proportion of neurons responsive to specific interventions between infarcted and normal animals, the only significant difference was in response to noxious chemicals (normal 13% vs. MI 60%; P<0.0001).20
The study concluded that the increased prevalence of chemosensitive neurons in MI animals suggests a potential role for these nervous systems in the pathophysiology of MI.20 These findings provide insights into the complex interactions between the heart and the nervous system.
In recent years, the mechanisms by which higher brain functions, such as emotions, control the brain-cardiovascular system interaction have been elucidated using recently developed technologies. Hsueh et al investigated the impact of heart rate on anxiety and fear responses.21 That study explored the idea that there is a dominant flow of information from the body to the brain, which may influence emotional states. The research team developed a non-invasive optogenetic pacemaker that accurately and specifically controls the heart rhythm of freely behaving mice based on cell type. This pacemaker was realized by a wearable micro-LED harness and systemic viral delivery of a powerful pump-like channelrhodopsin. The research team discovered that optically induced tachycardia (fast heart rate) strongly enhances anxious behavior, but only under threatening situations, suggesting that both central (brain) and peripheral (body) processes may be involved in the expression of emotional states.21
To identify potential mechanisms underlying these results, the researchers used whole-brain activity screening and electrophysiological methods to identify brain regions activated by load heartbeats. It was found that the posterior insular cortex could mediate the bottom-up interoceptive processing of the heart and that when this brain region was optogenetically inhibited, anxiety-like behavior induced by optical heart pacing was attenuated.21 This research concluded that to understand the origins of emotions and emotional states, it is necessary to consider both the body and the brain together. These results provide a generalizable approach to non-invasive and temporally accurate biological interactions between target cells during behavior.
In the field of human research, Mezue et al discussed the impact of mild/moderate alcohol consumption on cardiovascular risk and its relationship with stress-related neural network activity.22 In that study, a U-shaped association was found between alcohol consumption and major adverse cardiovascular events (MACE). After considering potential confounding factors, such as demographic and socioeconomic factors and health behavior, mild/moderate alcohol consumption was associated with a reduction in MACE risk compared with non-consumption/minimal consumption.22
That study also revealed that mild/moderate alcohol consumption is associated with a reduction in stress-related neural network activity.22 This was evaluated by the relative uptake of PET-FDG in the amygdala and cortex and was associated with a reduction in cardiovascular risk.22
Understanding the impact of alcohol on stress-related neural network activity may help in the development of new treatments that achieve similar cardiovascular risk reductions without the side effects.
This paper reviews recent cardiovascular research into the connection between the cardiovascular system and brain nervous system. With the development of analytical tools for the central and peripheral nervous systems and the development of analytical techniques at the single-cell level, advanced research to understand the essence of interorgan networks is rapidly being undertaken. It is not easy for cardiovascular researchers to use technology in this field at this time, but through research collaboration in different fields, their capabilities are sure to develop in the future.
The author expresses his profound appreciation to all the researchers in his team for their indispensable role in this work.
No research funding was provided for this review.
None.
Not applicable.