論文ID: CJ-23-0581
論文ID: CJ-23-0581
The gold standard graft for coronary artery bypass grafting (CABG) is the internal thoracic artery (ITA), and the second recommendation is the radial artery. However, complete revascularization with arterial grafts alone is often difficult, and the saphenous vein (SV) is the most commonly used autologous graft for CABG, because it is easier to use without restriction for the length of the graft. On the other hand, the patency of SV grafts (SVGs) is poor compared with that of arterial grafts. The SVG is conventionally harvested as a distended conduit with surrounding tissue removed, a procedure that may cause vascular damage. A no-touch technique of SVG harvesting has been reported to result in improved long-term patency in CABG comparable to that when using the ITA for grafting. Possible reasons for the excellent long-term patency of no-touch SVGs are the physical support provided by preserved surrounding perivascular adipose tissue, preservation of the vascular wall structure including the vasa vasorum, and production of adipocyte-derived factors. In this review, we discuss recent strategies aimed at improving the performance of SVGs, including no-touch harvesting, minimally invasive harvesting and mechanical support using external stents.
Coronary artery disease accounts for >7 million deaths per year worldwide with >800,000 coronary artery bypass grafting (CABG) procedures performed annually.1 A number of different conduits have been used for myocardial revascularization, but the main conduits are the internal thoracic artery (ITA), radial artery (RA), and saphenous vein (SV) (Figure 1). Currently, the SV is the most frequently used autologous graft, and its use eliminates the problems of tissue rejection and the need for tissue typing and matching.2 Other practical advantages of using the SV include the fact that lower limb venous drainage can rely solely on the deep venous system, that the SV is expendable and easily accessible due to its superficial position, and morphological features such as its thick media and adventitia, which facilitate its exposure at harvest.3,4 In addition, its long length of approximately 30 cm enables multiple grafts when harvesting for CABG.5 Although the ITA is the accepted ‘gold standard’ graft with superior patency, there is some disagreement about whether the SV or RA is the second graft of choice in patients undergoing CABG.6

Conduits used for coronary artery bypass grafting. Explants of pedicled and skeletonized grafts including the internal thoracic artery (ITA), radial artery (RA) and saphenous vein graft (SVG) at harvesting. We gratefully acknowledge Dr. Domingos Souza, Orebro University Hospital, for providing the photographs of bypass conduits.
PVAT is present at the outermost boundary of the vascular wall surrounding most blood vessels, termed either the vessel’s “6th layer”7 or the tunica adiposa.8 PVAT is comprised of discrete adipocytes with a network of capillaries and nerve fibers as well as macrophages, adipocyte stem/progenitor cells, lymphocytes and fibroblasts.9 It is no longer thought of as merely supporting but is now recognized as being a source of factors that potentially influence vascular tone and structure.10–12 Both relaxant and contractile factors may play a role in pathological conditions as well as having a beneficial role in situations such as improving graft patency in patients undergoing CABG.13,14
Depending on the vessel, PVAT displays phenotypic and functional heterogeneity, exhibiting several features associated with either brown adipose tissue with a high mitochondrial content or white adipose tissue with a low mitochondrial content.7 Considering its close proximity to the adventitia, the outermost layer of the vascular wall, PVAT is ideally situated to interact directly with the vasculature or in a bidirectional manner via the vasa vasorum.15–17 Regional differences, ranging from PVAT surrounding coronary and peripheral arteries to PVAT surrounding the SV and other conduits used for myocardial revascularization, may have an effect on vascular homeostasis.18–21 Furthermore, the vascular effects of PVAT are produced by adipocyte-derived relaxing factors (ADRFs), as first described by Lohn et al,22 and adipocyte-derived constricting factors.23
The SV has been the most commonly used conduit for myocardial revascularization2 since its introduction by Favaloro more than 50 years ago.24 In the methods section of the original publication, Favaloro stated that “Care must be taken to dissect only the vein, avoiding as much as possible the adventitia that surrounds it.”24 and this procedure has been adopted by cardiac surgeons as the conventional technique using the SV for CABG. In harvesting the SV by this conventional approach (Figure 2A,B; Left), the PVAT is removed, with inevitable damage to the adventitia. In addition, the vein is directly handled by surgical instruments, causing spasm that is overcome using high-pressure intraluminal distension.25 The combination of surgical trauma and distension results in damage to all layers of the graft from the innermost luminal endothelium to the outermost adventitia,26 affecting all aspects of graft performance ranging from early thrombus formation following endothelial damage at harvesting to proliferation and migration of vascular smooth muscle cells and formation of neointima and atheroma, leading to graft occlusion.27
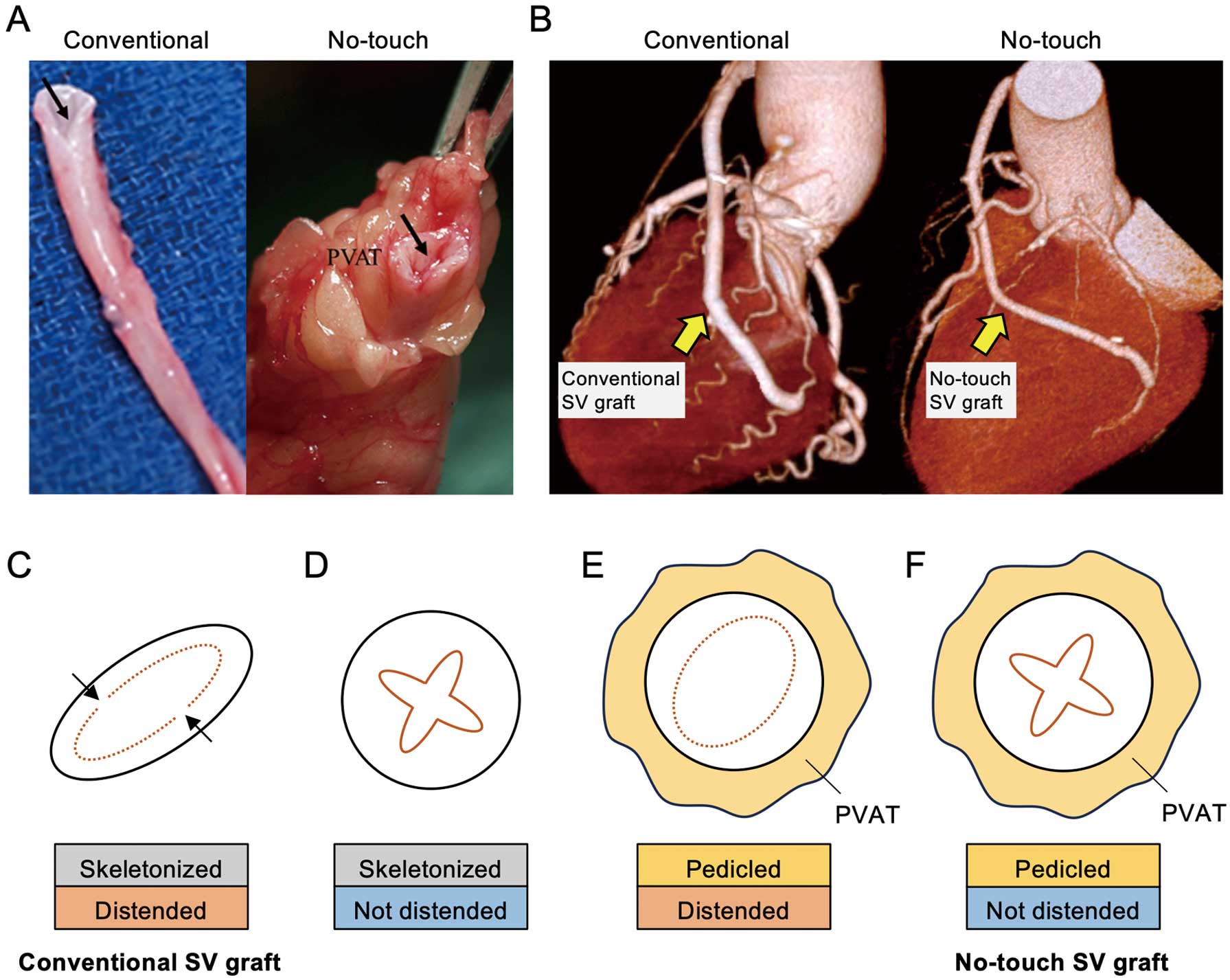
Perivascular adipose tissue as a saphenous vein graft protector. (A) Conventional (skeletonized) and no-touch (pedicled) saphenous vein (SV) grafts. The no-touch SV is surrounded by its cushion of perivascular adipose tissue (PVAT). The arrows show the lumen of the veins. (B) Computed tomography angiography of conventional and no-touch SV grafts in coronary artery bypass grafting. (C,D) Line drawings of a skeletonized SV graft with distension (C, conventional SV graft) and without distension (D). The arrows show endothelial denudation and exposure of the intima. (E,F) Line drawings of a pedicled SV graft with distension (E) and without distension (F, no-touch SV graft).
It has been reported that SV graft (SVG) patency is significantly improved when using a no-touch harvesting technique with the PVAT intact (Figure 2A,B; Right); the patency of such grafts (83%) is superior to that of conventionally harvested SVGs (64%) for up to 16 years.28 Although the results of that study were obtained from a single center, the superiority of no-touch SVGs for a 1-year follow-up period has been shown in more recent trials.29,30 The patency rates for the 1-year period in no-touch grafts/conventional SVGs were 97.4%/92.4% in Korea,29 and 96.3%/93.5% in China.30 Various other clinical aspects of no-touch SVGs, ranging from off-pump CABG31,32 to percutaneous coronary intervention have been reported,33,34 but there is limited information regarding the importance of maintaining normal vessel architecture for improved patency of no-touch SVGs compared with conventional SVGs. Based on studies of the improved performance of no-touch SVGs, the question about the role of PVAT arises.
The cushion of fat surrounding the SV used in CABG offers various forms of protection to the graft at both harvesting and post-implantation, and this protection becomes evident when the no-touch technique is used. By preserving the PVAT, direct handling of the SV by surgical instruments is avoided, spasm does not occur, high-pressure intraluminal distension is not required, and endothelial damage is minimized.25,26 Damage to the endothelium was previously investigated using conventionally harvested and skeletonized SVGs with distention (Figure 2C, conventional SVG) and without distention (Figure 2D) and using no-touch SVGs with distention (Figure 2E) and without distention (Figure 2F, no-touch SVG).14,25,35 Using immunostaining of CD31, a marker of endothelial cells, endothelial denudation and exposure of the intima were observed in conventionally harvested SVGs with distention (Figure 2C), whereas the endothelium of no-touch SVGs was intact (Figure 2F).35 An important finding was the minimal endothelial damage in skeletonized SVGs that had not been distended (Figure 2D). Furthermore, the endothelium was virtually intact in no-touch SVGs that had been distended at 300 mmHg for 1 min (Figure 2E). These findings suggest that PVAT provides mechanical support that protects the luminal endothelium of no-touch SVGs against the higher arterial pressure of ~100 mmHg than the normal venous pressure of ~10 mmHg that the graft is exposed to when it is subjected to coronary artery hemodynamics.26 Furthermore, it has been shown that the pressure within the SV is ~5–10 mmHg in the supine posture but rises to ~75 mmHg on standing, and the SV may thus be preconditioned to withstand such pressure changes once grafted.4 Although this is the case for the SV in vivo, where PVAT is intact, vascular damage that occurs on removal of the PVAT surrounding the SV used as a conventional graft stimulates several mechanisms involved in the process of graft occlusion.27 In addition, the cushion of PVAT surrounding the no-touch SV provides mechanical support that prevents longer grafts from kinking, as shown by both angiography and computed tomography angiography (Figure 2B).25,35
Vasa Vasorum Within PVAT Surrounding the SVBy preserving the PVAT and minimizing direct handling of the SV, the adventitia is not damaged, and the vasa vasorum remain intact. Experimental occlusion of the vasa vasorum has been shown to cause neointimal hyperplasia and atheroma formation due to a reduction in transmural blood flow,36 which is reversed on the appearance of neoadventitia.37 The vasa vasorum of veins are more pronounced and reach deeper into the media of veins than of arteries due to the differences between veins and arteries in the supply of oxygen and nutrients.38 It has also been shown that the density of the vasa vasorum of the SV is much higher than that in arteries and that the density of no-touch SVGs is higher than that of conventional SVGs.15,16,39,40
There is supporting evidence for inside-out/outside-in pathways from studies using a combination of scanning electron microscopy and immunohistochemistry.41,42 Scanning electron micrographs have revealed what appear to be terminations within the luminal endothelium.41 In addition, endothelial cells of the capillary network within PVAT surrounding the SV have been identified, and it has been shown that the surrounding adipocytes contain various vasodilator factors, including nitric oxide, leptin and adiponectin.13,35 An inside-out/outside-in transport system may exist to connect the innermost vessel surface of the lumen via the vasa vasorum of the media and adventitia to the capillary network within the PVAT.13,16,42 Given the close proximity of PVAT to the adventitia, no-touch SVGs may be affected by ADRFs that are beneficial for graft patency. It is also possible that ADRFs are transmurally transported via the vasa vasorum to the vein lumen.13,15,16
Vasoactive Action of PVAT-Derived FactorsThere has been an increased interest in PVAT-derived factors and their effects on a variety of blood vessels both in vitro and in vivo.43–45 It has been reported that the PVAT surrounding the ITA releases a relaxant factor that acts through activation of calcium-dependent potassium channels.46 In addition, the PVAT surrounding the ITA was shown to be a source of nitric oxide and prostacyclin-independent anticontractile factors, which affect the vascular tone of the ITA.47 It has also been shown that the PVAT surrounding the ITA exerts its anticontractile effect independently from that of prostanoids.48 Another study showed that the PVAT surrounding the RA exhibited anticontractile/vasorelaxant properties that are inherently associated with secretion of ADRFs and endothelial-independent activity of ADRFs.49
An interaction between PVAT and the vascular wall in the regulation of endothelial nitric oxide synthase via adiponectin in the SV and ITA from patients who underwent CABG has been reported.50 A previous study using SVGs derived by no-touch and conventional harvesting showed that endothelial cells in the no-touch SVGs were predominantly involved in the greater production of nitric oxide than those derived from conventional SVGs.51 Because nitric oxide plays crucial roles in protecting against the development of atherosclerosis, preserving the PVAT may greatly contribute to the excellent patency of no-touch SVGs. However, it remains unclear whether graft patency depends on how much fat pad remains over the SVG.
We recently investigated the role of PVAT surrounding the SV, ITA and coronary artery in CABG.18–20 PVAT surrounding the SV and ITA had less metabolic dysfunction-driven chronic inflammation or “metaflammation” and consequent adipose tissue remodeling including fibrosis than did PVAT surrounding the coronary artery (Figure 3). This phenotypic difference may contribute to the improved long-term patency of SVGs when the no-touch technique of harvesting is used. Furthermore, PVAT surrounding the SV had healthy expansion with less fibrosis in the fat than in subcutaneous fat, and the properties of the PVAT surrounding the SV were similar to, but not the same as, those of subcutaneous fat, possibly resulting from inherent differences in adipocytes.21 Taken together, preserving the PVAT surrounding grafts, including SV, ITA and RA grafts, may improve graft patency through several mechanisms.

Chronic inflammation and fibrosis in perivascular adipose tissue. Representative hematoxylin-eosin (HE) staining, Masson trichrome (MT) staining, and immunohistological staining, including CD68 (a marker of macrophages), and CD11c (a marker of M1 macrophages) of perivascular adipose tissue (PVAT) surrounding the coronary artery (CA-PVAT), the saphenous vein (SV-PVAT), and the internal thoracic artery (ITA-PVAT). Bar=100 μm.
When using the conventional technique, the SV is harvested via a large open incision and its excision causes both vascular damage and wound healing complications (Figure 4A). As a consequence, both vein graft patency and the surgical site are compromised. Although graft patency is dramatically improved when the SV is atraumatically harvested with the surrounding cushion of PVAT intact, wound healing and infection remain a problem.52 Wound healing can be reduced by using endoscopic vein harvesting (EVH), a technique that was introduced almost 30 years ago53 and was used in ≈80% of all CABG procedures in the USA in 2005.54 However, this form of harvesting may have a negative effect on graft performance because of vascular damage associated with forces to the vein that are usually avoided in open vein harvesting (OVH), including traction, adventitial stripping and venous compression.55 To date, there have been only a few short- and mid-term follow-up trials focused on the effects of EVH and OVH of SVGs on graft patency, and the patency of EVH grafts was at best only comparable to that of OVH grafts.56

Open and endoscopic vein harvesting of saphenous vein grafts. (A) Representative image of open vein harvesting of the saphenous vein (SV). (B) Screen shot from video footage taken during endoscopic vein harvesting showing exposure of the SV, separation of perivascular adipose tissue (PVAT) and vasa vasorum (arrow). (C) Screen shot from video footage taken during no-touch SV graft using endoscopic vein harvesting with the PVAT surrounding the SV remaining intact.
As in cases of OVH, PVAT is also removed when using EVH (Figure 4B). Other structural changes in endoscopically harvested SVGs, including damage to the adventitia, intima and endothelium, have also been reported.57–59 EVH is associated with reduced scarring and postoperative pain, reduced inflammation and infection, and greater patient mobility as well as reduced wound complications when performed by experienced surgeons. Although compromised wound healing is reduced by using EVH, damage to the SVG is similar or more pronounced than that when harvesting by the conventional/open technique. Given the improved patency of no-touch SVGs, there have been recent attempts to develop minimally invasive methods of harvesting with the PVAT intact.
A method similar to no-touch SV harvesting using an electrothermal bipolar vessel sealing device via small incisions was used in a recent study, and the SVG was harvested with intact PVAT and preservation of the normal vein architecture as confirmed by histological examination.60 Video footage of an endoscopic no-touch technique using a reusable retractor and a closed tunnel system and an electrothermal bipolar vessel sealing device was obtained in a subsequent study by that group.61
Another recent study using a novel technique of EVH with preserved PVAT showed that the SV can be harvested complete with the PVAT intact via a small incision made in the medial thigh just above the knee using an EVH system (Figure 4C).62 The SV was shown to have its normal architecture, and there was neither surgical site infection nor requirement for antibiotic treatment.62
A small randomized trial using an SVG with a surrounding macroporous Dacron sheath reinforced with polytetrafluoroethylene ribs (EXTENT trial) (Figure 5) to either the right or left coronary system was performed in patients undergoing CABG (n=20).63 At 6 and 19 months after CABG, the EXTENT-supported grafts were found to be thrombosed on angiographic assessment, whereas all of the left ITA grafts and non-stented ‘conventional’ SVGs remained patent, suggesting that a combination of factors associated with the EXTENT ‘tube’ design caused kinking and graft failure. Because problems associated with loose-fitting Dacron stents had been recognized in a pig model,27 it is surprising that such grafts were used on patients undergoing CABG and underscores the ‘translational’, bench to bedside, limitations of the experimental pig model.26

External support of saphenous vein grafts. (Left to Right) Representative images and hematoxylin-eosin staining of saphenous vein (SV) grafts: no-touch SV graft with perivascular adipose tissue (PVAT) intact, conventional SV graft with PVAT removed, loose-fitting Dacron EXTENT fitment surrounding a conventional SV graft, and a metal external and close-fitting VEST fitment surrounding a conventional SV graft. A, adventitia; L, lumen; M, media. Red area indicates ‘exudate’.
After confirming the results of experiments using an external support made from braided cobalt-chromium-nickel-molybdenum-iron alloy fibers, forming an expandable external SV support, in a sheep model (n=20),64 the first randomized clinical study, the Venous External Support Trial (VEST), was carried out to investigate whether external stenting of the SVG inhibited intimal hyperplasia in 30 patients undergoing CABG.65 In addition to an ITA graft, patients received a VEST SVG (Figure 5), to either the right or left coronary territory, and ≥1 non-stented SVG served as a control. At 1 year follow-up, all ITA grafts were patent, and SVG failure did not significantly differ between the VEST and non-stented grafts. The mean intimal hyperplasia area was significantly reduced in the VEST grafts compared with the non-stented grafts, and the VEST grafts showed an improvement in lumen uniformity.
A more recent and longer term (4.5 years) study, the VEST-IV study using coronary angiography and intravascular ultrasound, was performed in 21 patients.66 Vein graft failure rates were comparable in the VEST and non-stented groups. In the patent grafts, the Fitzgibbon perfect patency rate remained significantly higher in the VEST grafts than in the non-stented grafts, and intimal hyperplasia and thickness were significantly reduced in the VEST grafts. Intimal hyperplasia correlated with lumen uniformity and with the distance between the VEST graft and the lumen. It was concluded that VEST fitment may mitigate SVG remodeling and reduce intimal hyperplasia and the development of lumen irregularities after CABG.66
The effect of VEST fitment to SVGs harvested by OVH and EVH techniques was also assessed by computed tomography angiology at 6 months and coronary angiology at 2 years in a post hoc analysis of the VEST-III study.67 OVH was associated with improved overall patency of SVGs and reduced intimal hyperplasia and thickness. The authors concluded that external stenting reduced the progression of graft disease, particularly with OVH. However, the results of the REGROUP trial in a short follow-up (median follow-up of 2.7 years)68 and intermediate follow-up (median follow-up of 4.7 years)69 showed no difference between the OVH and EVH techniques in major cardiac adverse outcomes, though graft patency was not examined. In the VEST-III study, although patency was decreased in the VEST group compared with the non-stented group, the difference was not statistically significant.67 Therefore, it remains unclear if the effects of harvesting by OVH or EVH are ultimately linked to improved SVG patency.70
Flow Pattern of Externally Stented SVGsVein graft thickening was markedly asymmetric and produced alterations of blood flow through the graft that were associated with turbulent blood flow, leading to thrombosis and hyperplasia.27 The external stent or sheath imposes a cylindrical symmetry on the vein graft and keeps the graft contained in a way that prevents turbulent blood flow, inducing laminar and symmetric blood flow, leading to a reduction in hyperplasia due to asymmetric blood flow.27
Diffuse flow patterns were assessed in a previous study using various hemodynamic parameters, including time-averaged wall shear stress and oscillatory shear index, in VEST SVGs.71 Although there was no significant difference in time-averaged wall shear stress values, the oscillatory shear index was significantly lower in the VEST grafts than in the non-stented grafts.71
Although excellent ITA graft patency is well established, the SVG is still an important and the most widely used conduit for CABG. Conventional SV harvesting with removal of PVAT results in reduced patency due to damage to all layers of the vessel. No-touch SV harvesting minimizes vascular damage, and the PVAT surrounding the SV also contributes to the improvement of graft patency due to preservation of the vasa vasorum and secretion of ADRFs. Externally stented SVGs show reduced kinking and reduced oscillatory shear stress, resulting in a more homogenous and less eccentric graft lumen and reduced thrombus formation. It has been proposed that the use of external stents represents a strategy to ‘rectify’ the adverse effects of removing the PVAT when using conventional harvesting techniques. When the fat is left intact, PVAT surrounding no-touch SVGs inhibits the adverse effects of altered hemodynamics on the graft and obviates the need for synthetic external support. Recently, novel techniques of minimally invasive no-touch SV harvesting have been reported, and they may overcome the problem of wound complications and become a new strategy for CABG.