論文ID: CJ-66-0211
論文ID: CJ-66-0211
Dear Colleagues,
On behalf of the Editorial Team of Circulation Journal, I am pleased to announce the Circulation Journal Awards for the Year 2022.
The aim of these Awards is to recognize papers published in 2022, both clinical and experimental studies, that were highly appreciated by the Editorial Team. The selection process comprises 2 steps. In the first step, from 137 original papers published in the journal in 2022, our 44 Japanese Associate Editors selected papers with a high scientific level in their respective fields. In the second step, 5 groups each consisting of 5–12 Japanese Associate Editors further evaluated the selected papers in terms of originality, contribution to cardiovascular science, manner of paper preparation, and future possibilities.
In the year of 2022, the following 7 papers have been selected for the Circulation Journal Awards.
(Circ J 2022; 86: 986–992)1
| Clinical Implication of Smoking-Related Aryl-Hydrocarbon Receptor Repressor (AHRR) Hypomethylation in Japanese Adults Fumihiko Takeuchi, Kozue Takano, Masaya Yamamoto, Masato Isono, Wataru Miyake, Kotaro Mori, Hisao Hara, Yukio Hiroi, Norihiro Kato (Department of Gene Diagnostics and Therapeutics (F.T., M.I., N.K.), Medical Genomics Center (F.T., K.T., K.M., N.K.), Research Institute, National Center for Global Health and Medicine, Tokyo; Department of Genomic Medicine (K.T., N.K.), Department of Cardiology (M.Y., W.M., H.H., Y.H.), Center Hospital, National Center for Global Health and Medicine, Tokyo, Japan) |
 |

Effect of smoking status on DNA methylation. The role of AHRR in the xenobiotic detoxification pathway.

cg05575921 methylation among former smokers. The distribution of cg05575921 methylation extent is shown by years after smoking cessation.
Background: Tobacco smoking is a leading preventable cause of morbidity and mortality worldwide; still, the success rate of smoking cessation is low in general. From the viewpoint of public health and clinical care, an objective biomarker of long-term smoking behavior is sought.
Methods and Results: This study assessed DNA methylation as a biomarker of smoking in a hospital setting through a combination of molecular approaches including genetic, DNA methylation and mRNA expression analyses. First, in an epigenome-wide association study involving Japanese individuals with chronic cardiovascular disease (n=94), genome-wide significant smoking association was identified at 2 CpG sites on chromosome 5, with the strongest signal at cg05575921 located in intron 3 of the aryl-hydrocarbon receptor repressor (AHRR) gene. Highly significant (P<1×10−27) smoking–cg05575921 association was validated in 2 additional panels (n=339 and n=300). For the relationship of cg05575921 methylation extent with time after smoking cessation and cumulative cigarette consumption among former smokers, smoking-related hypomethylation was found to remain for ≥20 years after smoking cessation and to be affected by multiple factors, such as cis-interaction of genetic variation. There was a significant inverse correlation (P=0.0005) between cg05575921 methylation extent and AHRR mRNA expression.
Conclusions: The present study results support that reversion of AHRR hypomethylation can be a quantifiable biomarker for progress in and observance of smoking cessation, although some methodological points need to be considered.
(Circ J 2022; 86: 1982–1989)2
| Prognostic Effects of Changes in Right Ventricular Fractional Area Change in Patients With Heart Failure Yukiko Sugawara, Akiomi Yoshihisa, Ryohei Takeishi, Himika Ohara, Fumiya Anzai, Yu Hotsuki, Koichiro Watanabe, Yu Sato, Satoshi Abe, Tomofumi Misaka, Takamasa Sato, Masayoshi Oikawa, Atsushi Kobayashi, Kazuhiko Nakazato, Yasuchika Takeishi (Department of Cardiovascular Medicine, Fukushima Medical University, Fukushima (Y. Sugawara, A.Y., R.T., H.O, F.A., Y.H., K.W., Y. Sato, S.A., T.M., T.S., M.O., A.K., K.N., Y.T.); Department of Clinical Laboratory Sciences, Fukushima Medical University School of Health Science, Fukushima (A.Y.), Japan) |
 |

Changes in right ventricular fractional area change at discharge and post discharge in the outpatient setting. RVFAC, right ventricular fractional area change.

All-cause mortality stratified by baseline RVFAC and its changes from the (A) first to the (B) second examination. RVFAC, right ventricular fractional area change.
Background: It is still unclear whether changes in right ventricular function are associated with prognosis in heart failure (HF) patients. This study aimed to examine the prognostic effect of changes in right ventricular fractional area change (RVFAC).
Methods and Results: This study enrolled 480 hospitalized patients with decompensated HF, and measured RVFAC with echocardiography at discharge (first examination) and post-discharge in the outpatient setting (second examination). RVFAC was divided into 3 categories: >35% in 314 patients, 25–35% in 108 patients, and <25% in 58 patients. Next, based on changes in RVFAC from the first to the second examination, the patients were further classed into 4 groups: (1) Preserved/Unchanged (preserved and unchanged RVFAC, n=235); (2) Reduced/Improved (improved RVFAC in at least 1 category, n=106); (3) Reduced/Unchanged (reduced and unchanged RVFAC, n=47); and (4) Preserved or Reduced/Worsened (deteriorated RVAFC in at least 1 category, n=92). Multivariate logistic regression analysis revealed that chronic kidney disease and anemia were the predictors of the preserved or reduced/worsened RVFAC. In the Kaplan-Meier analysis, changes in RVFAC were associated with the cardiac event rate and all-cause mortality. In the multivariable Cox proportional hazard analysis, the preserved or reduced/worsened RVFAC was an independent predictor of cardiac events and all-cause mortality.
Conclusions: Changes in RVFAC were associated with post-discharge prognosis in hospitalized heart failure patients.
(Circ J 2022; 86: 1092–1101)3
| Myocardial T-Lymphocytes as a Prognostic Risk-Stratifying Marker of Dilated Cardiomyopathy ― Results of the Multicenter Registry to Investigate Inflammatory Cell Infiltration in Dilated Cardiomyopathy in Tissues of Endomyocardial Biopsy (INDICATE Study) ― Keiko Ohta-Ogo, Yasuo Sugano, Soshiro Ogata, Takafumi Nakayama, Takahiro Komori, Kazuo Eguchi, Kaoru Dohi, Tetsuro Yokokawa, Hiromitsu Kanamori, Shigeyuki Nishimura, Kazufumi Nakamura, Yoshihiko Ikeda, Kunihiro Nishimura, Genzou Takemura, Toshihisa Anzai, Michiaki Hiroe, Kinta Hatakeyama, Hatsue Ishibashi-Ueda, Kyoko Imanaka-Yoshida (Department of Pathology (K.O.-O., Y.I., K.H., H.I.-U.), Department of Preventive Medicine and Epidemiology (S.O., K. Nishimura), Department of Cardiovascular Medicine (T.A.), National Cerebral and Cardiovascular Center, Suita; Department of Cardiology, Keiyu Hospital, Yokohama (Y.S.); Department of Cardiology, Nagoya City University Graduate School of Medical Sciences, Nagoya (T.N.); Department of Cardiovascular Medicine, Jichi Medical University School of Medicine, Shimotsuke (T.K.); Department of General Internal Medicine, Saitama Red Cross Hospital, Saitama (K.E.); Department of Cardiology and Nephrology (K.D.), Department of Pathology and Matrix Biology (K.I.-Y.), Mie University Graduate School of Medicine, Tsu; Department of Cardiovascular Medicine, Fukushima Medical University, Fukushima (T.Y.); Department of Cardiology, Gifu University Graduate School of Medicine, Gifu (H.K.); Department of Cardiology, Saitama Medical University International Medical Center, Hidaka (S.N.); Department of Cardiovascular Medicine, Okayama University Graduate School of Medicine, Dentistry and Pharmaceuticals, Okayama (K. Nakamura); Department of Internal Medicine, Asahi University School of Dentistry, Gifu (G.T.); Department of Cardiovascular Medicine, Hokkaido University Graduate School of Medicine, Sapporo (T.A.); and Department of Cardiology, National Center for Global Health and Medicine, Tokyo (M.H.), Japan) |
 |

Variations of histological and immunohistological findings in dilated cardiomyopathy patients. Case 1 (CD3+: 4/mm2, CD68+: 32/mm2): no inflammation as per ESC-defined criteria. Case 2 (CD3+: 13/mm2, CD68+: 30/mm2) and 3 (CD3+: 124/mm2, CD68+: 58/mm2): inflammation positive. Case 2 represents histologically equivocal cases with inflammation identified by immunohistochemistry. Case 3 represents rare cases of histologically defined myocarditis with adjacent myocyte necrosis. From top to bottom: Hematoxylin and eosin (HE) stain, immunohistochemistry for CD3 and CD68.

Kaplan-Meier estimates of cardiac death or left ventricular assist device (LVAD) implantation-free survival in 261 dilated cardiomyopathy patients stratified by the 3 CD3+ count categories (log-rank test). Long-term prognosis was incrementally associated with CD3+ count. The CD3+-High ≥24/mm2 group had the worst outcome (P for trend; P<0.0001).
Background: Dilated cardiomyopathy (DCM) associated with inflammation is diagnosed by endomyocardial biopsy; patients with this have a poorer prognosis than patients without inflammation. To date, standard diagnostic criteria have not been established.
Methods and Results: This study analyzed clinical records and endomyocardial biopsy samples of 261 patients with DCM (201 males, median left ventricular ejection fraction; 28%) from 8 institutions in a multicenter retrospective study. Based on the European Society of Cardiology criteria and CD3 (T-lymphocytes) and CD68 (macrophages) immunohistochemistry, 48% of patients were categorized as having inflammatory DCM. For risk-stratification, we divided patients into 3 groups using Akaike Information Criterion/log-rank tests, which can determine multiple cut-off points: CD3+-Low, <13/mm2 (n=178, 68%); CD3+-Moderate, 13–24/mm2 (n=58, 22%); and CD3+-High, ≥24/mm2 (n=25, 10%). The survival curves for cardiac death or left ventricular assist device implantation differed significantly among the 3 groups (10-year survival rates: CD3+-Low: 83.4%; CD3+-Moderate: 68.4%; CD3+-High: 21.1%; Log-rank P<0.001). Multivariate Cox analysis revealed CD3+ count as a potent independent predictive factor for survival (fully adjusted hazard ratio: CD3+-High: 5.70, P<0.001; CD3+-Moderate: 2.64, P<0.01). CD3+-High was also associated with poor left ventricular functional and morphological recovery at short-term follow up.
Conclusions: Myocardial CD3+ T-lymphocyte infiltration has a significant prognostic impact in DCM and a 3-tiered risk-stratification model could be helpful to refine patient categorization.
(Circ J 2022; 86: 427–437)4
| Concomitant Mitral Regurgitation in Severe Aortic Stenosis ― A Report From the CURRENT AS Registry ― Ryosuke Murai*, Yuichi Kawase, Tomohiko Taniguchi, Takeshi Morimoto, Kazushige Kadota, Masanobu Ohya, Takenobu Shimada, Takeshi Maruo, Yasushi Fuku, Tatsuhiko Komiya, Kenji Ando, Michiya Hanyu, Norio Kanamori, Takeshi Aoyama, Koichiro Murata, Tomoya Onodera, Fumio Yamazaki, Takeshi Kitai, Yutaka Furukawa, Tadaaki Koyama, Makoto Miyake, Chisato Izumi, Yoshihisa Nakagawa, Kazuo Yamanaka, Hirokazu Mitsuoka, Manabu Shirotani, Masashi Kato, Shinji Miki, Hiroyuki Nakajima, Yutaka Hirano, Shunichi Miyazaki, Toshihiko Saga, Sachiko Sugioka, Shintaro Matsuda, Mitsuo Matsuda, Tatsuya Ogawa, Kazuya Nagao, Tsukasa Inada, Shogo Nakayama, Hiroshi Mabuchi, Yasuyo Takeuchi, Hiroki Sakamoto, Genichi Sakaguchi, Keiichiro Yamane, Hiroshi Eizawa, Mamoru Toyofuku, Takashi Tamura, Atsushi Iwakura, Mitsuru Ishii, Masaharu Akao, Kotaro Shiraga, Eri Minamino-Muta, Takao Kato, Moriaki Inoko, Koji Ueyama, Tomoyuki Ikeda, Yoshihiro Himura, Akihiro Komasa, Katsuhisa Ishii, Kozo Hotta, Yukihito Sato, Keiichi Fujiwara, Yoshihiro Kato, Ichiro Kouchi, Yasutaka Inuzuka, Shigeru Ikeguchi, Senri Miwa, Chiyo Maeda, Eiji Shinoda, Junichiro Nishizawa, Toshikazu Jinnai, Nobuya Higashitani, Mitsuru Kitano, Yuko Morikami, Shouji Kitaguchi, Kenji Minatoya, Takeshi Kimura on behalf of the CURRENT AS Registry Investigators (Department of Cardiology (R.M., Y. Kawase, K.K., M.O., T. Shimada, T. Maruo, Y. Fuku), Department of Cardiovascular Surgery (T. Komiya), Kurashiki Central Hospital, Kurashiki; Division of Cardiology (T.T., K.A.), Department of Cardiovascular Surgery (M.H.), Kokura Memorial Hospital, Kitakyushu; Department of Clinical Epidemiology, Hyogo College of Medicine, Nishinomiya (T. Morimoto); Division of Cardiology, Shimada Municipal Hospital, Shimada (N.K., T.A.); Department of Cardiology (K. Murata, T. Onodera), Department of Cardiovascular Surgery (F.Y.), Shizuoka City Shizuoka Hospital, Shizuoka; Department of Cardiovascular Medicine (T. Kitai, Y. Furukawa), Department of Cardiovascular Surgery (T. Koyama), Kobe City Medical Center General Hospital, Kobe; Department of Cardiology (M. Miyake), Department of Cardiovascular Surgery (K. Yamanaka), Tenri Hospital, Tenri; Division of Heart Failure, National Cerebral and Cardiovascular Center, Suita (C.I.); Department of Cardiovascular Medicine, Shiga University of Medical Science, Otsu (Y.N.); Division of Cardiology, Kinki University Faculty of Medicine, Ikoma (H. Mitsuoka, M.S.); Department of Cardiology (M. Kato, S. Miki), Department of Cardiovascular Surgery (H.N.), Mitsubishi Kyoto Hospital, Kyoto; Department of Cardiology (Y. Hirano, S. Miyazaki), Department of Cardiovascular Surgery (T. Saga), Kindai University Hospital, Osakasayama; Department of Cardiology (S.S., S. Matsuda, M. Matsuda), Department of Cardiovascular Surgery (T. Ogawa), Kishiwada City Hospital, Kishiwada; Department of Cardiovascular Center (K.N., T. Inada), Department of Cardiovascular Surgery (S.N.), Osaka Red Cross Hospital, Osaka; Department of Cardiology, Koto Memorial Hospital, Higashiomi (H. Mabuchi); Department of Cardiology (Y.T., H.S.), Department of Cardiovascular Surgery (G.S.), Shizuoka General Hospital, Shizuoka; Department of Cardiology, Nishikobe Medical Center, Kobe (K. Yamane, H.E.); Department of Cardiology (M.T., T. Tamura), Department of Cardiovascular Surgery (A.I.), Japanese Red Cross Wakayama Medical Center, Wakayama; Department of Cardiology (M. Ishii, M.A.), Department of Cardiovascular Surgery (K.S.), National Hospital Organization Kyoto Medical Center, Kyoto; Department of Cardiovascular Medicine, Kyoto University Graduate School of Medicine, Kyoto (E.M.-M., T. Kato, A.K., T. Kimura); Cardiovascular Center (M. Inoko, K.U.), Department of Cardiovascular Surgery, Cardiovascular Center (T. Ikeda), The Tazuke Kofukai Medical Research Institute, Kitano Hospital, Osaka; Department of Cardiology, Hikone Municipal Hospital, Hikone (Y. Himura); Department of Cardiology, Kansai Electric Power Hospital, Osaka (K.I.); Department of Cardiology (K.H., Y.S.), Department of Cardiovascular Surgery (K.F.), Hyogo Prefectural Amagasaki General Medical Center, Amagasaki; Department of Cardiology, Saiseikai Noe Hospital, Osaka (Y. Kato, I.K.); Department of Cardiology (Y.I., S.I.), Department of Cardiovascular Surgery (S. Miwa), Shiga General Hospital, Moriyama; Department of Cardiology (C.M., E.S.), Department of Cardiovascular Surgery (J.N.), Hamamatsu Rosai Hospital, Hamamatsu; Department of Cardiology (T.J., N.H.), Department of Cardiovascular Surgery (M. Kitano), Japanese Red Cross Otsu Hospital, Otsu; Department of Cardiology, Hirakata Kohsai Hospital, Hirakata (Y.M., S.K.); and Department of Cardiovascular Surgery, Kyoto University Graduate School of Medicine, Kyoto (K. Minatoya), Japan) *Current affiliation: Department of Cardiovascular Medicine, Kobe City Medical Center General Hospital, Hyogo |
 |

Study flowchart. AS, aortic stenosis; AVR, aortic valve replacement; MR, mitral regurgitation; SAVR, surgical AVR.

Kaplan-Meier curves for the composite endpoint of aortic valve-related death and heart failure hospitalization (primary outcome measure) in the initial AVR stratum (Left), and in the conservative stratum (Right). We estimated the adjusted hazard ratio for the primary outcome measure by multivariable Cox proportional hazard model. AVR, aortic valve replacement; MR, mitral regurgitation.
Background: The clinical significance of concomitant mitral regurgitation (MR) has not been well addressed in patients with severe aortic stenosis (AS).
Methods and Results: We analyzed 3,815 patients from a retrospective multicenter registry of severe AS in Japan (CURRENT AS registry). We compared the clinical outcomes between patients with moderate/severe MR and with none/mild MR according to the initial treatment strategy (initial aortic valve replacement [AVR] or conservative strategy). The primary outcome measure was a composite of aortic valve-related death or heart failure hospitalization. At baseline, moderate/severe MR was present in 227/1,197 (19%) patients with initial AVR strategy and in 536/2,618 (20%) patients with a conservative strategy. The crude cumulative 5-year incidence of the primary outcome measure was significantly higher in patients with moderate/severe MR than in those with none/mild MR, regardless of the initial treatment strategy (25.2% vs. 14.4%, P<0.001 in the initial AVR strategy, and 63.3% vs. 40.7%, P<0.001 in the conservative strategy). After adjusting confounders, moderate/severe MR was not independently associated with higher risk for the primary outcome measure in the initial AVR strategy (hazard ratio [HR] 1.11, 95% confidence interval [CI] 0.67–1.83, P=0.69), and in the conservative strategy (HR 1.13, 95% CI 0.93–1.37, P=0.22).
Conclusions: Concomitant moderate/severe MR was not independently associated with higher risk for the primary outcome measure regardless of the initial treatment strategy.
(Circ J 2022; 86: 319–329)5
| Artificially Created Reentry Circuit by Laser Irradiation Causes Atrial Tachycardia to Persist in Murine Atria Shunpei Horii, Hirotaka Yada, Kei Ito, Kazuhiro Tsujita, Ayumu Osaki, Kazuki Kagami, Atsushi Sato, Toyokazu Kimura, Risako Yasuda, Takumi Toya, Takayuki Namba, Yuji Nagatomo, Yasuo Ido, Koji Miyazaki, Nobuyuki Masaki, Miya Ishihara, Bonpei Takase, Takeshi Adachi (Department of Cardiology (S.H., K.I., A.O., K.K., A.S., T.K., R.Y., T.T., T.N., Y.N., Y.I., T.A.), Department of Medical Engineering (K.T., M.I.), Department of Intensive Care Medicine (N.M., B.T.), National Defense Medical College, Tokorozawa; Department of Cardiology, International University of Health and Welfare Mita Hospital, Tokyo (H.Y.); and Department of Comprehensive Internal Medicine, Tokai University Hachioji Hospital, Hachioji (K.M.), Japan) |

(A) Creation of an artificial arrhythmogenic substrate by laser irradiation. We irradiated the anterior part of the atrium by laser under an antegrade perfusion. The irradiation range was adjusted to a circle of ~1.2 mm in diameter by defocusing. The right-hand side lower panel shows a laser beam profile on a mouse atriumequivalent position (wedge-shaped dent irradiation pattern). (B) Representative electrocardiogram of long-lasting atrial arrhythmia (AA; duration >30 min) in a mouse heart under an antegrade perfusion method. (C) Long-lasting AA was not induced (0%) in the control (non-laser) and circular-shape laser-irradiated groups. Longlasting AA was greatly induced in 6 mice (75%) in the wedge-shaped dent laser-irradiated group. Fisher’s exact test was used for statistical analysis. ***P<0.001. (D) Dot plot of AA duration in each group; the duration of AA was significantly longer in the wedge-shaped dent laser-irradiated group than in other groups. One-way ANOVA was used for statistical analysis. ***P<0.001.

(A) Optical phase map and phase singularity space-time plots of short-duration spiral atrial fibrillation (AF) in wild-type (WT) mice (analysis of the left atrium (LA) in Figure 4). The singularity of the phase map was plotted with white spots in the upper panel. The singularity of the phase map was plotted with black dots in the middle and lower panels. It showed meandering with time. (B) Optical phase map and phase singularity space-time plots of long-lasting atrial arrhythmia (AA) post wedge-shaped dent laser irradiation in the right atrium (RA). The phase singularity drew almost the same trajectory rotating counterclockwise around the regularly laser irradiated area (Supplementary Movie).
Background: There is a gradual progression from paroxysmal to persistent atrial fibrillation (AF) in humans. To elucidate the mechanism involved, the creation of an artificial atrial substrate to persist AF in mice was attempted.
Methods and Results: This study used wild type (WT) mice, but it is difficult to induce AF in them. A novel antegrade perfusion method from the left ventricle (LV) to enlarge both atria for artificial atrial modification was proposed in this study. Short duration AF was induced by burst pacing under this method. Optical mapping analysis revealed non-sustained focal type and meandering spiral reentrants after short duration AF. A tiny artificial substrate (∼1.2 mm in diameter) was added in by laser irradiation to create a critical atrial arrhythmogenic substrate. Burst pacing was performed in a non-laser group (n=8), a circular-shape laser group (n=8), and a wedge-shaped dent laser group (n=8). We defined AF and atrial tachycardia (AT) as atrial arrhythmia (AA). Long-lasting AA was defined as lasting for ≥30 min. Long-lasting AA was observed in 0/8, 0/8, and 6/8 (75%) mice in each group. Optical mapping analysis revealed that the mechanism was AT with a stationary rotor around the irradiated margin.
Conclusions: Regrettably, this study failed to reproduce persistent AF, but succeeded in creating an arrhythmic substrate that causes sustained AT in WT mice.
(Circ J 2022; 86: 2029–2039)6
| Sodium Glucose Co-Transporter 2 Inhibitors Improve Renal Congestion and Left Ventricular Fibrosis in Rats With Hypertensive Heart Failure Tomofumi Nakatsukasa, Tomoko Ishizu, Masumi Ouchi, Nobuyuki Murakoshi, Kimi Sato, Masayoshi Yamamoto, Kunio Kawanishi, Yoshihiro Seo, Masaki Ieda (Department of Cardiology, Graduate School of Comprehensive Human Sciences, University of Tsukuba, Tsukuba (T.N.); Department of Cardiology (T.I., N.M., K.S., M.Y., M.I.), Department of Experimental Pathology (K.K.), Faculty of Medicine, University of Tsukuba, Tsukuba; Graduate School of Comprehensive Human Sciences, University of Tsukuba, Tsukuba (M.O.); and Department of Cardiology, Faculty of Medicine, Nagoya City University Graduate School of Medical Sciences, Nagoya (Y.S.), Japan) |
 |

Central volume pressure and renal medullary pressure. (A) Shows the correlation between CVP and RMP. (B) CVP and RMP value bars are the mean±standard error in each group. CVP, central venous pressure; RMP, renal medullary pressure. *P<0.05, **P<0.01, ***P<0.001 vs. the LS group; #P<0.05, ##P<0.01 vs. the HS group. Differences between the control or untreated group and the treatment groups were evaluated using Dunnett’s test.
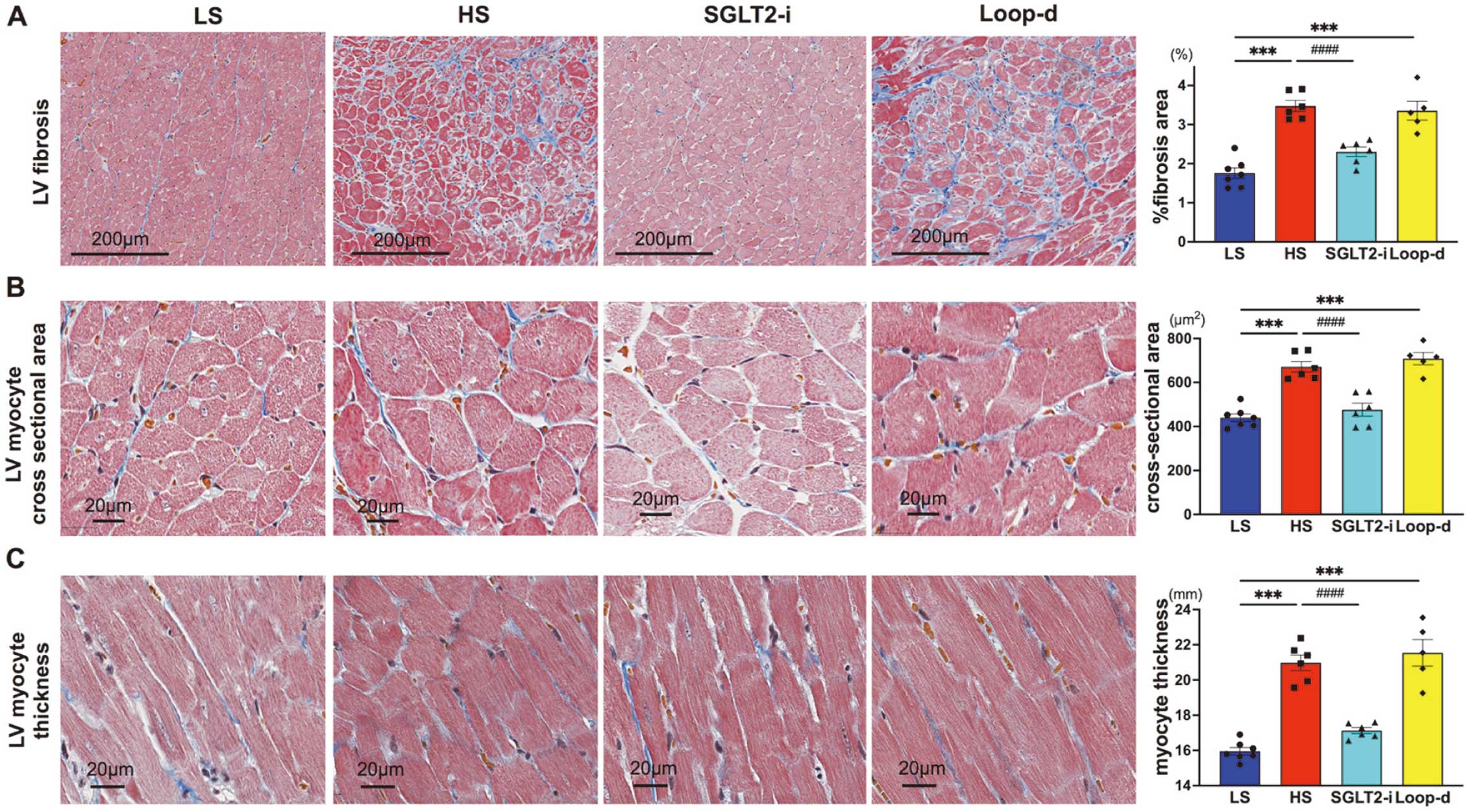
LV fibrosis and hypertrophy. (A–C) Left panels show representative images of LV fibrosis (A), LV myocyte cross-sectional area (B), and myocyte thickness (C). Figures on the right show comparisons among the 4 groups, corresponding to those shown in the left panels. ***P<0.001 vs. the LS group; ###P<0.001 vs. the HS group. Differences between the control or untreated group and the treatment groups were evaluated using Dunnett’s test (A–C).
Background: Elevated central venous pressure (CVP) in heart failure causes renal congestion, which deteriorates prognosis. Sodium glucose co-transporter 2 inhibitor (SGLT2-i) improves kidney function and heart failure prognosis; however, it is unknown whether they affect renal congestion. This study investigated the effect of SGLT2-i on the kidney and left ventricle using model rats with hypertensive heart failure.
Methods and Results: Eight rats were fed a 0.3% low-salt diet (n=7), and 24 rats were fed an 8% high-salt diet, and they were divided into 3 groups of untreated (n=6), SGLT2-i (canagliflozin; n=6), and loop diuretic (furosemide; n=5) groups after 11 weeks of age. At 18 weeks of age, CVP and renal intramedullary pressure (RMP) were monitored directly by catheterization. We performed contrast-enhanced ultrasonography to evaluate intrarenal perfusion. In all high-salt fed groups, systolic blood pressure was elevated (P=0.287). The left ventricular ejection fraction did not differ among high-salt groups. Although CVP decreased in both the furosemide (P=0.032) and the canagliflozin groups (P=0.030), RMP reduction (P=0.003) and preserved renal medulla perfusion were only observed in the canagliflozin group (P=0.001). Histological analysis showed less cast formation in the intrarenal tubule (P=0.032), left ventricle fibrosis (P<0.001), and myocyte thickness (P<0.001) in the canagliflozin group than in the control group.
Conclusions: These results suggest that SGLT2-i causes renal decongestion and prevents left ventricular hypertrophy, fibrosis, and dysfunction.
(Circ J 2022; 86: 1365–1375)7
| Long-Term Clinical Outcomes and Its Predictors Between the 1- and 2-Stent Strategy in Coronary Bifurcation Lesions ― A Baseline Clinical and Lesion Characteristic-Matched Analysis ― Albert Youngwoo Jang, Minsu Kim, Pyung Chun Oh, Soon Yong Suh, Kyounghoon Lee, Woong Chol Kang, Ki Hong Choi, Young Bin Song, Hyeon-Cheol Gwon, Hyo-Soo Kim, Woo Jung Chun, Seung-Ho Hur, Seung-Woon Rha, In-Ho Chae, Jin-Ok Jeong, Jung Ho Heo, Junghan Yoon, Soon Jun Hong, Jong-Seon Park, Myeong-Ki Hong, Joon-Hyung Doh, Kwang Soo Cha, Doo-Il Kim, Sang Yeub Lee, Kiyuk Chang, Byung-Hee Hwang, So-Yeon Choi, Myung Ho Jeong, Chang-Wook Nam, Bon-Kwon Koo, Seung Hwan Han (Division of Cardiology, Department of Internal Medicine, Gachon University Gil Medical Center, Incheon (A.Y.J., M.K., P.C.O., S.Y.S., K.L., W.C.K., S.H.H.); Division of Cardiology, Department of Internal Medicine, Heart Vascular Stroke Institute, Samsung Medical Center, Sungkyunkwan University School of Medicine, Seoul (K.H.C., Y.B.S., H.-C.G.); Department of Internal Medicine and Cardiovascular Center, Seoul National University Hospital, Seoul (H.-S.K., B.-K.K.); Division of Cardiology, Department of Internal Medicine, Samsung Changwon Hospital, Sungkyunkwan University School of Medicine, Changwon (W.J.C.); Division of Cardiology, Department of Internal Medicine, Keimyung University Dongsan Medical Center, Daegu (S.-H.H.); Division of Cardiology, Department of Internal Medicine, Korea University Guro Hospital, Seoul (S.-W.R.); Division of Cardiology, Department of Internal Medicine, Seoul National University Bundang Hospital, Seongnam, Gyeonggi-do (I.-H.C.); Division of Cardiology, Department of Medicine, Chungnam National University Hospital, Daejeon (J.-O.J.); Division of Cardiology, Department of Internal Medicine, Kosin University Gospel Hospital, Kosin University College of Medicine, Busan (J.H.H.); Division of Cardiology, Department of Internal Medicine, Wonju Severance Christian Hospital, Yonsei University Wonju College of Medicine, Wonju (J.Y.); Division of Cardiology, Department of Internal Medicine, Korea University Anam Hospital, Seoul (S.J.H.); Division of Cardiology, Department of Internal Medicine, Yeungnam University Medical Center, Daegu (J.-S.P.); Division of Cardiology, Department of Internal Medicine, Severance Cardiovascular Hospital, Yonsei University College of Medicine, Seoul (M.-K.H.); Division of Cardiology, Department of Internal Medicine, Inje University Ilsan Paik Hospital, Goyang (J.-H.D.); Division of Cardiology, Department of Internal Medicine, Pusan National University Hospital, Busan (K.S.C.); Division of Cardiology, Department of Internal Medicine, Inje University Haeundae Paik Hospital, Goyang (D.-I.K.); Division of Cardiology, Department of Internal Medicine, Chungbuk National University College of Medicine, Cheongju (S.Y.L.); Division of Cardiology, Department of Internal Medicine, Seoul St. Mary’s Hospital, The Catholic University of Korea, Seoul (K.C.); Division of Cardiology, Department of Internal Medicine, St. Paul’s Hospital, The Catholic University of Korea, Seoul (B.-H.H.); Division of Cardiology, Department of Internal Medicine, Ajou University Hospital, Suwon (S.-Y.C.); Division of Cardiology, Department of Internal Medicine, Chonnam National University Hospital, Gwangju (M.H.J.); and Division of Cardiology, Department of Internal Medicine, Keimyung University Dongsan Medical Center, Daegoo (C.-W.N.), Republic of Korea) |
 |

Comparison of 5-year clinical outcomes between the 1-stent and 2-stent strategies in the post-matched population. Kaplan-Meier curves comparing the risk of TLF (A), TLR (B), cardiac death (C), and target-vessel MI (D), cardiac death+TVMI (E), and cardiac death+TVMI+ST (F). ST, stent thrombosis; TLF, target lesion failure; TLR, target lesion revascularization; TVMI, target vessel myocardial infarction.

Outcomes and predictors between the 1- and 2-stent strategy in matched coronary bifurcation lesions. ACS, acute coronary syndrome; CAC, coronary artery calcification; CKD, chronic kidney disease; DES, drug-eluting stent; DS, diameter stenosis; HTN, hypertension; LDL-C, low-density lipoprotein cholesterol; LM, left main; LVEF, left ventricular ejection fraction; MI, myocardial infarction; MV, main vessel; MVD, multi-vessel disease; NC, non-compliant; PCI, percutaneous coronary intervention; PSM, propensity score matching; RD, reference diameter; SB, side branch.
Background: Differences in the impact of the 1- or 2-stent strategy in similar coronary bifurcation lesion conditions are not well understood. This study investigated the clinical outcomes and its predictors between 1 or 2 stents in propensity score-matched (PSM) complex bifurcation lesions.
Methods and Results: We analyzed the data of patients with bifurcation lesions, obtained from a multicenter registry of 2,648 patients (median follow up, 53 months). The patients were treated by second generation drug-eluting stents (DESs). The primary outcome was target lesion failure (TLF), composite of cardiac death, target vessel myocardial infarction (TVMI), and ischemia-driven target lesion revascularization (TLR). PSM was performed to balance baseline clinical and angiographic discrepancies between 1 and 2 stents. After PSM (N=333 from each group), the 2-stent group had more TLRs (hazard ratio [HR] 3.14, 95% confidence interval [CI] 1.42–6.97, P=0.005) and fewer hard endpoints (composite of cardiac death and TVMI; HR 0.44, 95% CI 0.19–1.01, P=0.054), which resulted in a similar TLF rate (HR 1.40, 95% CI 0.83–2.37, P=0.209) compared to the 1-stent group. Compared with 1-stent, the 2-stent technique was more frequently associated with less TLF in the presence of main vessel (pinteraction=0.008) and side branch calcification (pinteraction=0.010).
Conclusions: The 2-stent strategy should be considered to reduce hard clinical endpoints in complex bifurcation lesions, particularly those with calcifications.
Awards will be presented to the authors of the 7 papers above during the 87th Annual Scientific Meeting of the Japanese Circulation Society. We look forward to receiving manuscripts with high scientific impact for publication in Circulation Journal in 2023.
Toshihisa Anzai, MD, PhD
Editor-in-Chief
Circulation Journal