論文ID: CJ-23-0593
論文ID: CJ-23-0593
Background: Transcatheter aortic valve implantation (TAVI) is an established treatment for severe aortic stenosis (AS), but despite estimates of life expectancy after TAVI being essential in heart team discussion, these data are scarce. Therefore, the current study sought to assess long-term survival and its trends in relation to chronological age, surgical risk, and treatment period.
Methods and Results: We included 2,414 consecutive patients who underwent TAVI for severe symptomatic AS between 2008 and 2021 at 2 international centers. For the analysis, long-term survival was evaluated according to age, surgical risk, and treatment period categorized into 3 groups, respectively. The longest follow-up was 13.5 years. Overall survival was 67.6% at 5 years and 26.9% at 10 years. Younger patients, lower surgical risk, and later treatment period showed better survival (log-rank P<0.001, respectively). In the multivariate analysis, age <75years, lower surgical risk, and later time period were significantly associated with better survival. The incidence of paravalvular leakage ≥moderate, red blood cell transfusion, and acute kidney injury were independently associated with increasing risk of 5-year death.
Conclusions: In a real-world registry, survival was substantial following TAVI, especially in younger and lower surgical-risk patients, with improving outcomes over time. This should be considered in heart team discussions of life-long management for AS patients after TAVI.
Transcatheter aortic valve implantation (TAVI) is now an established treatment for patients with severe aortic stenosis (AS) across the entire spectrum of operative risks.1–4 Consequently, contemporary guidelines have formulated strong recommendations for TAVI in patients with symptomatic severe AS aged ≥75 years in Europe and Japan or ≥65 years in the USA.5–7 In this era of well-established TAVI, life-long management for AS patients after TAVI should be discussed by the heart team. Reliable and contemporary survival estimates are important for any long-term condition at the population level to keep track of prognosis trends and to commission appropriate medical services. At the individual level, these estimates assist patients to participate in informed discussion and shared decision-making about treatment options and advanced care planning.8 Previously, 5-year survival data after TAVI in comparison with after surgical aortic valve replacement (SAVR) with respect to each surgical-risk category have been reported in the clinical trial settings.9–11 However, longer-term survival over 10 years in real-world data remains unknown. For better life-long management of AS patients who undergo TAVI, knowledge of extended survival estimates and their trends in an all-comers TAVI population is now required.
We therefore explored the long-term survival in an all-comers TAVI population and the trends in outcomes in relation to treatment period. Moreover, we aimed to investigate how age and surgical risk categories at indexed TAVI affected survival rates.
The international TAVI registry (UMIN-CTR, Identifier: UMIN000040413) includes data from consecutive and unselected patients who have undergone TAVI for severe AS or degenerated surgical aortic valves at 2 centers: Shonan Kamakura General Hospital in Japan and Helsinki University Central Hospital in Finland. In the ongoing registry, 2,414 consecutive patients who underwent TAVI between December 2008 and December 2021 were evaluated for the current study (data from Kamakura n=527: October 2013 to March 2020; data from Helsinki n=1,887: December 2008 to December 2021). All patients were evaluated as eligible for TAVI by a multidisciplinary heart team. The selection of the transcatheter heart valve (THV) and its sizing were based on labeled indications, and the decision was left to the operators. TAVI was performed using a self-expanding valve (CoreValve, Evolut R, and Evolut Pro/Pro+: Medtronic Inc., Minneapolis, MN, USA; Acurate Neo/Neo2: Boston Scientific, Marlborough, MA, USA; Allegra; Biosensors, Singapore and New Valve Technology, Hechingen, Germany; Portico: Abbott Vascular, Santa Clara, CA, USA), balloon-expandable valve (Sapien, Sapien XT, Sapien 3, and Sapien 3 Ultra; Edwards Lifesciences, Irvine, CA, USA), or mechanical-expanding valve (Lotus/Lotus Edge; Boston Scientific). The operative risk of the patients was evaluated according to the Society of Thoracic Surgeons (STS) score.12,13 Data were both retrospectively and prospectively collected by the cardiologists, cardiac surgeons and trained research nurses using a dedicated case report form. Data were checked for completeness and quality. In Finland, data on the date and death were obtained from the National Registry Statistics Finland, which is based on death certificates reviewed by local and central authorities. In Japan, all data were retrospectively collected by reviewing medical records and by telephone contact. The study protocol conformed to the principles of the Declaration of Helsinki and was approved by the institutional clinical research and ethics committee of each participating center.
Definitions and OutcomesChronic kidney disease (CKD) was defined as an estimated glomerular filtration rate <60 mL/min/1.73 m2 according to the Modification of Diet in Renal Disease equation.14 Clinical outcomes were registered based on the VARC-2 criteria.15 Acute kidney injury (AKI) was defined in this registry according to the KDIGO classification criteria.16 Pre- and post-TAVI transthoracic echocardiography was performed by echocardiographers who were independent from the TAVI operators at each participating center. Device success was defined as correct implantation of a single THV, with intended performance of the valve (peak aortic flow velocity <3 m/s, mean pressure gradient <20 mmHg, and no moderate or severe paravalvular leakage [PVL]), and no intraprocedural death.15 For the purpose of this study, patients were divided into 3 time periods based on the procedure year (1st: 2008–2014, n=265; 2nd: 2015–2018, n=967; 3rd: 2019–2021, n=1,182). Patients’ age at the time of the procedure was also divided into 3 categories (<75 years, n=449; 75–≤85 years, n=1,332; >85 years, n=633).
The primary outcome of the current study was to elucidate long-term survival up to 10 years after TAVI. The secondary outcomes were to explore trends in survival after TAVI according to age, surgical risk, and time period, and to evaluate the factors that were independently associated with 5-year survival.
Statistical AnalysisContinuous variables are presented as mean±standard deviation. The Shapiro-Wilk test was used to assess data normality. Categorical variables are presented as numbers and percentages, and Fisher’s exact test was used to compare the data between groups. For continuous variables, differences between groups were assessed using the Mann-Whitney U test. Kaplan-Meier analysis was performed using the log-rank test to compare the endpoints between the groups. Covariates exhibiting a P value <0.10 in the univariate analysis and showing a variance inflation factor <5.0 were inserted into the logistic regression analysis (Model 1: baseline and procedural characteristics; and Model 2: baseline/procedural characteristics and early outcomes) to determine the predictive factors of survival at 5 years. A P value <0.05 was considered statistically significant. All statistical tests were 2-tailed and performed using JMP version 16.0 (SAS Institute Inc., Cary, NC, USA).
The study included all patients (n=2,414) who underwent TAVI at 2 centers in Japan and Finland, and the longest follow-up was 13.5 years. The number of TAVI procedures increased every 2 years until the coronavirus pandemic (Figure 1A). According to the STS scores, 7.4% of patients were high surgical risk, 37.0% were intermediate, and 55.6% were low. The proportion of patients with low surgical risk (45.3% vs. 50.0% vs. 62.7%, P<0.001) and those aged <75 years (9.1% vs. 17.0% vs. 22.1%, P<0.001) significantly increased over the 3 time periods (2008–2014, 2015–2018, and 2019–2021) (Figure 1B,C). Of the patients aged <75 years, 79.3% were low risk (Figure 1D). Table 1 shows the patients’ characteristics according to STS score categories. In the overall cohort, mean age was 81.0±6.4 years, STS score was 4.5±1.7%, and 54.8% were female. Among the STS categories, baseline variables were significantly different except for hypertension, aortic valve peak velocity, mean pressure gradient, preprocedural electrocardiographic findings, and the use of oral anticoagulants. In procedural characteristics, significant differences were observed in the use of transfemoral approach and the size of THV between STS categories. There were not significant differences in THV type (Table 2). In Supplementary Tables 1 and 2, significant differences in baseline and procedural characteristics between Japan and Finland were observed.
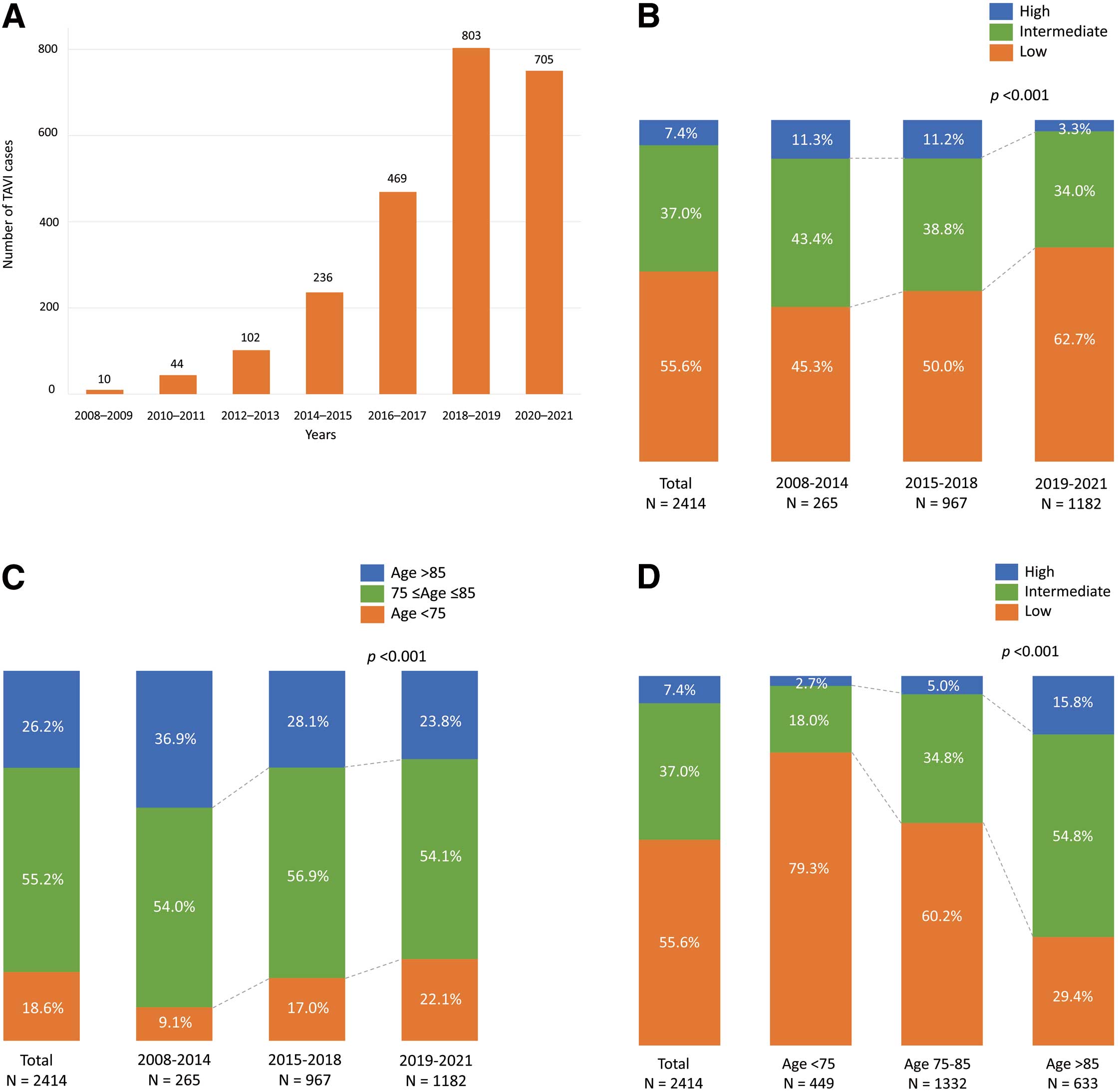
Proportions of TAVI patients. (A) Changes in number of TAVI cases at the 2 centers. (B) Transitions in the proportions of surgical risk over the 3 time periods. (C) Transitions in the proportions of age groups over the 3 time periods. (D) Surgical risk according to age categories. TAVI, transcatheter aortic valve implantation.
Baseline Clinical Characteristics According to Surgical Risk
| Variable | Overall (n=2,414) |
High (n=178) |
Intermediate (n=892) |
Low (n=1,344) |
P value |
|---|---|---|---|---|---|
| Age, years | 81.0±6.4 | 85.2±5.7 | 83.4±5.9 | 78.8±6.8 | <0.001 |
| Female, n | 1,322 (54.8) | 123 (69.1) | 573 (64.2) | 626 (46.6) | <0.001 |
| Height, cm | 164.6±10.4 | 158.5±11.7 | 160.9±10.8 | 167.9±9.9 | <0.001 |
| Weight, kg | 70.5±17.0 | 58.8±18.1 | 64.1±16.9 | 76.2±16.9 | <0.001 |
| Body mass index, kg/m2 | 25.8±5.1 | 23.0±4.9 | 24.5±4.9 | 26.9±5.2 | <0.001 |
| Body surface area, m2 | 1.77±0.24 | 1.59±0.28 | 1.67±0.25 | 1.85±0.23 | <0.001 |
| Region | <0.001 | ||||
| Japan, n | 527 (21.8) | 88 (16.7) | 291 (55.2) | 148 (28.1) | |
| Finland, n | 1,887 (78.2) | 90 (4.8) | 601 (31.9) | 1,196 (63.4) | |
| Hypertension, n | 2,137 (88.5) | 156 (87.6) | 806 (90.4) | 1,175 (87.4) | 0.10 |
| Diabetes mellitus, n | 676 (28.0) | 67 (37.6) | 235 (26.4) | 374 (27.8) | 0.009 |
| Chronic kidney disease, n | 1,039 (43.0) | 133 (74.7) | 468 (52.5) | 438 (32.6) | <0.001 |
| Hemoglobin, g/L | 124.4±15.6 | 113.2±17.2 | 120.1±15.3 | 128.7±15.6 | <0.001 |
| eGFR, mL/min/1.73 m2 (n=2,413) | 62.0±19.7 | 46.2±21.4 | 56.8±19.8 | 67.5±19.4 | <0.001 |
| Atrial fibrillation, n | 878 (36.4) | 75 (42.1) | 352 (39.5) | 451 (33.6) | 0.004 |
| Peripheral artery disease, n | 315 (13.1) | 45 (25.3) | 131 (14.7) | 139 (10.3) | <0.001 |
| Prior pacemaker implantation, n | 220 (9.1) | 21 (11.8) | 102 (11.4) | 97 (7.2) | 0.001 |
| Prior MI, n (n=2,186) | 197 (9.0) | 18 (11.2) | 90 (11.5) | 89 (7.2) | <0.001 |
| Prior CABG, n | 245 (10.2) | 27 (15.2) | 116 (13.0) | 102 (7.6) | <0.001 |
| Prior PCI, n | 555 (23.0) | 46 (25.8) | 235 (26.4) | 274 (20.4) | 0.003 |
| Prior stroke, n | 255 (10.6) | 27 (15.2) | 89 (10.0) | 69 (5.2) | 0.012 |
| NYHA ≥III, n | 1,556 (64.5) | 141 (79.2) | 588 (65.9) | 827 (61.5) | <0.001 |
| STS score, % | 4.5±1.7 | 13.0±5.1 | 5.8±1.3 | 2.6±0.80 | <0.001 |
| LVEF, % | 57.8±11.5 | 54.0±13.0 | 57.6±12.1 | 58.4±10.8 | <0.001 |
| AVA, cm2 (n=2,294) | 0.68±0.19 | 0.60±0.21 | 0.66±0.20 | 0.71±0.18 | <0.001 |
| Peak aortic velocity, m/s | 4.3±0.69 | 4.4±0.88 | 4.3±0.73 | 4.3±0.64 | 0.15 |
| Mean pressure gradient, mmHg | 45.9±15.6 | 47.8±19.5 | 45.2±16.4 | 46.1±14.3 | 0.11 |
| Bicuspid aortic valve, n | 309 (14.6) | 8 (5.3) | 65 (8.3) | 236 (20.0) | <0.001 |
| CRBBB, n | 271 (11.3) | 22 (12.4) | 93 (10.5) | 156 (11.8) | 0.57 |
| CLBBB, n | 175 (7.3) | 6 (3.4) | 71 (8.0) | 98 (7.4) | 0.10 |
| Aspirin, n | 1,136 (47.1) | 96 (53.9) | 443 (50.0) | 597 (44.4) | 0.008 |
| P2Y12 inhibitor, n | 569 (23.6) | 64 (36.0) | 266 (29.8) | 239 (17.8) | <0.001 |
| Oral anticoagulants | 805 (33.4) | 60 (33.7) | 312 (35.0) | 433 (32.2) | 0.40 |
| Warfarin, n | 423 (17.5) | 37 (20.8) | 164 (18.4) | 222 (16.5) | 0.26 |
| DOAC, n | 385 (16.0) | 23 (12.9) | 150 (16.8) | 212 (15.8) | 0.42 |
Values are expressed as n (%) or mean±standard deviation. AVA, aortic valve area; CABG, coronary artery bypass grafting; CLBBB, complete left bundle branch block; CRBBB, complete right bundle branch block; DOAC, direct oral anti-coagulant; eGFR, estimated glomerular filtration rate; LVEF, left ventricular ejection fraction; MI, myocardial infarction; NYHA, New York Heart Association; PCI, percutaneous coronary intervention; STS, Society of Thoracic Surgeons.
Procedural Variables According to Surgical Risk
| Variable | Overall | High | Intermediate | Low | P value |
|---|---|---|---|---|---|
| TF approach | 2,304 (95.4) | 158 (88.8) | 834 (93.5) | 1,312 (97.6) | <0.001 |
| Predilatation | 1,552 (64.5) | 100 (56.2) | 533 (59.9) | 919 (68.6) | <0.001 |
| Postdilatation | 212 (8.9) | 29 (16.3) | 90 (10.2) | 93 (7.0) | <0.001 |
| THV type | 0.14 | ||||
| BEV | 1,346 (55.9) | 108 (61.4) | 506 (56.8) | 732 (54.6) | |
| SEV | 944 (39.2) | 62 (35.1) | 341 (38.3) | 541 (40.02) | |
| MEV | 117 (4.9) | 6 (3.4) | 40 (4.5) | 71 (5.3) | |
| THV size, mm | 26.0±2.8 | 25.3±2.5 | 25.6±2.8 | 26.4±2.8 | <0.001 |
| Second valve implantation | 24 (1.0) | 2 (1.1) | 11 (1.2) | 11 (0.82) | 0.62 |
Values are expressed as n (%) or mean±standard deviation. BEV, balloon-expandable valve; MEV, mechanically expanding valve; SEV, self-expanding valve; TF, transfemoral; THV, transcatheter heart valve.
30-Day Outcomes
The 30-day outcomes after TAVI according to STS categories are shown in Table 3. In the overall cohort, 30-day all-cause and cardiovascular mortality rates were 2.1% and 1.7%, respectively. For the 30-day mortality rate, there was a significant difference among the STS catego-ries (6.2% in high risk, 2.5% in intermediate, and 1.3% in low; P<0.001). The significant difference in STS categories was also detected for the 30-day cardiovascular mortality rate (5.6% in high risk, 2.0% in intermediate, and 1.0% in low; P<0.001). The incidence of PVL ≥moderate, vascular complications, stroke, and new-onset pacemaker implantation were statistically similar among the risk categories. On the other hand, significant differences were observed in the incidence of bleeding complications, red blood cell (RBC) transfusion, and AKI. In the Japanese cohort, the incidences of PVL ≥moderate, vascular complications, and major bleeding were lower than those in the Finnish cohort; however, the incidence of RBC transfusion was more frequent, and length of hospital stay was longer in the Japanese cohort (Supplementary Table 3). According to time period, although the incidence of procedural complications significantly decreased, significant differences were not observed in the incidence of all-cause death and PVL ≥moderate (Supplementary Table 4). The 30-day outcomes according to STS score by time period are shown in Supplementary Table 5.
30-Day Clinical Outcomes According to Surgical Risk
| Variable | Overall (n=2,414) |
High (n=178) |
Intermediate (n=892) |
Low (n=1,344) |
P value |
|---|---|---|---|---|---|
| All-cause death, n | 50 (2.1) | 11 (6.2) | 22 (2.5) | 17 (1.3) | <0.001 |
| Cardiovascular death, n | 41 (1.7) | 10 (5.6) | 18 (2.0) | 13 (1.0) | <0.001 |
| Mean pressure gradient, mmHg | 10.6±5.4 | 9.9±5.6 | 10.1±5.4 | 11.0±5.3 | <0.001 |
| Peak aortic velocity, m/s | 2.1±0.50 | 2.0±0.52 | 2.1±0.51 | 2.2±0.49 | 0.009 |
| PVL ≥moderate (n=2,366) | 65 (2.8) | 4 (2.3) | 29 (3.3) | 32 (2.4) | 0.43 |
| Vascular complications, n | |||||
| Major | 166 (6.9) | 14 (7.9) | 72 (8.1) | 80 (6.0) | 0.13 |
| Minor | 126 (5.2) | 7 (3.9) | 53 (6.0) | 66 (4.9) | 0.40 |
| Bleeding complications, n | |||||
| Life-threatening or disabling | 81 (3.4) | 6 (3.4) | 42 (4.7) | 33 (2.5) | 0.015 |
| Major | 213 (8.9) | 24 (13.5) | 72 (8.1) | 117 (8.7) | 0.068 |
| Minor | 176 (7.3) | 20 (11.2) | 85 (9.6) | 71 (5.3) | <0.001 |
| RBC transfusion | 215 (8.9) | 43 (24.2) | 91 (10.3) | 81 (6.0) | <0.001 |
| Stroke/TIA, n | 62 (2.6) | 8 (4.5) | 23 (2.6) | 31 (2.3) | 0.22 |
| AKI, n | 105 (4.4) | 20 (11.2) | 44 (5.0) | 41 (3.1) | <0.001 |
| PMI, n | 175 (7.3) | 14 (7.9) | 65 (7.3) | 96 (7.2) | 0.94 |
| LOS, days | 6.8±11.8 | 16.4±28.0 | 8.0±11.6 | 4.8±7.7 | <0.001 |
| LOS after TAVI, days | 5.0±7.5 | 9.4±9.2 | 6.1±9.7 | 3.7±5.3 | <0.001 |
Values are expressed as n (%) or mean±standard deviation. AKI, acute kidney injury; LOS, length of hospital stay; PMI, pacemaker implantation; PVL, paravalvular leak; RBC, red blood cell; STS, Society of Thoracic Surgeons; TAVI, transcatheter aortic valve implantation.
Long-Term Outcomes
The overall survival in the entire cohort was 67.6% at 5 years, 44.8% at 8 years, and 26.9% at 10 years (Figure 2). Median survival was 6.98 years in this population and survival was significantly affected by age and surgical risk profile; that is, the lower the surgical risk, the better the long-term survival (log-rank P<0.001) (Figure 3A). Age categories were also significantly associated with survival (log-rank P<0.001) (Figure 3B). A comparison of survival curves between patients in Japan and those in Finland is shown in Supplementary Figure 1.
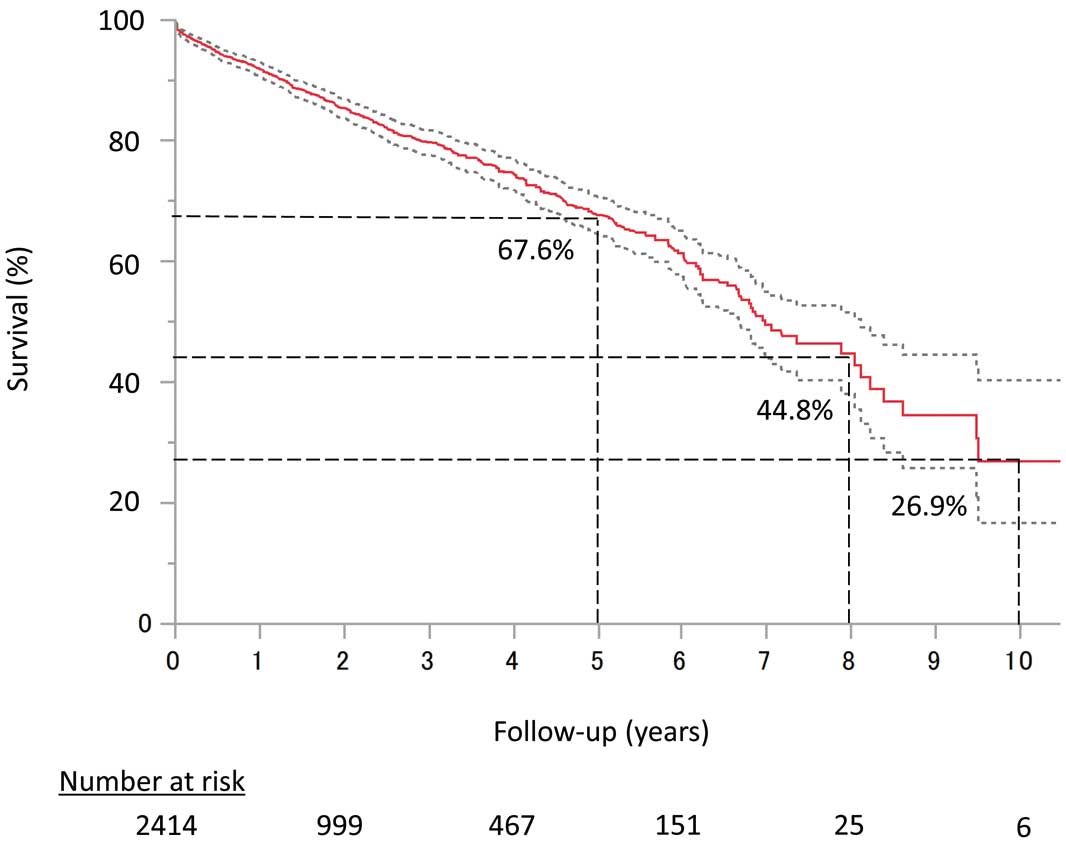
Overall survival up to 10 years by Kaplan-Meier analysis.
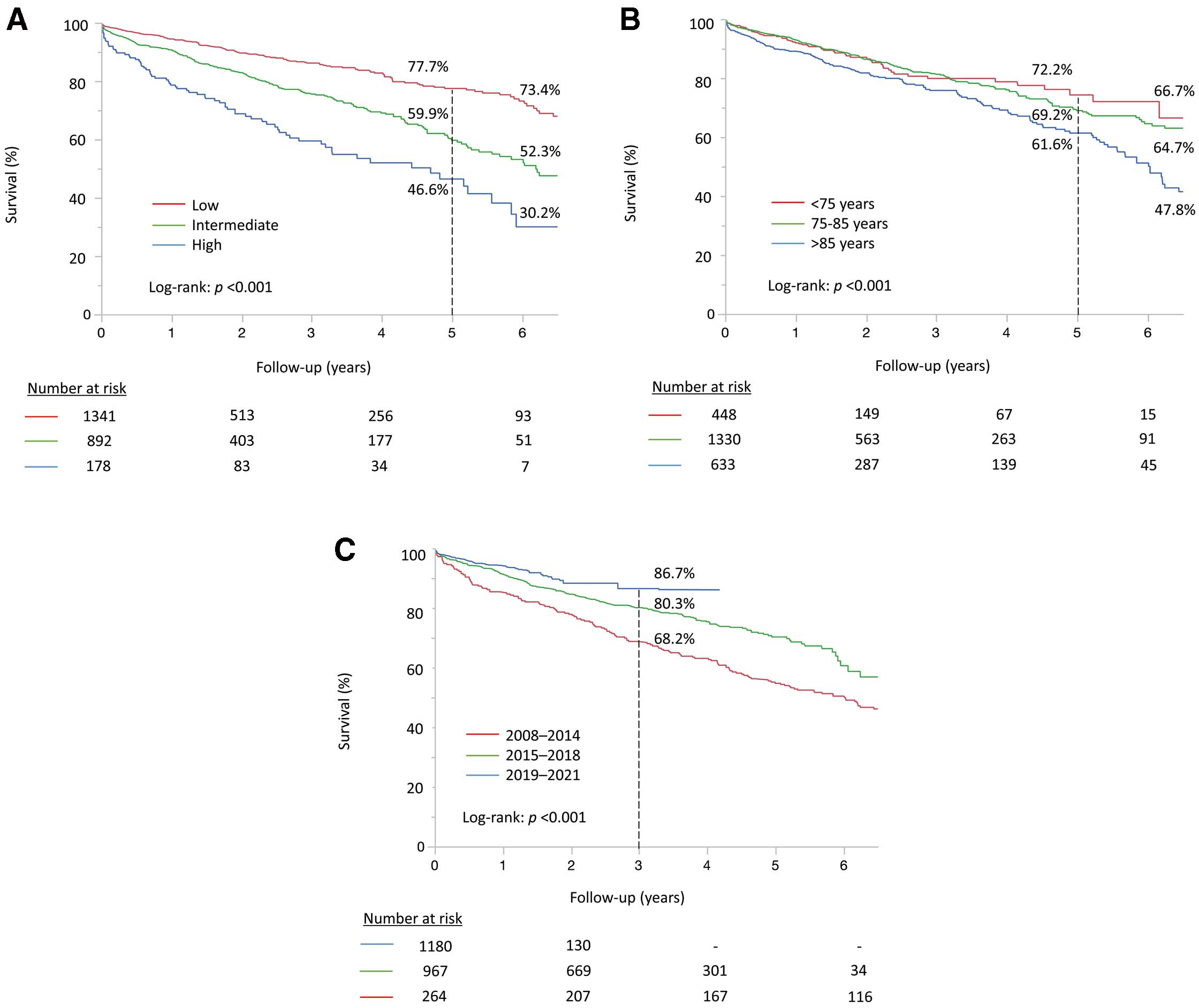
Comparison of survival curves according to (A) risk category, (B) age category and (C) treatment time period.
When comparing trends in survival between the 3 time periods, patients treated in the 3rd period (2019–2021) had a significantly better survival than those in the earlier periods (log-rank P<0.001) (Figure 3C). The better survival trend was observed in the 3rd period according to age categories except for patients aged >85 years (Figure 4A). In terms of surgical risk, time-related improvement in survival was observed in patients with intermediate risk. On the other hand, a significant effect in survival was not observed in patients with low risk (Figure 4B). Similar survival trends were observed in both the Japanese and Finnish cohorts (Supplementary Figure 2A–C).
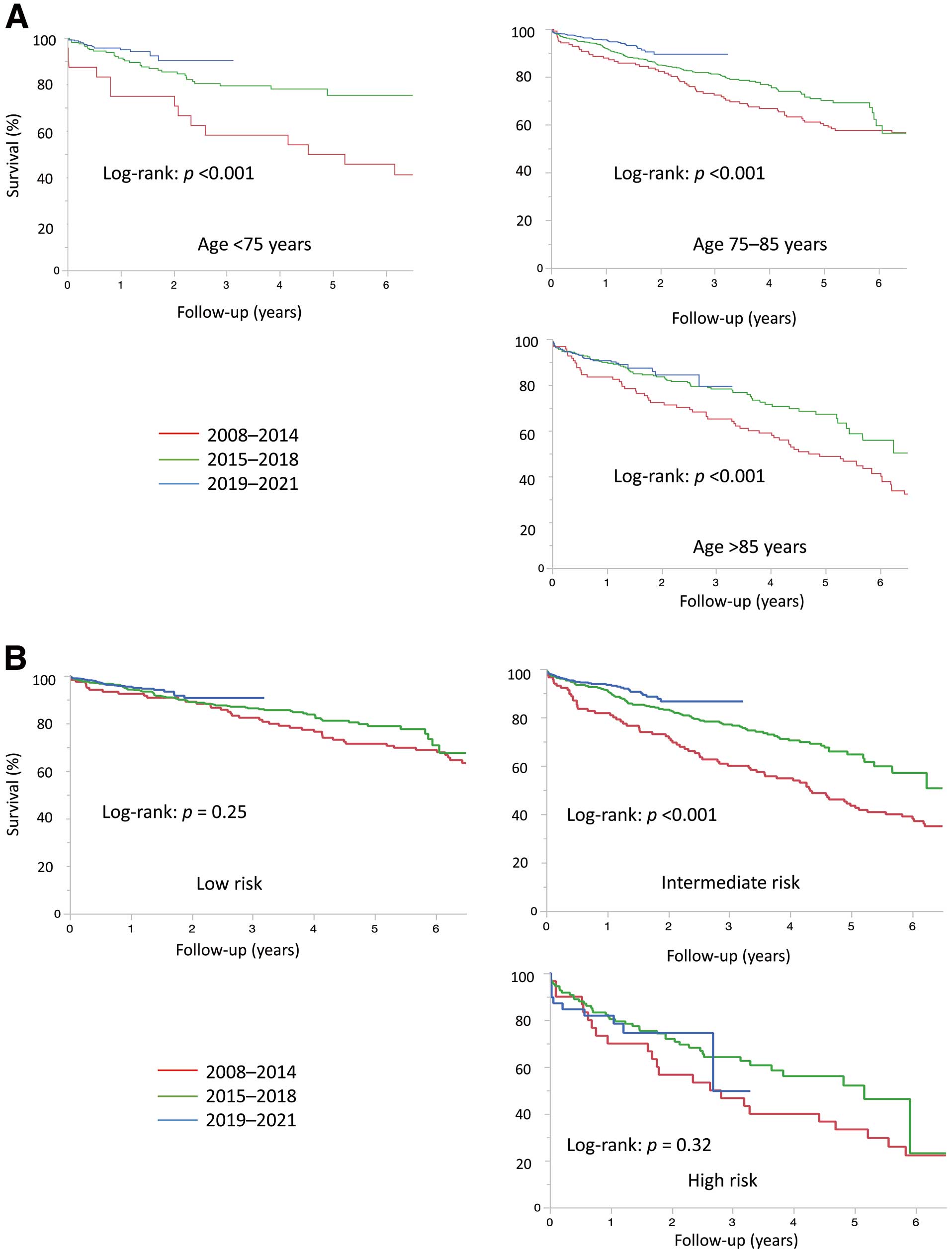
Trends in survival in (A) each age category and (B) each risk category.
Multivariate Analysis of 5-Year Survival
In Model 1, age <75 (vs. >85), lower STS score, and the 3rd time period were significantly associated with better survival (Figure 5A). In Model 2, age <75 (vs. >85), lower STS score, and the 3rd time period identically showed a significant association with better survival. Moreover, the incidence of PVL ≥moderate, RBC transfusion, and AKI were independently associated with the risk of 5-year death (Figure 5B). The survival curves in patients with and without PVL ≥moderate, RBC transfusion, and AKI are shown in Supplementary Figure 3A–C.
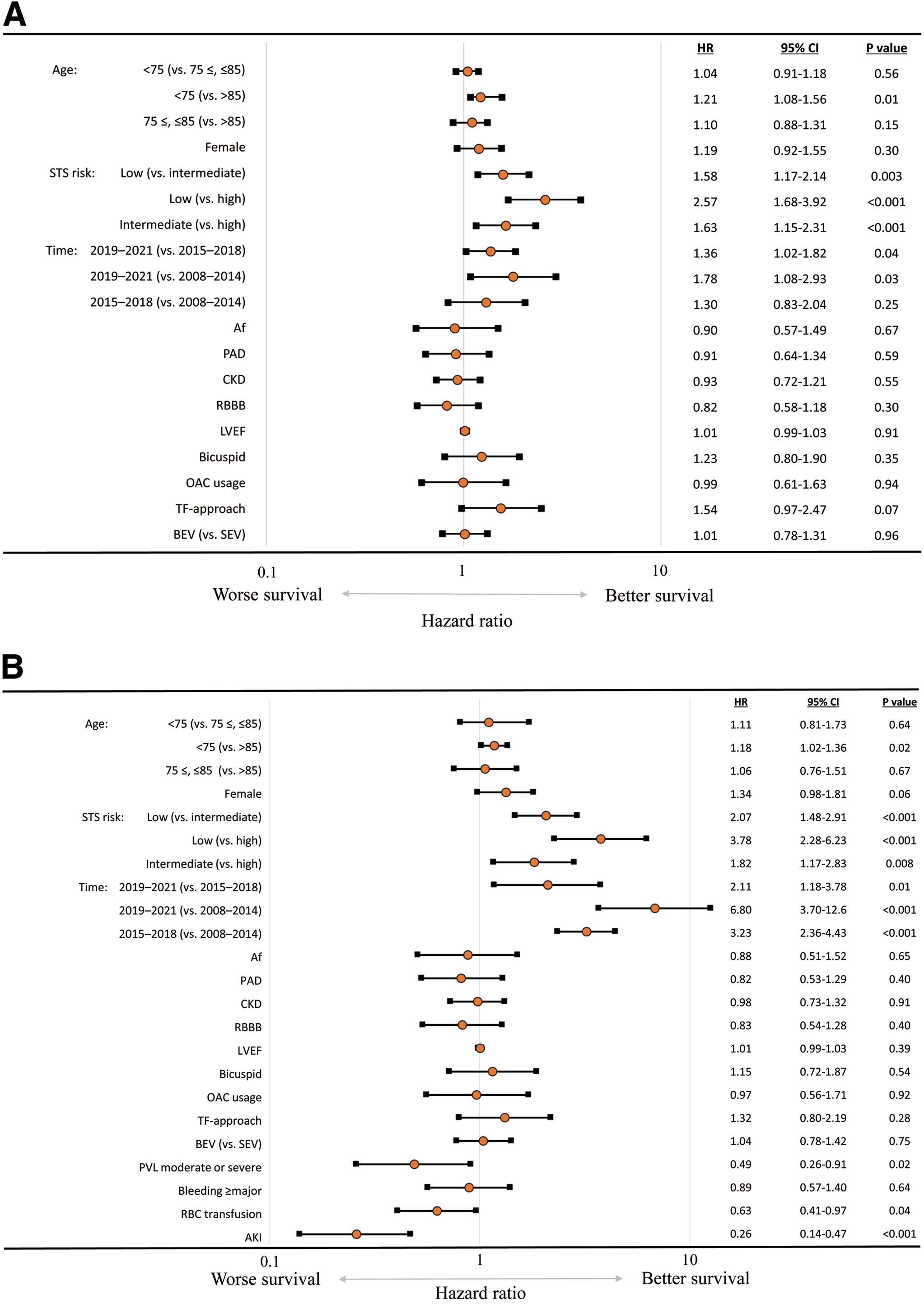
Multivariate analysis for 5-year survival. (A) Model 1 includes baseline and procedural characteristics. (B) Model 2 includes baseline/procedural characteristics and early outcomes. Af, atrial fibrillation; AKI, acute kidney injury; BEV, balloon-expandable valve; CKD, chronic kidney disease; LVEF, left ventricular ejection fraction; OAC, oral anti-coagulant; PAD, peripheral artery disease; PVL, paravalvular leak; RBBB, right bundle branch block; RBC, red blood cell; SEV, self-expanding valve; STS, Society of Thoracic Surgeons; TF, transfemoral.
The present international study has provided essential perspectives on survival and its trend after TAVI in patients with AS and various surgical risks, ages, and time periods. The main findings include: (1) the proportion of younger and/or lower risk patients who underwent TAVI significantly increased over the study time period; (2) 10-year survival after TAVI was 26.9%; (3) age and surgical risk categories were independently associated with survival; (4) survival improved over time, and the trend was observed over all age categories; and (5) the 5-year survival was significantly affected by PVL ≥moderate, RBC transfusion, and AKI.
Long-Term Survival After TAVIGiven the rapid expansion in the indication of TAVI across all surgical categories, attention has shifted toward strategies for lifetime management of patients with AS who undergo the initial intervention. For this point of view, reliable data on estimates in life expectancy after TAVI are essential. Moreover, stratification of expected prognosis at the time of the index procedure may be beneficial for discussion by the institutional heart team. Recently, 5-year survival data were reported from the PARTNER 1 and 2 trials.9,10 The 5-year survival rates were 32.2% in high-risk patients (mean age: 83.6±6.8 years; STS score: 11.8±3.3%) and 54.0% in intermediate-risk (mean age: 81.5±6.7 years; STS score: 5.8±2.1%), respectively. Today, the only randomized TAVI vs. SAVR trial in low-risk patients with 5-year follow-up is the NOTION trial.11 In that study, the 5-year survival in low-risk patients was 72.4% (mean age: 79.2±4.9 years; STS score: 2.9±1.6%). Data on longer-term survival are emerging, although, considering lifetime management includes re-do aortic valve replacement, little is known about >10-year survival after TAVI and the durability of THV. In the current study, 10-year survival in an all-comer AS population was 26.9%. Vanhaverbeke et al reported 20.0% survival at 10 years in a single-center study assessing an AS population of 2,670.17,18 Moreover, 5-year survival according to surgical risk categories in the present study was broadly consistent with those reported in the clinical trials just discussed, which suggest reliability of our analysis of survival. Long-term survival at 10 years after TAVI in the real-world setting is helpful for treatment selection to achieve better life-long management in AS patients, because it would allow informed discussion and shared decision-making about both treatment options and advanced care planning for the individual patient.
In the present study, 55.6%, 37.0%, and 7.4% of patients who underwent TAVI were classified as low, intermediate, and high risk, respectively, using STS scores. This was a marginally smaller proportion of low-risk patients than was included in the study by Vanhaverbeke et al, in which the risk profile was 64.3%, 27.1%, and 8.6%, respectively.18 However, whether this reflects an actual risk of the TAVI procedure remains unclear because anatomic variables such as hostile femoral access and annulus calcification were not considered in the risk assessment. The 30-day mortality rate of low-risk patients in the current study (1.3%) was lower than that in NOTION trial (2.1%), which was conducted from 2009 to 2013,19 and 3-fold higher than in the PARTNER 3 trial (0.4%), which was conducted from 2016 to 2017.1 These findings suggest improvements in both patient selection that considers anatomic characteristics and the institution’s level of experience, which is supported by the observed survival trend according to chronological treatment periods.
Use of Surgical Risk Score in TAVISurgical risk was a key inclusion criterion in landmark randomized studies that have compared the safety and efficacy of SAVR vs. TAVI in patients with severe AS. TAVI is now an established treatment across the entire spectrum of surgical risk in several guidelines.5,6 Therefore, regardless of surgical risk, the patient’s age has gained more attention for treatment selection between TAVI and SAVR. Indeed, age was an independent factor for better survival rates in the current study. In their study, Vanhaverbeke et al also showed that survival of TAVI patients with low surgical risk deteriorated as age increased to >80 years.18 These findings suggest that survival rates after TAVI could be stratified by patient’s age regardless of surgical risk category, as was described in a recent SAVR study.20 However, the surgical risk score does not consider frailty, which is associated with age and might influence life expectancy after the procedure. For optimal treatment selection, the heart team should refer to not only the surgical risk score but also the patient’s age. In conclusion, long-term survival could be stratified simply by age and surgical risk category before the index TAVI procedure. In combination with future data on the durability of transcatheter bioprosthetic valves and repeat accessibility to coronary arteries, more effective life-long management could be executable.
Survival Trends in TAVI PatientsIn terms of survival trends after TAVI, patients treated in the 3rd time period showed better survival than those in earlier categories, and it was observed over all age categories and even after multivariate analysis including variables such as age and surgical risk, as shown in a previous report.18 These findings suggest that better outcomes followed improvements in procedural technique and the THV system in addition to improved patient selection. When analyzed in each surgical-risk category, the significant time-related improvement in survival was observed only in patients with intermediate risk. On the other hand, there was a decreasing trend in procedural complications according to chronological treatment period in all surgical risk categories. Statistical underpowering might lead to no significant differences between time periods in the high-risk population. Additionally, the lower rates of complications in the low-risk subset, even in the 1st time period might diminish the effect of improvements in procedural technique over time on the time-related improvement in survival.
In contrast, early outcomes such as PVL ≥moderate, RBC transfusion, and AKI were independent predictors for worse 5-year survival irrespective of the time period. Of note, the relationship between RBC transfusion and death might be affected by baseline confounders such as frailty, malnutrition, anemia of chronic disease, and subclinical bleeding.21 On the other hand, transfusion itself might result in a worse outcome. A previous study reported that RBC transfusion increased the risk of early and late adverse events after TAVI, including death, stroke, and AKI, and the negative effect of RBC transfusion was independent of other significant comorbidities.22 The authors of that study proposed immune-mediated transfusion reactions as the basis for these adverse events. Therefore, we should carefully consider the indication of RBC transfusion and prevent bleeding complications. Regarding the comparison of TAVI and SAVR, a previous study showed that TAVI for patients without CKD was significantly associated with lower incidence of both AKI and RBC transfusion than with SAVR. Additionally, RBC transfusion was an independent predictor for the incidence of AKI, and AKI significantly increased the risk of 5-year death in both TAVI and SAVR cohorts.23 Considering these complications, treatment selection between TAVI and SAVR should be carefully decided for each patient. Furthermore, it is worth noting that these adverse events remained significant predictors for worse long-term outcome regardless of time period. Prognosis will improve with increasing experience of comprehensive management of AS patients in individual centers. However, specific adverse events of TAVI should be further mitigated.
Study LimitationsOur findings should be interpreted in terms of several limitations. First, this was a retrospective observational study. The indication of TAVI instead of SAVR and the selection of THV for low-risk patients might have differed between the 2 centers. Second, the current analysis of long-term survival was limited by the patients treated in the 3rd time period because they had the shortest follow-up. The 5-year mortality rate might be especially affected by a bias of patients in the 3rd time period not achieving a 5-year follow-up. Third, life expectancy of the entire population in Japan is the longest in the world, which could influence life expectancy after TAVI in our study; thus, the current study might not apply to other regions. Fourth, the current study included patients undergoing valve-in-valve procedure, whose life expectancy might be shorter than for TAVI patients with a native aortic valve. Fifth, the logistic regression analysis for 5-year survival did not include several variables such as B-type natriuretic peptide, serum albumin, and indexed effective orifice area, which could affect long-term survival after TAVI, because these data were not sufficient for the analysis. Sixth, the long-term mortality rate was defined by the incidence of all-cause death, not the incidence of cardiac death or cardiovascular death because the registry lacked these data. Lastly, we did not perform an analysis of echocardiographic outcomes. Therefore, the long-term durability of THV is still unclear.
Long-term survival at 10 years after TAVI was clarified in real-world settings. Younger age, lower surgical risk, and the later time period were significantly associated with better survival rates. These data might be beneficial for heart team discussion of life-long management of AS patients.
The registry is granted by the Japanese Circulation Society.
N.M. has received honoraria for lectures from Abbott, Edwards Lifesciences and Medtronic, is a clinical proctor of Edwards Lifesciences (SAPIEN) and Boston Scientific (Acurate Neo), and received a research grant from the Japanese Circulation Society. T.O. has received honoraria for lectures from Edwards Lifesciences and Medtronic. T.V. is a clinical proctor of Edwards Lifesciences (SAPIEN). S.S. is a clinical proctor of Edwards Lifesciences (SAPIEN), Medtronic (CoreValve) and Abbott (Navitor). M.L. reports receiving non-regulatory research grants from Teleflex and consultant fees from Boston Scientific, Edwards Lifesciences, and Medtronic. The other authors have no potential conflicts of interest to declare.
The study protocol conformed to the principles of the Declaration of Helsinki and was approved by the Tokushukai Group Ethics Committee (Reference no. TGE01492-024).
The deidentified participant data will be shared on a request basis. We can share all deidentified participant data collected during this study for anyone after this article is accepted and for 10 years. The data will be shared as Excel files via email for any legitimate purpose. Please directly contact the corresponding author to request data sharing.
Please find supplementary file(s);
https://doi.org/10.1253/circj.CJ-23-0593