2013 Volume 61 Issue 6 Pages 604-610
2013 Volume 61 Issue 6 Pages 604-610
Three phenyl derivatives of butyrate, 2-phenylbutyrate (2-PB), 3-phenylbutyrate (3-PB) and 4-phenylbutyrate (4-PB), were evaluated in terms of their antibacterial and cytotoxic activities. Our results indicated that PBs demonstrated specific inhibitory activity against Helicobacter pylori and Escherichia coli but did not influence the growth of Bifidobacterium bifidium and Lactobacillus reuteri. PBs also exhibited synergistic effects on H. pylori ATCC 43504 especially at pH 5.5. In the protein expression profiles in H. pylori treated by phenylbutyrates, we also found that three protein spots identified as oxidative stress-related proteins were significantly up-regulated, confirming the response of H. pylori when exposed to PBs. Due to their antibacterial activities and low or slight cytotoxicities, PBs are potential candidates for the treatment of H. pylori infection. This is the first study to discover the antibiotic effects of 2-PB, 3-PB and 4-PB (Buphenyl).
Helicobacter pylori is a spiral-shaped, Gram-negative rod that has developed sophisticated strategies to colonize the epithelial cells of the stomach. H. pylori colonies in the stomach synthesize virulence factors such as VacA and CagA. These are toxic products, and as a result they can often stimulate irregular secretion of gastric acid and cause inflammation of gastric mucosa.1) It also has been proven that H. pylori infection has a very close relationship with many diseases, including chronic atrophic gastritis, gastric or duodenal ulcer, postgastric cancer resection, mucosa-associated lymphoid tissue lymphomas and first degree relatives of gastric cancer patients. In many clinical cases, previous diseases have obviously improved when H. pylori infection was eradicated.2)
Many antibiotics including metronidazole, tetracycline, amoxicillin and clarithromycin are generally used medicate patients with H. pylori-associated diseases for the inhibition of growth.2,3) The resistance of H. pylori to the antibiotics used in current regimens is a major reason for the failure to eradicate H. pylori infection, and the failure rates of H. pylori therapy are rising steadily due to increasing antibiotic resistance rates. Antibiotic resistance increased from 12.9 to 31% during 1999–2003 in north America, and >70% of clinic isolates of H. pylori were found to be resistant to metronidazole in Korea, Bangladesh and Kenya.4) In addition, side effects of antibiotics treatment, which include intestinal disturbances, nausea, vomiting and abdominal pain, are often found in clinical cases and are problematic for patients. For these reasons, novel therapeutic agents have been developed as the drugs to improve the curative effect in H. pylori infection.5,6)
Short-chain organic acids such as acetic acid, propionic acid, and butyric acid are widely used as food preservatives. They also appear to be involved in controlling the number of pathogenic enterobacteria in the large intestine.7) Butyrate was found to possess a bacteriostatic effect at pH ≤5.0.8) I showed a better inhibitive effect on the growth of H. pylori than lactic acid and hydrochloric acid, but the effect of butyrate on H. pylori is still not sufficient.
Modification of antimicrobial compounds has been applied to enlarge the antimicrobial spectrum, improve absorption after administration, reduce side effects and acquire activity against pathogens.9) The introduction of phenyl acetamido groups to the cephem nucleus and the derivatives effectively improved the inhibitory effect on H. pylori.3)
4-Phenylbutyrate (4-PB) is a phenyl derivative of butyrate that has been applied in the treatment of urea cycle disorders by oral administration. It has also been confirmed that therapeutic levels can be achieved without significant toxicity to various cells.10) Although it has been suggested that 4-PB could be safely used in clinical trials for specific diseases, its antimicrobial ability has rarely been examined in previous research. In this study, 4-PB and two phenyl derivatives of butyrate, 2-phenylbutyrate (2-PB), 3-phenylbutyrate (3-PB), were selected for examination of their anti-H. pylori activity. Finally, the cellular protein profiles of H. pylori treated with 3 kinds of PB were investigated using proteomic technologies to assess the possible effects of PB treatment on H. pylori.
Butyrate and its phenyl derivatives including butyric acid (Fluka, Switzerland), 2-phenylbutyric acid (Fluka), 3-phenylbutyric acid (Wako Pure Chemical Industries, Ltd., Osaka, Japan), and 4-phenylbutyric acid (Fluka) were the selected compounds examined in terms of their inhibitive effects on H. pylori in this study. The antibiotic metronidazole (Sigma-Aldrich, St. Louis, MO, U.S.A.) was used as the positive control agent for the examination of H. pylori inhibition and synergistic effects with the tested compounds. Solutions of butyrate, 2-PB and 4-PB were prepared by diluting or dissolving in 0.05 m phosphate-buffered saline (PBS). The solutions, after adjustment to pH 7.0 with 2.5 m NaOH, were used as anti-H. pylori reagents.
Bacterial Strains and Culture ConditionsThe bacteria and media used in this study are shown in Table 1. H. pylori No. 238, a clinical isolate, was provided by the National Chung Kung University Hospital (Tainan, Taiwan). Salmonella enterica, Proteus mirabilis and Klebsiella pneumonias were also isolated from patients of National Chung Kung University Hospital. H. pylori ATCC43504 and the other tested strains were purchased from the Bioresource Collection and Research Centre (Shinchu, Taiwan). H. pylori strains were grown on blood agar plates (Becton Dickinson, Cockeysville, MD, U.S.A.) in a microaerophilic environment (5% O2, 10% CO2, 85% N2) at 37°C for 48 h.11)
| Bacterial strain/compound | Medium | Inhibition zone (mm) | ||||
|---|---|---|---|---|---|---|
| 2-PBc) | 3-PBc) | 4-PBc) | Butyratec) | Metronidazoled) | ||
| Helicobacter pylori ATCC43504 | Brucella | 32.7±2.5 | 33.5±2.5 | 34.0±2.6 | 8.0±0a) | 21.0±2.0 |
| Helicobacter pylori No. 238b) | Brucella | 40.0±1.7 | 43.0±1.0 | 41.7±0.6 | 10.0±1.0 | 70.7±2.1 |
| Escherichia coli ATCC25922 | Mueller Hinton II | 32.3±0.6 | 22.5±1.5 | 21.7±1.2 | 8.0±0 | 8.0±0 |
| Klebsiella pneumoniasb) | Mueller Hinton II | 8.0±0 | 8.0±0 | 8.0±0 | 8.0±0 | 8.0±0 |
| Pseudomonas aeruginosa ATCC27853 | Mueller Hinton II | 8.0±0 | 8.0±0 | 8.0±0 | 8.0±0 | 8.0±0 |
| Proteus mirabilisb) | Mueller Hinton II | 8.0±0 | 8.0±0 | 8.0±0 | 8.0±0 | 8.0±0 |
| Salmonella entericab) | Mueller Hinton II | 8.0±0 | 8.0±0 | 8.0±0 | 8.0±0 | 8.0±0 |
| Staphylococcus aureus ATCC29213 | Mueller Hinton II | 8.0±0 | 8.0±0 | 8.0±0 | 8.0±0 | 8.0±0 |
| Bifidobacterium bifidium ATCC14614 | MRS | 8.0±0 | 8.0±0 | 8.0±0 | 8.0±0 | 35.0±0 |
| Lactobacillus reuteri JC71112 | MRS | 8.0±0 | 8.0±0 | 8.0±0 | 8.0±0 | 23.0±0.6 |
a) A disk of 8 mm in diameter was used . A diameter of 8.0±0 mm indicates no inhibition. b) H. pylori No. 238, S. enterica, P. mirabilis and K. pneumonias are clinical strains. c) The dosage of 2-phenylbutyrate (2-PB), 3-phenylbutyrate (3-PB), 4-phenylbutyrate (4-PB) and butyrate used in the test was 4 mg/disc. d) The dosage of metronidazole used in the test was 0.4 mg/disc.
H. pylori was grown in Brucella broth (Difco, Detroit, MI, U.S.A.) and collected by centrifugation (4000×g for 5 min). The pellet was resuspended in Brucella broth, and 1×107 H. pylori cells were spread on Brucella agar containing 7% horse serum. The paper disc containing 0.4 mg of metronidazole or 4 mg of butyrate or its derivatives was placed on the agar. The plates were then incubated at 37°C under microaerobic conditions. The diameter of the inhibition zone in the bacterial lawn was measured after 48 h of incubation. The other bacteria were incubated in the specific media shown in Table 1 and examined according to the description of Jorgensen et al.12)
Susceptibility TestingThe method used for the determination of the minimum bactericidal concentration (MBC) for H. pylori ATCC43504 and H. pylori No. 238 was modified from the protocols proposed by Paraschos et al.13) 0.25 to 16 mg/mL of butyrate and its phenyl derivatives were dissolved in Brucella broth containing 7% horse serum. 107 H. pylori cells were inoculated into the broth and incubated at 37°C with continuous shaking (225 rpm) under microaerophilic conditions. After 24 h, the viability of H. pylori was evaluated by counting the number of colony-forming units (CFUs) in Brucella agar plates containing 7% horse serum after incubation at 37°C for 48 h under microaerophilic conditions. The MBC was defined as the lowest concentration of the compound at which at least 99.9% of the cells contained in the original inoculation were killed.12)
Synergistic Inhibition of Phenylbutyrates Associated with Metronidazole on H. pyloriBrucella agar plates containing 0–0.4 mg/mL of the tested compound were adjusted to pH 6.5, 6.0 and 5.5. Discs containing 200 µg of metronidazole were placed on the plates after 107 cells of H. pylori had been spread. The diameter of the inhibition zone in the bacterial lawn was measured after 48 h of microaerophilic incubation. The effect of phenylbutyrates and metronidazole combination in H. pylori was determined by checkerboard assay and fractional inhibitory concentration (FIC) index evaluation described by Botelho.14) Statistic analysis was performed by Student’s t-test and one-way analysis of variance (ANOVA). Significance was accepted when p<0.01.
Two-Dimensional Electrophoresis and Protein IdentificationH. pylori ATCC43504 was grown in Brucella broth supplemented with 7% horse serum in the presence of 0.1 mg/mL phenyl derivatives of butyrate or the same volume of phosphate buffer saline (PBS). The bacterium was incubated at 37°C for 72 h and harvested by centrifugation (4000×g, 5 min, 4°C). The pellets were resuspended in 10 mm Tris-buffer (pH 7.5) and sonicated, and the lysates were collected by centrifugation (14400×g, 1 h, 4°C). The lysates were dehydrated using a freeze dryer, and washed with ice-cold 100% acetone. The dried sample (250 g) was dissolved in 350 μL of immobilized pH gradient (IPG) rehydration buffer (7 m urea/4% (3-[(3-cholamidopropyl)-dimethylammonio]-1-propane sulfonate) (CHAPS)/2 m thiourea/0.5% pH 4–7 IPG buffer/65m m dithiothreitol). IPG strips (13 cm, pH 3 to 10, Immobiline™ DryStrip, GE Healthcare Bio-Sciences AB, Sweden) were used in the first dimensional separation. Denaturing solution containing the protein sample was used to passively hydrate the IPG strips. The IPG strips were then placed on an IPGphor (GE Healthcare Bio-Sciences AB) for the isoelectric focusing separation procedure. The IPG strips were equilibrated for 20 min in 10 mL of equilibration solution and subsequently separated on polyacrylamide gels for the second dimensional electrophoresis. Electrophoresis was carried out using a Hoefer SE600 Ruby system (GE Healthcare Bio-Sciences AB). The polyacrylamide gel was visualized using a protein silver-staining kit (GE Healthcare Bio-Sciences AB). The differentially-expressed protein spots were excised and subjected to in-gel reduction and trypsin hydrolysis. Mass spectrometry analysis of the peptides was performed on an ESI-Q-TOF mass spectrometer (ABI QSTAR Pulsar i System, Applied Biosystems, CA, U.S.A.). Mass spectrometry data were compared with data in the the NCBInr databases using the Mascot search algorithm.15)
Reverse Transcriptase-Polymerase Chain Reaction (RT-PCR)The bacterial cells were cultured in Brucella broth supplemented with 7% horse serum in the presence of 0.1 mg/mL phenyl derivatives of butyrate or the same volume of PBS and collected by centrifugation (6000×g, 4°C, 10 min). Total RNA was extracted by the TRIzol reagent (Invitrogen, Carlsbad, CA, U.S.A.), dissolved in diethyl pyrocarbonate-treated water. One microgram of RNA samples were used for the detections of transcription of 23s rRNA, 3-dehydroquinate dehydratase, non-heme iron-containing ferritin and inorganic pyrophosphatase genes with the SuperScript III One-Step RT-PCR kit (Invitrogen). PCR primers were designed on the basis of published sequences of H. pylori and given in Table 2.16) The products in every 5 cycles of RT-PCR were collected for the estimation of transcript levels in the extracted RNA samples. Agarose gel electrophoresis with ethidium bromide staining was used to visualize the amplified products.
| Gene | Sequence (5′→3′) | |
|---|---|---|
| 23s rRNA | forward | TGTGTGCTACCCAGCGATGC |
| reverse | GCGTTGAATTGAAGCCCGAG | |
| Non-heme iron-containing ferritin | forward | GCGCATATGTTATCAAAAGACATC |
| reverse | CTCGAGAGATTTCCTGCTTTTAG | |
| 3-Dehydroquinate dehydratase | forward | GACCCAAGGCTTTATGGTATG |
| reverse | CACGCCTCCACAAGCCGCTCC | |
| Inorganic pyrophosphatase | forward | ACGCTGATTCTTTGT GCGTG |
| reverse | TTTCACTACGCTTCCGGCTT | |
For the cytotoxicity assays after butyrate or PB treatment, we conducted 3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyltetrazolium bromide (MTT) assays as described previously.17) BHK-21 cells, purchased from the Bioresource Collection and Research Centre, were grown and counted to 10000 cells in a 96-well microplate. The cells were then treated with various concentrations (0 to 20 mm) of butyrate, 2-PB, 3-PB and 4-PB for 24 h in quadruplicate. The media were decanted, 100 µL of MTT in medium were added to each well, and cells were incubated at 37°C for 1 h. Formazan crystals in the wells were solubilized in 100 µL of dimethyl sulfoxide (DMSO) by shaking at room temperature for 1 h. Absorbance was measured at a wavelength of 570 nm using a microplate reader (SpectraMax Plus384, Molecular Devices, CA, U.S.A.). The MTT values of the treatment groups were normalized to, and expressed as percentages of, the mean MTT values of PBS-treated cells. The experiment was performed in triplicate. The average percentage±standard deviation of viable cells was calculated against the control.
Two H. pylori strains and other bacteria were selected for testing of their susceptibility to butyrate, 2-PB, 3-PB, 4-PB and metronidazole in disc diffusion assays. The average diameters of the inhibition zones are listed in Table 2. Phosphate-buffered saline, as a negative control, did not inhibit the growth of any of the tested bacteria (data not shown). Butyrate exhibited weak inhibition (10 mm) of H. pylori No.238 but did not inhibit H. pylori ATCC43504 and the other selected bacteria during incubation. The phenyl derivatives of butyrate showed a more defined inhibition of H. pylori than butyrate, resulting in inhibitory zones of 32.7 to 43.0 mm. 2-PB, 3-PB and 4-PB also interfered with the growth of Escherichia coli and formed inhibitory lawns of a size of 21.7 mm to 32.3 mm. The growth of Klebsiella pneumonias, Pseudomonas aeruginosa, Pseudomonas mirabilis, Staphylococcus typhi and Staphylococcus aureus and two probiotic strains were not interfered with by the phenyl derivatives of butyrate. H. pylori No. 238 was sensitive to metronidazole (0.4 mg/disc), and metronidazole did not show an obviously suppressive effect on H. pylori ATCC43504. Metronidazole also restricted the growth of Bifidobacterium bifidium and Lactobacillus reuteri. Therefore, the phenyl derivatives of butyrate not only exerted better anti-H. pylori and anti-E. coli activities than butyrate, but probiotic strains were insensitive.
Susceptibility TestingTwo strains of H. pylori were tested in terms of their susceptibility to different concentrations of butyrate and its phenyl derivatives, and the results were as shown in Fig. 1. The anti-H. pylori potency of the phenyl derivatives of butyrate was considerably greater (8- to 62-fold-lower MBCs) than that of butyrate. All the phenylbutyrates displayed a dose-dependent bactericidal activity against the H. pylori strains. The MBC values of butyrate 2-PB, 3-PB and 4-PB for H. pylori ATCC43504 were 16 mg/mL, 2 mg/mL, 1 mg/mL, and 1 mg/mL, respectively. The MBC values of butyrate 2-PB, 3-PB and 4-PB for H. pylori No. 238 were 16 mg/mL, 0.5 mg/mL, 0.25 mg/mL and 0.25 mg/mL, respectively. The susceptibilities of H. pylori ATCC43504 and to H. pylori No. 238 2-PB did not differ significantly from that of 3-PB and 4-PB.

Different dilutions of the tested compounds were incubated with 108 CFU/mL H. pylori. Cell viability was measured by counting the number of CFU on the plates. The MBCs were determined when a 99.9% decrease was observed as compared with the control.
H. pylori ATCC43504 was used in the testing of the inhibition abilities of 2-PB, 3-PB and 4-PB in agar dilution assays under several acidic environments, and the results are given in Table 3. When a disc containing 100 µg metronidazole was placed on a Brucella plate (pH 7) without 4-PB, H. pylori ATCC43504 was able to grow on the plate and insignificant clear zones (12±0 mm) were formed around the disk. With increasing 4-PB concentration, the diameter of the inhibitory lawn was enlarged. When the concentration reached 0.4 mg/mL, the growth of H. pylori ATCC43504 was halted. The inhibitory effect also increased as the pH level lowered. The bacterium failed to grow on a Brucella plate with a low concentration of 4-PB (0.05 mg/mL) when the pH of the plate was adjusted to pH 5.5. A similar and more powerful effect was observed when 4-PB was replaced by 2-PB or 3-PB. The FIC indices shown in Table 4 were also used to estimate the effect of phenylbutyrates and metronidazole combination for H. pylori ATCC43504. The additive effect of PBs and metronidazole for HP43504 were found when the pH value was higher than 6.5. The synergistic effect (FIC<0.5) of mixture combined with PBs and metronidazole appeared in pH 5.5. These results appear to indicate that the PBs augmented the bactericidal effect of metronidazole and the synergistic effects were increased under acidic conditions.
| Chemical | Concentration (mg/mL) | ||||
|---|---|---|---|---|---|
| 0 | 0.05 | 0.1 | 0.2 | 0.4 | |
| 2-Phenylbutyrate | |||||
| pH 7.0 | 11±0.0 | 11±0.0 | 11±0.1 | 14±0.6* | N.G. |
| pH 6.5 | 10±0.6 | 13±0.6* | 13±0.6* | 14±0.1** | N.G. |
| pH 6.0 | 13±0.6 | 13±0.0 | 15±0.6 | 21±1.2** | N.G. |
| pH 5.5 | 16±0.6 | N.G. | N.G. | N.G. | N.G. |
| 3-Phenylbutyrate | |||||
| pH 7.0 | 11±0.3 | 12±1.0 | 11±0.1 | 14±1.0 | N.G. |
| pH 6.5 | 12±0.0 | 13±0.1** | 13±0.2** | 13±0.1** | N.G. |
| pH 6.0 | 12±0.6 | 13±0.6 | 13±0.0 | 24±1.0** | N.G. |
| pH 5.5 | 16±0.3 | N.G. | N.G. | N.G. | N.G. |
| 4-Phenylbutyrate | |||||
| pH 7.0 | 12±0.6 | 13±1.0 | 14±0.0* | 19±0.0** | N.G. |
| pH 6.5 | 12±0.0 | 12±0.6 | 19±0.6** | N.G. | N.G. |
| pH 6.0 | 16±1.2 | 19±1.7 | 31±1.2** | N.G. | N.G. |
| pH 5.5 | 17±1.2 | N.G. | N.G. | N.G. | N.G. |
1. Each disk contained 100 µg of metronidazole. 2. A disk of 8 mm in diameter was used. 3. N.G. represents No Growth. 4. Asterisks * and ** represent p<0.01 and p<0.001, respectively, between phenylbutyrate treated and untreated groups.
| Chemical | pH 7.0 | pH 6.5 | pH 6.0 | pH 5.5 |
|---|---|---|---|---|
| 2-Phenylbutyrate | 0.87 | 0.87 | 0.75 | 0.38 |
| 3-Phenylbutyrate | 0.87 | 0.87 | 0.75 | 0.38 |
| 4-Phenylbutyrate | 0.87 | 0.75 | 0.63 | 0.38 |
Metronidazole is one of the major antibiotics chosen for the eradication of H. pylori infection.18) A cocktail of antibiotics is used to improve the effect of H. pylori treatment, but unfortunately, some side effects such as vomiting, constipation and diarrhea are difficult to avoid.19) In this study, an inhibitory ability of PBs against H. pylori (Table 2) was observed, and it was also shown that PBs can act synergistically with metronidazole (Table 3) on H. pylori strains.
More importantly, PBs showed selective inhibition of H. pylori and E. coli but not of bifidobacteria and lactobacillus (Table 2). E. coli is generally considered an opportunistic pathogen, but Bifidobacterium and lactobacillus are probiotics. The ratios of E. coli and Bifidobacteria are significantly changed in bowel flora after antibiotics treatment.20) Our results suggested that PBs could be applied in the treatment of H. pylori infection, and the side-effects caused by in the inhibition of probiotics might be diminished, when some antibiotics are altered by PBs.
Changes in the Protein Profiles of H. pylori Exposed to PBsCellular proteins from lysates of H. pylori ATCC43504 cells treated by PBS, butyrate and phenylbutyrates were separated by 2-DE and are shown in Fig. 2. The protein profiles were similar when H. pylori ATCC43504 treated by PBS and butyrate. Three proteins, as shown in Fig. 2, were found to be significantly differently expressed in the protein samples extracted from the H. pylori cells treated with 2-PB, 3-PB or 4-PB. Three protein spots were identified as 3-dehydroquinate dehydratase (DHQase, spot 1), non-heme iron-containing ferritin (Pfr, spot 2) and inorganic pyrophosphatase (Ppa, spot 3) after the identification of mass spectrometry analyses and database-searching.
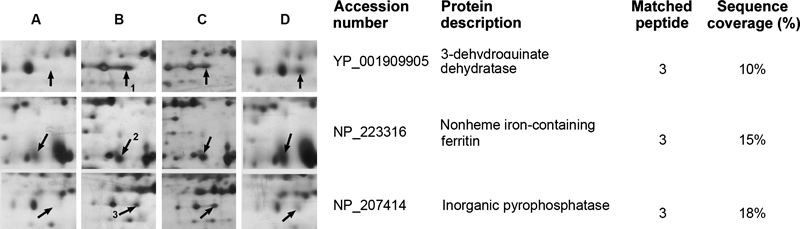
Total proteins from lysates of H. pylori cells were loaded on IPG strips (pH 4–7, 13 cm). After IEF, the IPG strips were rehydrated and separated in 12.5% acrylamide gels. The protein spots marked 1–3 on the silver-stained gels were found to be differentially-expressed. The spots were removed and identified by LC-MS/MS. Mass spectrometry data were compared with data in the NCBInr database using the Mascot program.
DHQase is the key enzyme in the shikimate pathway, which is an essential route in the synthesis of aromatic compounds in bacteria. Oxidative stress caused by oxidants such as superoxide imposes auxotrophies for branched-chain, sulfur-containing, and aromatic amino acids on bacteria. In this study, the levels of DHQase (spot 1) were remarkably increased when H. pylori cells were treated with PBs. The up-regulated expression of 3-dehydroquinate dehydratase gene was able to provide an extra amount for the synthesis of chorismate and aromatic amino acids.21) Among the differentially-expressed proteins, Pfr spots of H. pylori were also found to be consistently up-regulated under PBs-supplemented conditions as compared with phosphate-buffered saline-supplemented conditions. Pfr appears to be a non-specific DNA-binding protein, and has been shown to protect cells from oxidative stress.22) It is believed that the protection of DNA by preventing DNA scission from reactive oxygen species (ROS) generated by the cellular metabolite hydrogen peroxide in the presence of reactive metal ions is afforded by the ferroxidase activity of Pfr.16)
Spot 3 was identified as inorganic pyrophosphatase. The enzyme is widely distributed in many organisms and catalyzes the hydrolysis of one molecule of pyrophosphate into two phosphate ions. The enzyme generally plays a critical role in lipid metabolism. It catalyzes the hydrolysis reaction in the early steps of fatty acid degradation, and provides the driving force for the activation of fatty acids destined for oxidation.23,24) The increased expression levels of Ppa might be essential for the metabolism of PBs and suggest that PBs might be recognized as a fatty acids and metabolized through the pathway of fatty oxidation.
Oxidative stress- and fatty acid utilization-related proteins (Fig. 2) suggest the role of PBs in H. pylori inhibition. Weak organic acids including benzoic acid, propionic acid, and butyric acid could enhance the detrimental effects of endogenous reactive oxygen species production from another chemical that generates oxidative stress by redox cycling, and these organic acids could be used as pro-oxidants for the inhibition of microbes and food preservation.25) This supposition is able to reasonably explain the inhibitory action of organic acids fermented by bacteria against H. pylori.26) The effects of PBs are more significant than that of butyrate because the phenyl group of PBs contributes to a higher lipophilicity and enables PBs to permeate the bacterial cell membrane more easily than butyrate. Metronidazole was found to be able to affect the functions of ferredoxin mediated enzymes in anaerobic bacteria like H. pylori.27) Synergistic effect of PBs and metronidazole may be attributed to the PBs could augment the oxidative stress and multiplied the hinder of electron transfer in critical biochemical reaction in bacterial cells treated by metronidazole.
The transcripts of Pfr, DHQase and Ppa genes in H. pylori stimulated by phenylbutyrates were amplified by RT-PCR and shown in Fig. 3. The mRNA of DHQase gene was significantly transcribed when the bacterial cells were exposed to 2-PB, 3-PB and 4-PB. The transcription of Pfr and Ppa genes in H. pylori were manifested in all conditions, however, their mRNA levels were higher when the bacterial cells were exposed to phenylbutyrates in the results of semi-quantitative RT-PCR (Fig .4). In addition, the intensities of Pfr and Ppa spots were much significant higher when the bacterial exposed to phenylbutyrates (Fig. 2). The transcription of Pfr gene is constitutive in H. pylori, but the expression level of Pfr would be up-regulated when the cells were exposed to acid stress.16) Our results also suggested the phenylbutyrates also brought the same reaction like the bacterial cells grown in acidic environment.

Molecular weight markers (lane M) are shown in the left lane of each gel. RT-PCR products were separated in 2% agarose gel and stained with 0.4 mg/mL ethidium bromide.
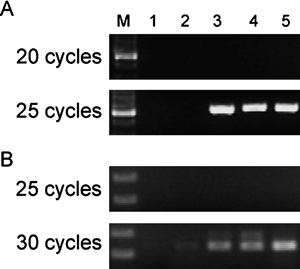
Molecular weight markers (lane M) are shown in the lane M. The numbers of PCR cycle are presented in the left of each gel. RT-PCR products were separated in 2% agarose gel and stained with 0.4 mg/mL ethidium bromide.
The effects of 2-PB, 3-PB and 4-PB on BHK21 cell proliferation after exposing cells to increasing concentrations of the tested compounds for one day are shown in Fig. 5. The results showed that 5 mm 4-PB has reduced the percentage of viable cells to 23.6%, and 49.8% of cells survived when the concentration of 4-PB was 10 mm. 2-PB and 3-PB were less toxic towards BHK-21 cells than 4-PB. When BHK-21 cells were treated with 10 mm 2-PB or 3-PB, 79.8% and 88.7% of the cells survived in the cultures, respectively. A comparison of treated cells to water-treated control cells revealed that these three phenyl derivatives had no significant immediate effect on cell viability when applied to BHK21 cell lines at any concentration (1–20 µg/mL).
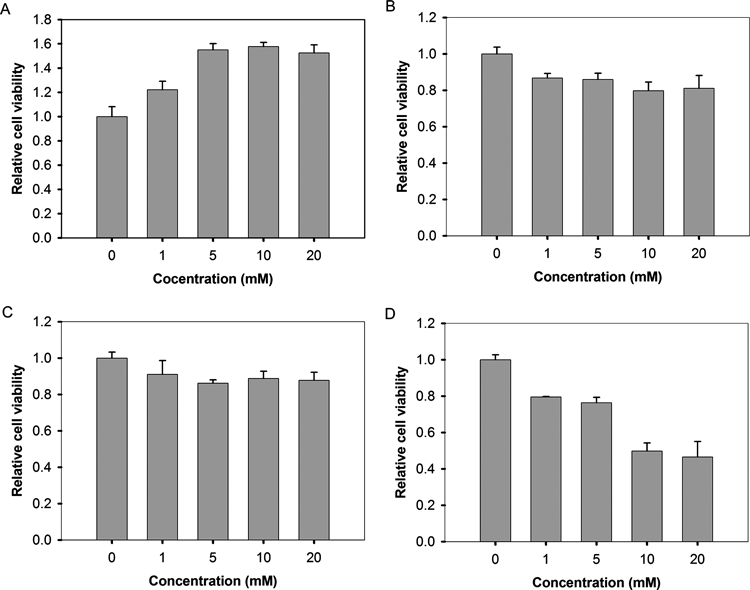
Cells were plated in 96-well plates. Butyric acid and its phenyl derivatives or control (the same volume of water) were added the next morning. After 24 h, MTT assays were performed. The results represent the means of four experiments±S.D. and are normalized to that of the control.
Many therapeutic properties of 4-PB have been identified. Its commercialized product (Brand name: Buphenyl) has been approved by the U.S. Food and Drug Administration as an orphan drug for treating hyperammonemia caused by urea cycle disorder, and has also been tested in the treatment of several diseases. 4-PB was reported to be very safe during a 26-month follow-up in ornithine transcarbamylase- deficient patients.28) In our study, 2-PB and 3-PB demonstrated a lower level of cell toxicity than 4-PB, and are better potential candidates for the treatment of H. pylori infection.
In this study, 4-PB was for the first time demonstrated to have functions other than in the treatment of urea cycle disorder. 2-PB, 3-PB and 4-PB were able to generate oxidative stress and inhibit the growth of H. pylori. The PBs also displayed synergistic effects in H. pylori, and lowered the antibiotic dosage required in patients. Our results suggested that PBs are effective assistant agents for the eradication of H. pylori infection and provide an alternative potential for clinical use.
The financial support of the National Science Council of Taiwan (Grant No. NSC NSC 99-2313-B-020-006-MY3) is greatly appreciated.