論文ID: CJ-23-0818
論文ID: CJ-23-0818
Background: The effectiveness and safety of edoxaban for venous thromboembolism (VTE) in unselected real-world patients have not been fully evaluated.
Methods and Results: In the Japanese nationwide administrative database, we identified 6,262 VTE patients in whom edoxaban was initiated; these patients were divided into 3 groups based on their index doses: 15 mg/day (n=235), 30 mg/day (n=4,532), and 60 mg/day (n=1,495). We evaluated patient characteristics, recurrent VTEs, and a composite endpoint of intracranial hemorrhage (ICH) and gastrointestinal (GI) bleeding. Patient characteristics among the 15-, 30-, and 60-mg edoxaban groups varied widely regarding several aspects, including age (mean 81.0, 76.2, and 65.0 years, respectively) and body weight (mean 49.5, 51.8, and 70.3 kg, respectively). At 180 days, the cumulative incidence of recurrent VTEs in the 15-, 30-, and 60-mg edoxaban groups was 4.4%, 2.6%, and 1.8%, respectively, whereas that of ICH or GI bleeding was 7.3%, 5.4%, and 3.3%, respectively. Subgroup analyses showed that the cumulative incidence of ICH or GI bleeding in patients in the 15-mg edoxaban group was 3.6% for patients aged ≥80 years, 8.4% for those with a body weight <60 kg, and 31.3% for those with renal dysfunction.
Conclusions: Only a minority of patients with VTEs received a super low dose (15 mg) of edoxaban, and these patients may be at higher risk of bleeding as well as VTE recurrence.
Venous thromboembolism (VTE), including deep vein thrombosis (DVT) and pulmonary embolism (PE), is a major health problem worldwide.1 VTE has a long-term risk of recurrence, which can be prevented by anticoagulation therapy.2 Current guidelines recommend prolonged anticoagulation therapy for patients with VTE, except for patients at a low risk of recurrence.3–5 Recently, direct oral anticoagulants (DOACs) have become widely available for VTE6–10 and they could be safer than a vitamin K antagonist (VKA) in terms of bleeding risk, thus favoring a longer duration of anticoagulation therapy.11 Although prolonged anticoagulation therapy could be effective for preventing VTE recurrence, clinicians are sometimes reluctant to continue anticoagulation therapy for patients at a high risk of bleeding.
In the Hokusai-VTE trial, both a high (60 mg) and low (30 mg) once-daily dose of edoxaban were non-inferior to VKAs for the prevention of recurrent VTE and were favorable in terms of fewer bleeding events compared with VKAs.10 Based on results of randomized clinical trials (RCT) and ease of use, edoxaban has prevailed in daily clinical practice in Japan.12 However, RCTs are conducted under idealized and rigorously controlled conditions, with highly selected patients, which may not be broadly representative of real-world practice. Because the clinical outcomes of edoxaban in the real world may not necessarily coincide with the clinical outcomes in RCTs, the effectiveness and safety of edoxaban in unselected real-world patients could be important. Thus, the aim of the present study was to investigate clinical characteristics and outcomes of VTE patients receiving edoxaban in a large-scale all-comer cohort, using a large nationwide administrative database in Japan.
We conducted a new-user cohort study using a nationwide hospital administrative database provided by Medical Data Vision Co., Ltd. (MDV), covering the period April 1, 2008 to July 31, 2020. As of 2021, the database included data on over 36 million patients treated in 449 acute care hospitals adopting the Diagnosis Procedure Combination/Per-Diem Payment System (DPC/PDPS), which is a Japanese case-mix classification system for inpatient reimbursement.13
The structure of the database has been reported in detail elsewhere.14–16 Briefly, the database contains longitudinal information on patient demographics, diagnoses coded using the International Classification of Diseases, 10th Revision (ICD-10), drug prescriptions coded using the Anatomical Therapeutic Chemical (ATC) classification system, procedures coded using the National Health Insurance reimbursement system, and laboratory results. The database can link inpatient and outpatient data, allowing for tracking of patients as long as they are treated in the same hospital.
The present study was conducted in accordance with the principles of the Declaration of Helsinki. The research protocol was approved by the Ethics Committee of Kyoto University Graduate School and Faculty of Medicine (Approval no. R3817). In accordance with the current guidelines for epidemiological studies issued by the Ministry of Health, Labor, and Welfare in Japan, the need for written informed consent from each patient was waived because the data were anonymous.
Study PopulationWe identified patients hospitalized with VTE using ICD-10 codes (Supplementary Appendix 1). Patients were required to undergo at least 1 diagnostic imaging during hospitalization. Imaging modalities included computed tomography for DVT and PE, ultrasound examination of leg veins for DVT, or ventilation-perfusion lung scanning for PE.14,15,17 Patients aged 18–99 years in whom edoxaban was initiated between September 26, 2014 (approval date of edoxaban for VTE in Japan) and February 2, 2020 (180 days before the end of the study period) were included in the present study. The cohort entry date was the date of the first prescription of edoxaban, and patients were required to have started the index dose of edoxaban within 30 days after the onset of VTE. In addition, all patients were required to have ≥90 days of continuous enrollment in the database before entry into the study cohort.
Patients were excluded from the study if they had been prescribed any oral anticoagulant (warfarin, dabigatran, apixaban, and rivaroxaban) before study entry; had received parenteral anticoagulation (unfractionated heparin or fondaparinux) in the 90 days before the onset VTE; had another indication for edoxaban (atrial fibrillation, atrial flutter, or prophylaxis of VTE after total knee arthroplasty, total hip arthroplasty, or hip fracture surgery); had endocarditis, heart valve disease, or were pregnant; or had missing data on body weight in the 90 days before study entry (Supplementary Appendix 1).14 In addition, patients were excluded if they had been prescribed edoxaban by a department of orthopedic surgery at cohort entry (because orthopedic surgeons often use edoxaban for VTE prophylaxis) and if they had an intracranial hemorrhage (ICH) or gastrointestinal (GI) bleeding at study entry. Patients were allowed to have received parenteral anticoagulation between VTE onset and study entry.
Patients included in the present study were divided into 3 mutually exclusive groups based on the index dose of edoxaban: 15, 30, or 60 mg/day. Although 15 mg edoxaban for the prevention of recurrent VTE has not been approved in Japan, some patients with VTE received 15 mg edoxaban off-label.
Patient Characteristics and DefinitionsWe evaluated baseline patient characteristics, including demographics, comorbidities, medications, and laboratory test results, where available.14 Active cancer was defined as at least one record of cancer that was treated with either pharmacotherapy, radiotherapy, or surgery. Renal dysfunction was defined as at least one record of renal disease or dialysis. Detailed definitions of patient characteristics are provided in Supplementary Appendix 2.
Clinical Outcomes and DefinitionsThe primary effectiveness outcome was recurrent VTE, defined as a DVT and/or PE occurring subsequent to the index VTE (Supplementary Appendix 3). Based on previous studies,14,15,17 recurrent VTE were determined by the combination of the following 2 components: inpatient diagnosis of VTE; and diagnostic imaging during hospitalization. We excluded recurrent VTE during the hospitalization for the index VTE to ensure that there was only one VTE episode per hospitalization. Following a previous study,18 we did not include outpatient VTE diagnoses because they may not have indicated a recurrence, but rather a follow-up visit.19
The primary safety outcome was the composite of ICH or GI bleeding, as in previous studies (Supplementary Appendix 3).18,20 ICH and GI bleeding were determined by a combination of a diagnosis and either a procedure or hospitalization.14,21 Secondary safety outcomes were separate incidences of ICH and GI bleeding.
Clinical Follow-upFollow-up for the study outcomes began on the day after study entry and continued until Day 180,22 discontinuation of edoxaban, switching to another dose from the index dose of edoxaban, a treatment switch to any other oral anticoagulant, transferal to another hospital or clinic, or death, whichever came first. Discontinuation of edoxaban was defined as no subsequent prescription for edoxaban within a 14-day grace period after the end of the supply provided in the last prescription. To account for medication stockpiling, when the period covered by 2 prescriptions overlapped, the number of overlapping days was added to the end date of the latter prescription.14 Separate follow-up times were calculated for each outcome.
Statistical AnalysisCategorical variables are presented as numbers and percentages. Continuous variables are presented as the mean±SD or as a median with interquartile range according to data distribution. To examine the significance of differences in patient characteristics among the 3 groups, we calculated standardized mean differences (SMDs) for 3 pairwise comparisons and averaged them for each patient characteristic. Patient characteristics with an SMD ≥0.2 were considered significantly different.23,24
We estimated the cumulative incidence function with 95% confidence intervals (CIs) for each outcome accounting for the competing risk of death. No statistical tests were performed because of the large differences in the patient characteristics among the 3 groups. Subgroup analyses were conducted by age (<80, ≥80 years), body weight (<60, ≥60 kg), active cancer (with/without), and renal dysfunction (with/without). Furthermore, sensitivity analyses were performed by changing the grace period from 14 to 7 or 28 days to assess for any potential misclassification of the exposure. All analyses were performed by one author (T.F.) using SAS version 9.4 (SAS Institute Inc., Cary, NC, USA) and R version 4.1.0 (R Foundation for Statistical Computing, Vienna, Austria).
We identified 27,397 patients with VTE in whom edoxaban was initiated between September 26, 2014 and February 2, 2020, and 15,650 patients met the inclusion criteria (Figure 1). After excluding 9,388 patients who met the exclusion criteria, the study population consisted of 6,262 patients with VTE in whom edoxaban was initiated. Among these patients, 235 (3.8%), 4,532 (72%), and 1,495 (24%) received 15, 30, and 60 mg edoxaban, respectively, as the index dose.

Study flow chart. VTE, venous thromboembolism.
Patient Characteristics
The patient characteristics varied widely regarding several aspects among the 3 groups (Table 1). Specifically, patients in the 15-mg edoxaban group were older than those in the 30- and 60-mg groups (mean age 81.0, 76.2, and 65.0 years, respectively), and the proportion of patients aged ≥80 years was highest in the 15-mg edoxaban group (62.6%, 45.7%, and 12.4%, respectively). Patients in the 15-mg edoxaban group had a lower body weight than those in the 30- and 60-mg groups (mean 49.5, 51.8, and 70.3 kg, respectively), and the proportion of patients with body weight <60 kg was highest in the 15-mg edoxaban group (82.1%, 81.5%, and 11.0%, respectively). Patients in the 15-mg edoxaban group more often presented with DVT as a VTE presentation than those in the 30- and 60-mg groups (78.7%, 65.4%, and 50.8%, respectively). Active cancer trended to be least frequent in patients in the 15-mg edoxaban group than in the 30- and 60-mg groups (21.7%, 25.4%, and 29.7%, respectively), whereas renal dysfunction trended to be most frequent in the 15-mg edoxaban group (5.1%, 2.2%, and 0.5%, respectively).
Patient Characteristics
| Edoxaban (mg) | SMD | |||
|---|---|---|---|---|
| 15 (n=235) | 30 (n=4,532) | 60 (n=1,495) | ||
| Demographics | ||||
| Age (years) | 81.0±10.4 | 76.2±11.8 | 65.0±13.2 | 0.89 |
| Age category (tears) | ||||
| 18–64 | 17 (7.2) | 635 (14.0) | 633 (42.3) | 0.86 |
| 65–79 | 71 (30.2) | 1,828 (40.3) | 677 (45.3) | |
| 80–99 | 147 (62.6) | 2,069 (45.7) | 185 (12.4) | |
| Female sex | 174 (74.0) | 3,132 (69.1) | 502 (33.6) | 0.59 |
| Body weight (kg) | 49.5±11.6 | 51.8±10.2 | 70.3±12.0 | 1.21 |
| <60 kg | 193 (82.1) | 3,694 (81.5) | 164 (11.0) | 1.35 |
| ≥60 kg | 42 (17.9) | 838 (18.5) | 1,331 (89.0) | |
| BMI (kg/m2) | 21.54±3.87 | 21.85±3.71 | 26.15±4.40 | 0.75 |
| BMI category (kg/m2) | ||||
| <18.5 | 49 (20.9) | 780 (17.2) | 15 (1.0) | 0.70 |
| 18.5–25 | 140 (59.6) | 2,858 (63.1) | 637 (42.6) | |
| 25–30 | 35 (14.9) | 670 (14.8) | 598 (40.0) | |
| ≥30 | 6 (2.6) | 106 (2.3) | 216 (14.4) | |
| Missing | 5 (2.1) | 118 (2.6) | 29 (1.9) | |
| Calendar time of the index date | ||||
| September 2014–December 2015 | 24 (10.2) | 589 (13.0) | 209 (14.0) | 0.13 |
| January 2016–December 2016 | 28 (11.9) | 628 (13.9) | 230 (15.4) | |
| January 2017–December 2017 | 44 (18.7) | 830 (18.3) | 294 (19.7) | |
| January 2018–December 2018 | 61 (26.0) | 1,130 (24.9) | 348 (23.3) | |
| January 2019–February 2020 | 78 (33.2) | 1,355 (29.9) | 414 (27.7) | |
| Type of index VTE | ||||
| DVT only | 185 (78.7) | 2,962 (65.4) | 759 (50.8) | 0.40 |
| PE with or without a DVT | 50 (21.3) | 1,570 (34.6) | 736 (49.2) | |
| Acute VTE treatment | ||||
| Initial parenteral anticoagulation | 101 (43.0) | 2,389 (52.7) | 1,001 (67.0) | 0.33 |
| Thrombolysis | 3 (1.3) | 159 (3.5) | 90 (6.0) | 0.17 |
| Inferior vena cava filter placement | 8 (3.4) | 294 (6.5) | 126 (8.4) | 0.14 |
| Comorbidities | ||||
| Active cancer | 51 (21.7) | 1,152 (25.4) | 444 (29.7) | 0.12 |
| Fracture or trauma | 51 (21.7) | 611 (13.5) | 149 (10.0) | 0.22 |
| Moderate- to high-risk surgery for PEs | 19 (8.1) | 408 (9.0) | 180 (12.0) | 0.09 |
| Previous VTE | 18 (7.7) | 306 (6.8) | 100 (6.7) | 0.03 |
| COPD | 17 (7.2) | 351 (7.7) | 107 (7.2) | 0.02 |
| Heart failure | 53 (22.6) | 829 (18.3) | 212 (14.2) | 0.15 |
| Stroke or transient ischemic attack | 41 (17.4) | 782 (17.3) | 190 (12.7) | 0.09 |
| Varicose veins of lower extremities | 4 (1.7) | 65 (1.4) | 29 (1.9) | 0.03 |
| Peptic ulcer disease | 51 (21.7) | 811 (17.9) | 249 (16.7) | 0.09 |
| Previous bleeding | 39 (16.6) | 640 (14.1) | 212 (14.2) | 0.05 |
| Previous gastrointestinal bleeding | 10 (4.3) | 220 (4.9) | 69 (4.6) | 0.02 |
| Hypertension | 134 (57.0) | 1,951 (43.0) | 610 (40.8) | 0.22 |
| Hyperlipidemia | 52 (22.1) | 923 (20.4) | 347 (23.2) | 0.05 |
| Diabetes | 51 (21.7) | 998 (22.0) | 388 (26.0) | 0.07 |
| Renal dysfunction | 12 (5.1) | 99 (2.2) | 8 (0.5) | 0.19 |
| Liver dysfunction | 36 (15.3) | 559 (12.3) | 202 (13.5) | 0.06 |
| Concomitant medication | ||||
| Antiplatelet agents | 56 (23.8) | 796 (17.6) | 202 (13.5) | 0.18 |
| NSAIDs | 88 (37.4) | 1,525 (33.6) | 574 (38.4) | 0.07 |
| H2 receptor antagonists | 45 (19.1) | 782 (17.3) | 234 (15.7) | 0.06 |
| Proton pump inhibitors | 135 (57.4) | 2,426 (53.5) | 774 (51.8) | 0.08 |
| Statins | 51 (21.7) | 709 (15.6) | 239 (16.0) | 0.10 |
| Laboratory testing | ||||
| eGFR (mL/min/1.73 m2) | 63.6±33.4 | 70.2±25.0 | 75.2±18.5 | 0.29 |
| eGFR results available | 27 (11.5) | 373 (8.2) | 121 (8.1) | 0.08 |
Unless indicated otherwise, data are given as the mean±SD or n (%). BMI, body mass index; COPD, chronic obstructive pulmonary disease; DVT, deep vein thrombosis; eGFR, estimated glomerular filtration rate; NSAIDs, non-steroidal anti-inflammatory drugs; PE, pulmonary embolism; SMD, standardized mean difference; VTE, venous thromboembolism.
Follow-up Information
The respective median follow-up times for the effectiveness and safety outcomes were 47 and 44 days for the 15-mg edoxaban group; 49 and 48 days for the 30-mg edoxaban group; and 60 and 60 days for the 60-mg edoxaban group. The reasons for censoring differed somewhat across the 3 groups (Supplementary Table 1). Treatment discontinuation, death, and transfer to another hospital or clinic were the most common reasons for censoring in the 15-mg edoxaban group.
Effectiveness OutcomesAt 180 days, the cumulative incidence of VTE recurrence in the 15-, 30-, and 60-mg edoxaban groups was 4.4% (95% CI 1.3–10.7%), 2.6% (95% CI 1.9–3.4%), and 1.8% (95% CI 1.0–2.8%), respectively (Table 2). The time course of VTE recurrence is shown in Figure 2A. Subgroup analyses stratified by age, body weight, active cancer, and renal dysfunction showed that the cumulative incidence of recurrent VTE among patients in the 15-mg edoxaban group was 7.2% (95% CI 1.7–18.3%) in those aged ≥80 years, 5.6% (95% CI 1.6–13.4%) in those with a body weight <60 kg, 7.0% (95% CI 0.9–22.2%) in those with active cancer, and 20.0% (95% CI 0.4–62.1%) in those with renal dysfunction (Table 3).
Clinical Outcomes According to Patients Receiving 15, 30, or 60 mg Edoxaban
| Outcome | Edoxaban (mg) |
No. patients | No. events in 7 days |
7-day CIF (95% CI) |
No. events in 30 days |
30-day CIF (95% CI) |
No. events in 90 days |
90-day CIF (95% CI) |
No. events in 180 days |
180-day CIF (95% CI) |
|---|---|---|---|---|---|---|---|---|---|---|
| Recurrent VTE | 15 | 235 | 1 | 0.4 (0.0–2.2) | 1 | 0.4 (0.0–2.2) | 2 | 1.5 (0.2–5.2) | 4 | 4.4 (1.3–10.7) |
| 30 | 4,532 | 1 | 0.0 (0.0–0.1) | 16 | 0.4 (0.2–0.6) | 38 | 1.3 (0.9–1.8) | 55 | 2.6 (1.9–3.4) | |
| 60 | 1,495 | 0 | 0.0 (0.0–0.0) | 8 | 0.7 (0.3–1.3) | 15 | 1.6 (0.9–2.6) | 16 | 1.8 (1.0–2.8) | |
| ICH or GI bleeding | 15 | 235 | 3 | 1.3 (0.4–3.5) | 9 | 4.2 (2.0–7.5) | 11 | 6.1 (3.1–10.6) | 12 | 7.3 (3.7–12.4) |
| 30 | 4,532 | 58 | 1.3 (1.0–1.7) | 132 | 3.1 (2.6–3.7) | 157 | 4.3 (3.6–5.0) | 171 | 5.4 (4.5–6.3) | |
| 60 | 1,495 | 13 | 0.9 (0.5–1.5) | 29 | 2.2 (1.5–3.1) | 35 | 2.9 (2.0–4.0) | 37 | 3.3 (2.3–4.5) | |
| ICH | 15 | 235 | 0 | 0.0 (0.0–0.0) | 4 | 2.0 (0.6–4.6) | 4 | 2.0 (0.6–4.6) | 4 | 2.0 (0.6–4.6) |
| 30 | 4,532 | 21 | 0.5 (0.3–0.7) | 47 | 1.1 (0.8–1.5) | 51 | 1.3 (1.0–1.7) | 54 | 1.5 (1.1–2.0) | |
| 60 | 1,495 | 3 | 0.2 (0.1–0.6) | 11 | 0.8 (0.4–1.5) | 11 | 0.8 (0.4–1.5) | 12 | 1.1 (0.5–1.9) | |
| GI bleeding | 15 | 235 | 3 | 1.3 (0.4–3.5) | 5 | 2.2 (0.8–4.8) | 7 | 4.2 (1.7–8.4) | 8 | 5.3 (2.3–10.3) |
| 30 | 4,532 | 38 | 0.9 (0.6–1.2) | 86 | 2.1 (1.7–2.5) | 107 | 3.0 (2.4–3.6) | 119 | 4.0 (3.2–4.8) | |
| 60 | 1,495 | 10 | 0.7 (0.4–1.3) | 19 | 1.4 (0.9–2.2) | 25 | 2.1 (1.4–3.1) | 26 | 2.3 (1.5–3.4) |
CI, confidence interval; CIF, cumulative incidence function; GI, gastrointestinal; ICH, intracranial hemorrhage; VTE, venous thromboembolism.
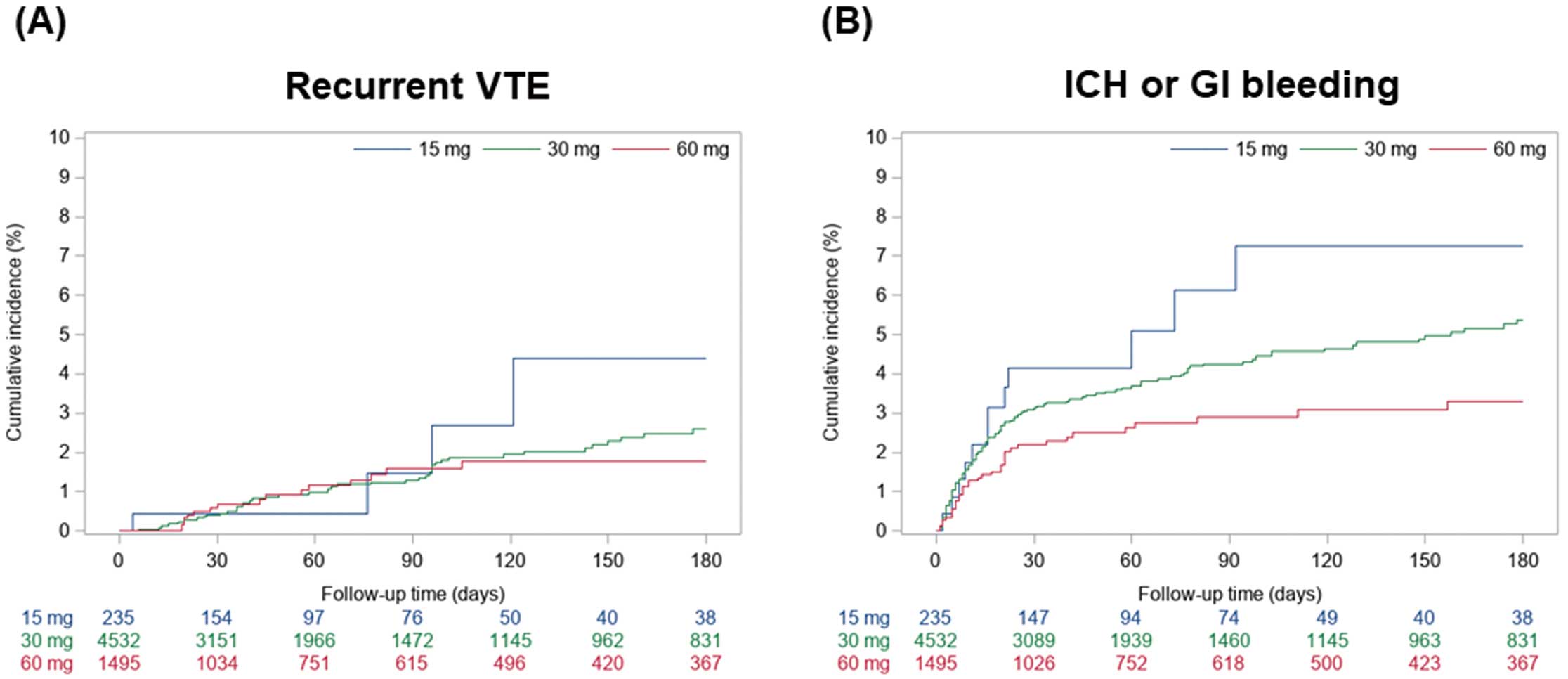
Cumulative incidence curves for (A) recurrent venous thromboembolism (VTE) and (B) intracranial hemorrhage (ICH) or gastrointestinal (GI) bleeding over a period of 180 days among patients receiving 15, 30, or 60 mg edoxaban.
Clinical Outcomes in Subgroup Analyses According to Edoxaban Dose
| Outcome / Subgroup | Edoxaban (mg) | No. patients | No. events in 180 days | 180-day CIF (95% CI) | |
|---|---|---|---|---|---|
| Recurrent VTE | |||||
| Age (years) | <80 | 15 | 88 | 1 | 1.1 (0.1–5.6) |
| 30 | 2,463 | 37 | 2.9 (2.0–4.0) | ||
| 60 | 1,310 | 15 | 1.7 (1.0–2.8) | ||
| ≥80 | 15 | 147 | 3 | 7.2 (1.7–18.3) | |
| 30 | 2,069 | 18 | 1.9 (1.1–3.1) | ||
| 60 | 185 | 1 | 2.4 (0.2–11.2) | ||
| Body weight (kg) | <60 | 15 | 193 | 4 | 5.6 (1.6–13.4) |
| 30 | 3,694 | 43 | 2.4 (1.7–3.3) | ||
| 60 | 164 | 1 | 1.2 (0.1–6.0) | ||
| ≥60 | 15 | 42 | 0 | 0.0 (0.0–0.0) | |
| 30 | 838 | 12 | 3.2 (1.6–5.7) | ||
| 60 | 1,331 | 15 | 1.8 (1.0–2.9) | ||
| Active cancer | With | 15 | 51 | 2 | 7.0 (0.9–22.2) |
| 30 | 1,152 | 24 | 3.5 (2.2–5.2) | ||
| 60 | 444 | 9 | 3.0 (1.5–5.5) | ||
| Without | 15 | 184 | 2 | 3.1 (0.6–9.6) | |
| 30 | 3,380 | 31 | 2.2 (1.4–3.2) | ||
| 60 | 1,051 | 7 | 1.1 (0.5–2.3) | ||
| Renal dysfunction | With | 15 | 12 | 1 | 20.0 (0.4–62.1) |
| 30 | 99 | 2 | 2.4 (0.5–7.6) | ||
| 60 | 8 | 0 | 0.0 (0.0–0.0) | ||
| Without | 15 | 223 | 3 | 3.5 (0.8–9.8) | |
| 30 | 4,433 | 53 | 2.6 (1.9–3.4) | ||
| 60 | 1,487 | 16 | 1.8 (1.0–2.8) | ||
| ICH or GI bleeding | |||||
| Age (years) | <80 | 15 | 88 | 7 | 12.1 (5.0–22.7) |
| 30 | 2,463 | 98 | 5.3 (4.3–6.6) | ||
| 60 | 1,310 | 32 | 3.2 (2.2–4.6) | ||
| ≥80 | 15 | 147 | 5 | 3.6 (1.3–7.8) | |
| 30 | 2,069 | 73 | 5.4 (4.0–7.1) | ||
| 60 | 185 | 5 | 3.4 (1.2–7.4) | ||
| Body weight (kg) | <60 | 15 | 193 | 11 | 8.4 (4.2–14.6) |
| 30 | 3,694 | 130 | 5.2 (4.2–6.2) | ||
| 60 | 164 | 6 | 4.4 (1.8–8.9) | ||
| ≥60 | 15 | 42 | 1 | 2.4 (0.2–10.9) | |
| 30 | 838 | 41 | 6.3 (4.4–8.6) | ||
| 60 | 1,331 | 31 | 3.1 (2.1–4.4) | ||
| Active cancer | With | 15 | 51 | 2 | 4.0 (0.7–12.2) |
| 30 | 1,152 | 42 | 5.1 (3.6–6.9) | ||
| 60 | 444 | 11 | 2.9 (1.5–5.1) | ||
| Without | 15 | 184 | 10 | 8.4 (4.0–15.1) | |
| 30 | 3,380 | 129 | 5.4 (4.4–6.5) | ||
| 60 | 1,051 | 26 | 3.5 (2.3–5.2) | ||
| Renal dysfunction | With | 15 | 12 | 2 | 31.3 (1.5–72.4) |
| 30 | 99 | 2 | 3.3 (0.6–10.6) | ||
| 60 | 8 | 0 | 0.0 (0.0–0.0) | ||
| Without | 15 | 223 | 10 | 6.1 (2.9–11.0) | |
| 30 | 4,433 | 169 | 5.4 (4.5–6.4) | ||
| 60 | 1,487 | 37 | 3.3 (2.3–4.6) | ||
Abbreviations as in Table 2.
Safety Outcomes
At 180 days, the cumulative incidence of ICH or GI bleeding in the 15-, 30-, and 60-mg edoxaban groups was 7.3% (95% CI 3.7–12.4%), 5.4% (95% CI 4.5–6.3%), and 3.3% (95% CI 2.3–4.5%), respectively (Table 2). The time course of ICH or GI bleeding is shown in Figure 2B. These results were generally consistent for the individual outcomes of ICH (2.0%, 1.5%, and 1.1%) and GI bleeding (5.3%, 4.0%, and 2.3%) at 180 days in the 15-, 30-, and 60-mg edoxaban groups (Table 2). Subgroup analyses stratified by age, body weight, active cancer, and renal dysfunction showed that the cumulative incidence of ICH or GI bleeding in patients with an index dose of 15 mg edoxaban was 3.6% (95% CI 1.3–7.8%) in those aged ≥80 years, 8.4% (95% CI 4.2–14.6%) in those with a body weight <60 kg, 4.0% (95% CI 0.7–12.2%) in those with active cancer, and 31.3% (95% CI 1.5–72.4%) in those with renal dysfunction (Table 3).
Sensitivity AnalysesThe sensitivity analyses that changed the grace period from 14 to 7 or 28 days showed consistent results with the main analysis (Supplementary Table 2).
The main findings of the present study are as follows: (1) patients with VTE frequently have characteristics of high bleeding risk, including an old age, low body weight, and renal dysfunction, and most patients with VTE received 30 mg edoxaban as current daily clinical practice; (2) the risks of recurrent VTE and ICH or GI bleeding differed in patients according to different doses of edoxaban; and (3) patients with a low body weight and renal dysfunction appeared to be at a higher risk of bleeding.
The mainstay of the prevention of recurrent VTE is anticoagulation therapy, which could also cause major concern regarding bleeding events, especially in patients at high bleeding risk. Thus, it may be important to obtain a good balance between the risk of recurrence and bleeding through long-term anticoagulation strategies including the duration and dose of anticoagulation therapy. In the Hokusai-VTE trial, edoxaban was given once daily at a dose of 60 mg, which was reduced to 30 mg in patients with a creatinine clearance of 30–50 mL/min, body weight ≤60 kg, or receiving certain P-glycoprotein inhibitors, and the reduced dose of 30 mg was reported to have a similar efficacy and safety as the 60-mg dose in patients meeting the criteria for a dose reduction.25 However, clinicians can sometimes be reluctant to prescribe even the reduced dose of 30 mg edoxaban, especially for patients at high bleeding risk, including those with very old age, severe renal dysfunction, very low body weight, or an overlapping of those risk factors. In fact, the present study showed that patients with VTE frequently had characteristics of high bleeding risk in daily clinical practice. Although a super low dose of edoxaban of 15 mg for the prevention of recurrent VTE has not been approved anywhere in the world, including in Japan, the ELDERCARE-AF trial reported that a 15-mg super low dose of edoxaban could be a potential alternative for the prevention of stroke in very elderly patients with atrial fibrillation who are at high bleeding risk.26 The large nationwide administrative database used in the present study revealed that only a minority of patients with VTE received 15 mg edoxaban as off-label use, and old age, low body weight, and renal dysfunction seemed to be the clinical characteristics for receiving 15 mg edoxaban.
When considering an optimal dosage of edoxaban from a pharmacological point of view, identifying patients at high bleeding risk during anticoagulation therapy could be particularly important. Previous studies reported several risk factors for bleeding in VTE patients, including old age, sex, cancer, uncontrolled hypertension, previous bleeding, renal dysfunction, anemia, thrombocytopenia, a labile international normalized ratio for warfarin users, and the use of platelet inhibitors.27–29 In addition, several factors related to the pharmacological metabolism of DOACs could be especially important for optimal doses of DOACs, including age, body weight, renal function, and concomitant drugs.30–33 In line with previous reports, the present study revealed that the risk of ICH or GI bleeding in patients receiving 15 mg edoxaban was numerically greater in patients with low body weight and renal dysfunction. Low body weight and renal dysfunction may be especially notable factors in terms of the bleeding risk related to the use of edoxaban.
No RCT to date has evaluated the efficacy and safety of a 15-mg super low dose of edoxaban for the prevention of recurrent VTE. The present observational study revealed that patients receiving 15 mg edoxaban had a numerically higher incidence of both recurrent VTE and ICH or GI bleeding compared with the other groups, which suggests that patients at potential high bleeding risk may also be at a high risk of recurrent VTE. Considering the quite different baseline characteristics of patients receiving 15 mg edoxaban compared with the other groups, including old age, low body weight, and renal dysfunction, these clinical features could have a considerable influence on bleeding risk and the risk of VTE recurrence. Although the present analysis was primarily exploratory due to the observational study design, making it subject to various biases, the risk of recurrent VTE should be noted when prescribing the super low dose of 15 mg edoxaban. Thus, it may be clinically relevant to identify which patients would most likely benefit from the super low dose of 15 mg edoxaban. Considering the clinical relevance of the issues, future RCTs evaluating the efficacy and safety of super low-dose edoxaban would be warranted.
Study LimitationsThe present study has several limitations. First, this study was an observational study, making it subject to various biases inherent to an observational study design. In particular, the therapeutic decision making was left to the discretion of the attending physicians, which could have had a considerable influence on the clinical outcomes. Second, the MDV database collected data only from acute care hospitals, and data were unavailable for patients who had also been treated in other clinics and hospitals. Thus, the present results may be only partially applicable to patients treated in clinics and long-term care hospitals. In addition, this may have led to an outcome misclassification and underestimation of cumulative incidences. However, the majority of VTE patients are seen in acute care hospitals, and the treatment for VTE is likely to continue in the same hospital. Third, the MDV database lacked body weight data for <5% of the total cohort, and we excluded those patients. However, when key confounders are missing in <5% of cases, a complete-case analysis may be acceptable because the results would be nearly identical, regardless of how missing confounders are treated.34 Fourth, we excluded recurrent VTE during the hospitalization for the index VTE to ensure the validity and accuracy of the recurrent VTE, which could underestimate recurrent VTE in the acute phase. Fifth, we defined baseline characteristics based on ICD-10 codes. Renal dysfunction was defined as at least one record of renal disease or dialysis. However, considering the relatively low prevalence of renal dysfunction, there could be some concerns regarding the accuracy of the identification of renal dysfunction, meaning the results should be interpreted with caution. Active cancer was defined as at least one record of cancer that was treated with either pharmacotherapy, radiotherapy, or surgery. However, if a patient refused any treatment for cancer, we would not have included them as patients with active cancer. Sixth, we could not evaluate patients with edoxaban under- or overdosing based on the packet insert in Japan, because there were only a few patients in the MDV database with data on serum creatine. Finally, the demographics, practice patterns, and clinical outcomes for patients with VTE in Japan may differ from those outside Japan.
In the Japanese nationwide administrative database, patients with VTE frequently had characteristics of high bleeding risk, with risks of clinical outcomes differing according to the different doses of edoxaban. Only a minority of patients with VTE received the super low dose of 15 mg edoxaban, and these patients may be at higher risk of bleeding and VTE recurrence, and patients with a low body weight and renal dysfunction appeared to be at a markedly higher risk of bleeding.
The authors are grateful to Medical Data Vision Co., Ltd. for the generous provision of the medical data and Mr. John Martin (Exclusive Consultant at Japan Lifeline Co., Ltd.) for his grammatical assistance.
This study was supported, in part, by research funding for the investigator-initiated studies by Daiichi Sankyo Co., Ltd. The research funder had no role in the design and conduct of the study; the collection, management, analysis, and interpretation of the data; and in the preparation, review, or approval of the manuscript.
K.O. is a member of Circulation Journal’s Editorial Team.
Y.Y. has received lecture fees from Bayer Healthcare, Bristol-Myers Squibb, Pfizer, and Daiichi Sankyo, and grant support from Bayer Healthcare and Daiichi Sankyo. T.F. has been employed by the Department of Digital Health and Epidemiology, an Industry-Academia Collaboration Course supported by Eisai Co., Ltd., Kyowa Kirin Co., Ltd., and Real World Data Co., Ltd.; and has received consulting fees from Real World Data Co., Ltd. and speaker fees from Asahi Kasei Pharma Corporation and EPS Corporation. K.K. has received research grants from Eisai Co., Ltd., Kyowa Kirin Co., Ltd., Sumitomo Pharma Co., Ltd., and Real World Data Co., Ltd.; consulting fees from LEBER Inc., JMDC Inc., Shin Nippon Biomedical Laboratories Ltd., and Advanced Medical Care Inc.; executive compensation from Cancer Intelligence Care Systems, Inc.; and speaker fees from Pharma Business Academy and Toppan Inc. All other authors have no relationships relevant to the contents of this paper to disclose.
Y.Y. and T.F. contributed equally to this work. Y.Y., T.F., and C.T. formulated the study concept and design. T.F. performed the statistical analysis and Y.Y. wrote the manuscript. All authors contributed to the discussion, reviewed, edited, and approved the final manuscript, and agreed to submission.
This study was conducted in accordance with the principles of the Declaration of Helsinki. The research protocol was approved by the Ethics Committee of Kyoto University Graduate School and Faculty of Medicine (Approval no. R3817). In accordance with the current guidelines for epidemiological studies issued by the Ministry of Health, Labor, and Welfare in Japan, The need for written informed consent from each patient was waived because the data are anonymous.
Deidentified participant data will not be shared.
Please find supplementary file(s);
https://doi.org/10.1253/circj.CJ-23-0818