2013 Volume 61 Issue 7 Pages 722-730
2013 Volume 61 Issue 7 Pages 722-730
A new class of sulfonamidomethane pyrrolyl–oxadiazoles/thiadiazoles and pyrazolyl–oxadiazoles/thiadiazoles was prepared from arylsulfonylaminoacetic acid hydrazides and E-cinnamic acid. The lead compounds were tested for antimicrobial and cytotoxic activities. The thiadiazole compounds having chloro substituent on the aromatic ring 4c, 8c and 10c exhibited comparable antibacterial activity against Pseudomonas aeruginosa and also antifungal activity against Penicillium chrysogenum. The styryl oxadiazole compound 3c showed appreciable cytotoxic activity on A549 lung carcinoma cells which can be used as a lead compound in the future studies.
Nitrogen containing heterocyclic molecules constitutes the largest portion of chemical entities which are part of many natural products, fine chemicals and biologically active pharmaceuticals vital for enhancing the quality of life. In fact the azole, a privileged structure endows with broad and potent biological functions.1) 1,3,4-Oxadiazole derivatives possess significant antibacterial2–4) and anti-inflammatory activities.5,6) 1,3,4-Oxadiazole scaffold can also act as hydrogen bond acceptors, which makes it possible to be used as an isosteric substituent for amide or ester groups.7) Literature survey reveals that 2,5-disubstituted 1,3,4-oxadiazoles have been synthesized either by thermal/acid catalysed cyclization of 1,2-diacylhydrazines8) or by oxidative cyclization of semicarbazone/hydrazone in the presence of an oxidant9) or by microwave irradiation of hydrazide and carboxylic acid mixture.10) 1,3,4-Thiadiazole nucleus constitutes the active part of several biologically active compounds including antibacterial, antimycotic and anti-inflammatory agents.11,12) Most frequently used methods for the synthesis of thiadiazoles include the reaction of acylthiosemicarbazides with acid reagents such as trifluoroacetic acid13) and methanesulfonic acid.14) Pyrazoles are one of the most prevalent heterocyclic compounds with a wide range of biological activities viz., antihyperglycemic,15) anti-inflammatory,16) antiobesity,17) antitumor18–20) and antimycobacterial.21) The 1,3-dipolar cycloaddition methodology is widely used for the syntheses of pyrazoles using diverse synthons such as nitrilimines and alkynes,22) hydrazones and nitroolefins23,24) and azomethine imines and alkynes.25,26) Pyrroles are the fundamental structural motifs in various classes of natural and biologically important molecules like porphyrins, bile pigments, coenzymes and alkaloids.27,28) Among the various synthetic methods, the notable classical methods are Knorr,29,30) Hantzsch31) and Paal–Knorr synthesis32,33) including multicomponent and metal-catalyzed routes.34–41) Recently we have studied the antimicrobial activity of pyrrolyl and pyrazolyl oxadiazoles, thiadiazoles and triazoles and observed that triazole compounds displayed high antimicrobial activity.42,43) Motivated by the above findings and our continued interest in the synthesis of biologically active heterocycles it is proposed to synthesize the molecules having two different pharmacophore units and to study their biological activity.
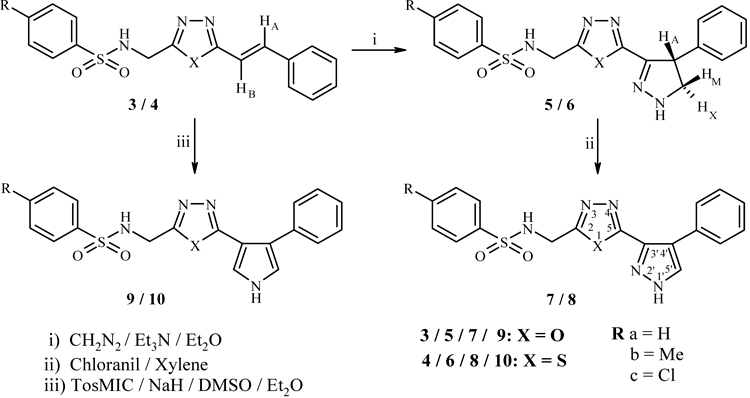
The general synthetic pathway to achieve the target molecules is depicted in Charts 1 and 2. The synthetic scheme involves the cyclocondensation of arylsulfonylaminoacetic acid hydrazides (1) with E-cinnamic acid (2) in the presence of POCl3 which led to the formation of 2-arylsulfonylaminomethyl-5-styryl-1,3,4-oxadiazoles (3). The oxadiazole moiety was interconverted to thiadiazole by treating with thiourea in tetrahydrofuran (THF). Thus 2-arylsulfonylaminomethyl-5-styryl-1,3,4-thiadiazoles (4) were prepared. The 1H-NMR spectra of 3a and 4a displayed a singlet at δ 4.12 and 4.30 due to methylene protons attached to C-2 of oxadiazole and thiadiazole moiety. In addition to this, one doublet was observed at 7.48 and 7.69 ppm in these compounds due to olefin proton HB. The other olefin proton, HA displayed a signal at downfield region and merged with aromatic protons. The coupling constant value J≈14.3 Hz indicated that they possess trans geometry. The broad signal appeared at 8.24 in 3a and at 8.45 ppm in 4a was assigned to SO2–NH which disappeared when D2O was added. The olefin moiety present in 3 and 4 was used to develop five membered heterocycles, pyrazoles and pyrroles. The 1,3-dipolar cycloaddition of diazomethane to 3 and 4 in the presence of Et3N in ether at −20°C for 40–48 h resulted in 2-(arylsulfonylaminomethyl)-5-(4-phenyl-4,5-dihydro-1H-pyrazol-3-yl)-1,3,4-oxadiazoles (5) and 2-(arylsulfonylaminomethyl)-5-(4-phenyl-4,5-dihydro-1H-pyrazol-3-yl)-1,3,4-thiadiazoles (6). The pyrazoline ring protons displayed an AMX splitting pattern in the 1H-NMR spectra of 5a and 6a. Thus three double doublets observed at δ 3.60, 4.23, 4.59 in 5a and at 3.79, 4.40, 4.75 ppm in 6a were assigned to HX, HM and HA, respectively. The coupling constant values JAX≈6.1, JMX≈10.2 and JAM≈12.1 Hz indicated that HA, HM are cis, HA, HX are trans and HM, HX are geminal. Oxidation of compounds 5 and 6 was effected with chloranil in xylene to afford the aromatized compounds, 2-(arylsulfonylaminomethyl)-5-(4-phenyl-1H-pyrazol-3-yl)-1,3,4-oxadiazoles (7) and 2-(arylsulfonylaminomethyl)-5-(4-phenyl-1H-pyrazol-3-yl)-1,3,4-thiadiazoles (8). The absence of AMX splitting pattern due to pyrazoline ring protons in 7 and 8 indicated that aromatization took place. On the other hand, the reaction of 3 and 4 with tosylmethyl isocyanide (TosMIC) in the presence of sodium hydride in a solvent mixture of dimethyl sulfoxide (DMSO) and ether gave 2-(arylsulfonylaminomethyl)-5-(4-phenyl-1H-pyrrol-3-yl)-1,3,4-oxadiazoles (9) and 2-(arylsulfonylaminomethyl)-5-(4-phenyl-1H-pyrrol-3-yl)-1,3,4-thiadiazoles (10). The 1H-NMR spectra of 9a and 10a displayed a singlet at δ 4.12 and 4.25 due to methylene protons attached to C-2 of oxadiazole and thiadiazole units. Apart from these, a singlet was observed at 6.63 and 6.85 ppm due to C5′-H of pyrrole ring whereas C2′-H appeared at downfield region and merged with aromatic protons. The compounds 9a and 10a showed two broad singlets at 9.12, 8.31 and at 9.29, 8.58 ppm due to NH of pyrrole ring and SO2–NH which disappeared on deuteration. The structures of these compounds were further established by IR and 13C-NMR spectra.

The compounds 3–10 were tested for antimicrobial activity at four different concentrations 12.5, 25, 50 and 100 µg/well. The results of antibacterial activity shown in Table 1 indicated that Gram-negative bacteria were more susceptible towards the tested compounds than Gram-positive ones. It was observed that mono heterocyclic compounds (3, 4) displayed higher activity than the respective bis heterocyclic systems. This may be due to the presence of electron withdrawing styryl moiety in mono heterocyclic derivatives. The compounds having a thiadiazole moiety (4a–c) were more active than those having an oxadiazole moiety (3a–c). In fact, the compound 4c displayed activity against Pseudomonas aeruginosa at 100 µg/well equal to the standard drug Chloramphenicol. This may be due to the presence of more electronegative atom viz., chlorine on the aromatic ring. Amongst pyrazolinyl and pyrazolyl derivatives, the aromatized systems 7 and 8 were more effective than the corresponding non-aromatized compounds 5 and 6. This may be due to the greater electron withdrawing capacity of the aromatized compounds than the non-aromatized compounds. Further, it was observed that pyrazole containing bis heterocycles 7 and 8 displayed slightly higher activity than the respective pyrrole containing bis heterocycles 9 and 10. Earlier, we have reported the antimicrobial activity of 2-(arylaminosulfonylmethyl)-5-(4′-aryl-1′H-pyrazol-3′-ylsulfonylmethyl)-1,3,4-thiadiazole42) and 2-(arylaminosulfonylmethyl)-5-(3′-arylsulfonyl-1′H-pyrazol-4′-ylsulfonylmethyl)-1,3,4-thiadiazole43) and observed that there was no marked difference in activity between the two compounds. It was noticed that the compound 8 in the present study, where the heterocyclic units are directly linked showed greater activity than those having sulfonylmethane moiety between the two heterocycles.42,43)
| Compound | Diameter of zone of inhibition (mm) | |||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Gram-positive bacteria | Gram-negative bacteria | |||||||||||||||
| Staphylococcus aureus | Bacillus subtilis | Pseudomonas aeruginosa | Klebsiella pneumoniae | |||||||||||||
| 12.5 µg/well | 25 µg/well | 50 µg/well | 100 µg/well | 12.5 µg/well | 25 µg/well | 50 µg/well | 100 µg/well | 12.5 µg/well | 25 µg/well | 50 µg/well | 100 µg/well | 12.5 µg/well | 25 µg/well | 50 µg/well | 100 µg/ well | |
| 3a | 08±2 | 10±1 | 13±3 | 15±3 | 9±1 | 11±3 | 12±3 | 14±3 | 11±2 | 13±1 | 15±3 | 17±1 | 10±2 | 12±1 | 13±3 | 16±1 |
| 3b | — | 07±1 | 10±3 | 12±1 | 07±2 | 8±2 | 9±1 | 12±3 | 09±2 | 10±1 | 12±3 | 14±2 | 08±1 | 09±2 | 10±1 | 13±2 |
| 3c | 11±2 | 13±3 | 15±2 | 17±3 | 12±1 | 13±2 | 14±3 | 16±1 | 14±1 | 16±2 | 17±3 | 19±1 | 13±1 | 15±3 | 17±2 | 18±1 |
| 4a | 19±1 | 20±2 | 23±1 | 25±3 | 25±2 | 27±1 | 29±2 | 31±1 | 20±2 | 23±1 | 24±3 | 27±2 | 26±2 | 28±1 | 30±2 | 32±1 |
| 4b | 16±2 | 18±1 | 19±1 | 22±3 | 23±3 | 25±1 | 27±2 | 29±3 | 19±1 | 21±2 | 23±1 | 28±3 | 24±2 | 26±1 | 28±3 | 30±1 |
| 4c | 20±1 | 23±2 | 24±2 | 26±1 | 28±1 | 30±2 | 32±3 | 35±1 | 21±1 | 24±3 | 25±2 | 32±1 | 33±2 | 35±1 | 37±2 | 39±1 |
| 5a | — | — | — | — | — | — | — | — | — | — | — | — | — | — | — | — |
| 5b | — | — | — | — | — | — | — | — | — | — | — | — | — | — | — | — |
| 5c | — | — | — | — | — | — | — | — | — | — | — | — | — | — | — | — |
| 6a | — | — | — | — | — | — | — | — | — | — | — | — | — | — | — | — |
| 6b | — | — | — | — | — | — | — | — | — | — | — | — | — | — | — | — |
| 6c | — | — | 07±1 | 09±2 | — | 07±1 | 08±3 | 10±2 | 07±3 | 09±1 | 10±2 | 12±1 | — | 08±3 | 09±1 | 11±3 |
| 7a | — | 07±1 | 09±2 | 11±1 | — | 08±2 | 09±3 | 11±1 | 09±2 | 10±1 | 12±3 | 14±2 | 07±1 | 09±3 | 10±1 | 12±1 |
| 7b | — | — | 07±2 | 09±1 | — | — | 08±1 | 09±2 | 07±1 | 08±3 | 09±2 | 11±1 | — | 07±1 | 08±2 | 10±1 |
| 7c | 08±2 | 10±3 | 11±1 | 13±1 | 08±1 | 09±1 | 12±3 | 14±2 | 10±1 | 11±3 | 14±2 | 16±1 | 09±2 | 10±1 | 13±2 | 15±3 |
| 8a | 18±2 | 19±1 | 21±3 | 23±1 | 24±2 | 25±1 | 27±2 | 29±1 | 19±2 | 22±3 | 23±1 | 25±2 | 25±1 | 26±1 | 28±2 | 30±3 |
| 8b | 15±3 | 16±3 | 18±2 | 20±1 | 19±3 | 22±3 | 24±2 | 26±2 | 18±1 | 19±2 | 21±1 | 24±2 | 21±1 | 23±2 | 25±3 | 27±1 |
| 8c | 19±2 | 22±2 | 23±3 | 25±1 | 26±1 | 29±3 | 30±2 | 34±1 | 20±1 | 23±3 | 24±1 | 26±3 | 30±2 | 34±3 | 35±1 | 37±2 |
| 9a | — | — | 07±3 | 09±1 | — | — | 08±3 | 10±1 | 07±2 | 09±1 | 10±1 | 12±2 | — | 07±1 | 09±2 | 11±1 |
| 9b | — | — | — | 07±1 | — | — | 07±3 | 08±1 | — | 07±1 | 08±1 | 10±2 | — | — | — | 09±1 |
| 9c | 07±3 | 08±1 | 09±3 | 11±1 | — | 08±3 | 10±1 | 12±2 | 08±3 | 10±3 | 12±1 | 14±2 | 07±1 | 09±2 | 11±3 | 13±1 |
| 10a | 15±1 | 17±3 | 20±1 | 21±2 | 18±3 | 20±2 | 23±1 | 26±3 | 18±1 | 20±2 | 22±1 | 23±3 | 22±3 | 24±2 | 26±1 | 28±3 |
| 10b | 13±3 | 15±1 | 17±1 | 18±2 | 16±2 | 18±1 | 20±2 | 23±1 | 15±1 | 17±3 | 18±1 | 22±2 | 18±2 | 20±1 | 23±2 | 26±1 |
| 10c | 16±3 | 19±1 | 21±2 | 23±1 | 22±3 | 26±2 | 28±2 | 32±1 | 19±2 | 21±3 | 23±1 | 24±1 | 27±3 | 29±1 | 32±3 | 35±1 |
| Chloramphenicol | 28±1 | 30±3 | 33±1 | 35±2 | 30±1 | 32±3 | 34±3 | 38±1 | 23±1 | 25±3 | 27±2 | 30±2 | 36±2 | 38±1 | 40±2 | 42±3 |
| Control (DMSO) | — | — | — | — | — | — | — | — | — | — | — | — | — | — | — | — |
(—) No activity. (±) Standard deviation.
All the tested compounds inhibited the spore germination against tested fungi except compound 5. In general, most of the compounds showed slightly higher antifungal activity towards Penicillium chrysogenum than Aspergillus niger. The compounds 4c and 8c displayed excellent activity particularly against Penicillium chrysogenum equivalent to the standard drug Ketoconazole at 100 µg/well (Table 2).
| Compound | Diameter of zone of inhibition (mm) | |||||||
|---|---|---|---|---|---|---|---|---|
| Aspergillus niger | Penicillium chrysogenum | |||||||
| 12.5 µg/ well | 25 µg/ well | 50 µg/ well | 100 µg/ well | 12.5 µg/ well | 25 µg/ well | 50 µg/ well | 100 µg/ well | |
| 3a | 08±2 | 10±3 | 12±1 | 15±2 | 10±2 | 12±3 | 13±1 | 15±2 |
| 3b | — | 07±3 | 09±1 | 12±2 | 07±3 | 08±1 | 10±2 | 12±3 |
| 3c | 12±1 | 14±3 | 15±2 | 17±2 | 14±2 | 15±1 | 17±2 | 19±3 |
| 4a | 25±3 | 27±1 | 30±1 | 33±3 | 29±1 | 30±2 | 32±2 | 35±3 |
| 4b | 23±2 | 24±1 | 27±2 | 30±1 | 26±1 | 28±2 | 30±3 | 33±1 |
| 4c | 27±3 | 30±1 | 32±3 | 35±1 | 31±3 | 33±2 | 35±1 | 38±2 |
| 5a | — | — | — | — | — | — | — | — |
| 5b | — | — | — | — | — | — | — | — |
| 5c | — | — | — | — | — | — | — | — |
| 6a | — | — | — | — | — | — | — | — |
| 6b | — | — | — | — | — | — | — | — |
| 6c | — | — | 08±3 | 10±2 | — | 07±1 | 09±2 | 11±1 |
| 7a | — | 08±3 | 10±1 | 12±2 | 07±3 | 09±1 | 11±3 | 13±1 |
| 7b | — | — | 07±1 | 09±3 | — | — | 08±2 | 10±1 |
| 7c | 08±2 | 09±1 | 11±2 | 14±1 | 11±1 | 12±2 | 14±1 | 16±2 |
| 8a | 23±2 | 25±1 | 28±3 | 31±1 | 27±3 | 29±1 | 31±2 | 33±1 |
| 8b | 20±2 | 22±1 | 25±2 | 27±3 | 25±2 | 27±1 | 29±2 | 32±2 |
| 8c | 25±1 | 29±3 | 30±2 | 32±1 | 28±1 | 32±1 | 34±1 | 38±2 |
| 9a | — | — | 08±1 | 10±2 | — | 08±2 | 09±1 | 11±2 |
| 9b | — | — | — | 08±1 | — | — | 07±2 | 09±3 |
| 9c | — | 07±3 | 09±3 | 13±1 | 09±1 | 11±2 | 12±3 | 14±1 |
| 10a | 18±2 | 21±3 | 23±1 | 26±2 | 25±2 | 27±1 | 28±2 | 31±1 |
| 10b | 16±1 | 19±1 | 21±3 | 23±3 | 22±2 | 24±2 | 25±1 | 27±3 |
| 10c | 22±1 | 25±2 | 26±1 | 29±2 | 27±1 | 29±2 | 30±3 | 34±1 |
| Ketoconazole | 29±1 | 31±2 | 33±3 | 36±3 | 33±3 | 35±1 | 36±2 | 38±3 |
| Control (DMSO) | — | — | — | — | — | — | — | — |
(—) No activity. (±) Standard deviation.
The minimum inhibitory concentration (MIC), minimum bactericidal (MBC) and minimum fungicidal concentration (MFC) values of the compounds tested are listed in Table 3. MIC is the lowest concentration of an antimicrobial that will inhibit the visible growth of a microorganism. (But it is not sure that the microorganisms are completely killed.) The MBC/MFC is the lowest concentration of antibiotic required to kill a particular bacterium/fungi. The MBC/MFC involve an additional set of steps performed once the minimum inhibitory concentration (MIC) is determined. The antimicrobials are usually regarded as bactericidal/fungicidal if the MBC/MFC is not greater than four times the MIC.44) The compound 4c exhibited low MIC values when compared with 8c and 10c. In addition MBC value is 2×MIC in case of Bacillus subtilis, Pseudomonas aeruginosa and MFC value is 2×MIC in case of P. chrysogenum. However, the other compounds showed bactericidal and fungicidal effects greater than 2×MIC. The structure–antimicrobial activity relationship of the synthesized compounds revealed that mono heterocyclic compounds with extended conjugation exhibited greater activity than the corresponding bis heterocycles. Amongst bis heterocyclic systems, the aromatized compounds having thiadiazole moiety (8, 10) were more effective when compared with those having oxadiazole unit (7, 9). The chloro substituted styryl-1,3,4-thiadiazole 4c exhibited strong antibacterial activity against P. aeruginosa with an inhibition zone of 32 mm at 100 µg and MIC and MBC of 6.25 and 12.5 µg, respectively. The compound 4c also displayed strong antifungal activity against P. chrysogenum with an inhibition zone of 38 mm at 100 µg and MIC and MFC of 12.5 and 25 µg, respectively.
| Compound | Minimum inhibitory concentration | |||||
|---|---|---|---|---|---|---|
| MIC (MBC/MFC) µg/well | ||||||
| S. aureus | B. subtilis | P. aeruginosa | K. pneumonia | A. niger | P. chrysogenum | |
| 4c | 12.5 (50) | 12.5 (25) | 6.25 (12.5) | 25 (100) | 12.5 (50) | 12.5 (25) |
| 8c | 50 (200) | 25 (100) | 50 (200) | 100 (>200) | 25 (100) | 12.5 (50) |
| 10c | 50 (200) | 25 (100) | 50 (>200) | 100 (>200) | 25 (100) | 50 (>200) |
| Chloramphenicol | 6.25 | 6.25 | 6.25 | 12.5 | — | — |
| Ketoconazole | — | — | — | — | 6.25 | 12.5 |
The compounds 3–10 were subjected to 3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyltetrazolium bromide (MTT) assay to determine cytotoxic activity. The compound 3c exhibited appreciable cytotoxic activity on A549 cells (IC50=30.5 µM). The results of cytotoxicity of 3c using MTT assay are shown in Fig. 1. The cytotoxic acitivity observed with the compound 3c is concentration dependent. The compound 3c at concentrations 100–200 µM showed lowest viability, while viability more than 75% is observed when this compound is used at concentrations below 12.50 µM. This suggests compound 3c is a potential lead molecule for cytotoxic activity against tumor cells. However, the other tested compounds did not show cytotoxic activity when used upto 0.2 mm concentration.
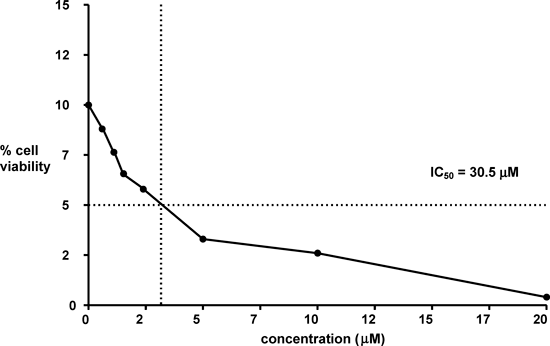
X-Axis shows the concentration of the compound and Y-axis, cell viability. (The scale of X- and Y-axes is 1/10 of actual values.)
In conclusion we have prepared a new class of sulfonamidomethane linked mono and bis heterocycles and studied their antimicrobial and cytotoxic activities. The styryl thiadiazole compound having chloro substituent on the aromatic ring 4c showed comparable antibacterial activity to Chloramphenicol against P. aeruginosa. The compounds 4c and 8c also exhibited comparable antifungal activity to Ketoconazole against P. chrysogenum. On the other hand the compound 3c displayed appreciable cytotoxic activity on A549 cells which can be used as a lead compound in the future studies.
Melting points were determined in open capillaries on a Mel-Temp apparatus and are uncorrected. The purity of the compounds was checked by TLC (silica gel H, British Drug House (BDH), ethyl acetate–hexane, 1 : 3). The IR spectra were recorded on a Thermo Nicolet IR 200 FT-IR spectrometer as KBr pellets and the wavenumbers were given in cm−1. The 1H-NMR spectra were recorded in DMSO-d6 on a Bruker-400 spectrometer (400 MHz). The 13C-NMR spectra were recorded in DMSO-d6 on a Bruker spectrometer operating at 100 MHz. All chemical shifts are reported in δ (ppm) using tetramethylsilane (TMS) as an internal standard. The mass spectra were recorded on Jeol JMS-D 300 and Finnigan Mat 1210 B at 70 eV with an emission current of 100 µA. The microanalyses were performed on a Perkin-Elmer 240C elemental analyzer.
General Procedure for the Synthesis of 2-Arylsulfonylaminomethyl-5-styryl-1,3,4-oxadiazole (3a–c)A mixture of arylsulfonylaminoacetic acid hydrazide (1) (5 mmol), E-cinnamic acid (2) (5 mmol) and POCl3 (6 mL) was heated under reflux for 3–5 h. The excess POCl3 was removed and the residue was poured onto crushed ice. The resulting precipitate was filtered, washed with saturated sodium bicarbonate solution followed by water and recrystallized from ethanol.
2-Phenylsulfonylaminomethyl-5-styryl-1,3,4-oxadiazole (3a): White solid, yield 68%, mp 196–198°C. IR (KBr) cm−1: 1152, 1312 (SO2), 1572 (C=N), 1630 (C=C), 3231 (NH). 1H-NMR (DMSO-d6) δ: 4.12 (s, 2H, CH2), 7.48 (d, 1H, HB, J=14.2 Hz), 7.67 (d, 1H, HA, J=14.2 Hz), 7.06–7.65 (m, 10H, Ar-H), 8.24 (br s, 1H, SO2–NH). 13C-NMR (DMSO-d6) δ: 44.5 (CH2), 126.9 (C-HB), 137.2 (C-HA), 167.8 (C-2), 178.2 (C-5), 124.6, 128.4, 129.7, 130.3, 131.1, 138.5, 144.2, 146.8 (aromatic carbons). MS m/z: 341.38 (M+). Anal. Calcd for C17H15N3O3S: C, 59.81; H, 4.43; N, 12.31. Found: C, 59.89; H, 4.37; N, 12.44.
2-(p-Methylphenylsulfonylaminomethyl)-5-styryl-1,3,4-oxadiazole (3b): White solid, yield 68%, mp 172–174°C. IR (KBr) cm−1: 1148, 1308 (SO2), 1565 (C=N), 1624 (C=C), 3220 (NH). 1H-NMR (DMSO-d6) δ: 2.37 (s, 3H, Ar-CH3), 4.07 (s, 2H, CH2), 7.41 (d, 1H, HB, J=14.0 Hz), 7.60 (d, 1H, HA, J=14.0 Hz), 7.03–7.67 (m, 9H, Ar-H), 8.19 (br s, 1H, SO2–NH). 13C-NMR (DMSO-d6) δ: 21.1 (Ar-CH3), 43.7 (CH2), 127.5 (C-HB), 136.7 (C-HA), 167.2 (C-2), 177.0 (C-5), 124.2, 128.1, 128.9, 130.7, 131.2, 137.4, 143.3, 145.5 (aromatic carbons). MS m/z: 355.41 (M+). Anal. Calcd for C18H17N3O3S: C, 60.83; H, 4.82; N, 11.82. Found: C, 60.77; H, 4.81; N, 11.90.
2-(p-Chlorophenylsulfonylaminomethyl)-5-styryl-1,3,4-oxadiazole (3c): White solid, yield 75%, mp 221–223°C. IR (KBr) cm−1: 1157, 1316 (SO2), 1570 (C=N), 1632 (C=C), 3238 (NH). 1H-NMR (DMSO-d6) δ: 4.19 (s, 2H, CH2), 7.55 (d, 1H, HB, J=14.4 Hz), 7.71 (d, 1H, HA, J=14.4 Hz), 7.05–7.68 (m, 9H, Ar-H), 8.31 (br s, 1H, SO2–NH). 13C-NMR (DMSO-d6) δ: 46.5 (CH2), 127.8 (C-HB), 137.9 (C-HA), 168.7 (C-2), 179.8 (C-5), 124.8, 129.6, 130.2, 131.2, 131.9, 139.8, 145.1, 147.2 (aromatic carbons). MS m/z: 375.83 (M+). Anal. Calcd for C17H14ClN3O3S: C, 54.33; H, 3.75; N, 11.18. Found: C, 54.40; H, 3.72; N, 11.28.
General Procedure for the Synthesis of 2-Arylsulfonylaminomethyl-5-styryl-1,3,4-thiadiazole (4a–c)In a sealed test tube, the compound 3 (5 mmol), thiourea (20 mmol) and tetrahydrofuran (6 mL) were taken and heated at 120–160°C in an oil bath for 17–20 h. After the reaction was completed, it was extracted with dichloromethane, washed with water, brine solution and dried over anhydrous Na2SO4. The solvent was removed under reduced pressure and the resultant solid was recrystallized from methanol.
2-Phenylsulfonylaminomethyl-5-styryl-1,3,4-thiadiazole (4a): White solid, yield 65%, mp 191–193°C. IR (KBr) cm−1: 1128, 1321 (SO2), 1568 (C=N), 1620 (C=C), 3245 (NH). 1H-NMR (DMSO-d6) δ: 4.30 (s, 2H, CH2), 7.69 (d, 1H, HB, J=14.6 Hz), 7.85 (d, 1H, HA, J=14.6 Hz), 7.26–7.80 (m, 10H, Ar-H), 8.45 (br s, 1H, SO2–NH). 13C-NMR (DMSO-d6) δ: 46.3 (CH2), 128.5 (C-HB), 139.0 (C-HA), 169.9 (C-2), 180.1 (C-5), 126.6, 130.8, 131.0, 132.1, 133.2, 140.2, 146.1, 148.5 (aromatic carbons). MS m/z: 357.45 (M+). Anal. Calcd for C17H15N3O2S2: C, 57.12; H, 4.23; N, 11.76. Found: C, 57.02; H, 4.28; N, 11.84.
2-(p-Methylphenylsulfonylaminomethyl)-5-styryl-1,3,4-thiadiazole (4b): White solid, yield 62%, mp 182–185°C. IR (KBr) cm−1: 1131, 1319 (SO2), 1560 (C=N), 1625 (C=C), 3237 (NH). 1H-NMR (DMSO-d6) δ: 2.48 (s, 3H, Ar-CH3), 4.26 (s, 2H, CH2), 7.61 (d, 1H, HB, J=14.5 Hz), 7.79 (d, 1H, HA, J=14.5 Hz), 7.21–7.77 (m, 9H, Ar-H), 8.38 (br s, 1H, SO2–NH). 13C-NMR (DMSO-d6) δ: 23.0 (Ar-CH3), 45.6 (CH2), 127.9 (C-HB), 138.5 (C-HA), 169.0 (C-2), 178.9 (C-5), 126.1, 130.2, 130.9, 131.5, 132.7, 139.2, 145.0, 147.0 (aromatic carbons). MS m/z: 371.48 (M+). Anal. Calcd for C18H17N3O2S2: C, 58.20; H, 4.61; N, 11.31. Found: C, 58.32; H, 4.58; N, 11.42.
2-(p-Chlorophenylsulfonylaminomethyl)-5-styryl-1,3,4-thiadiazole (4c): White solid, yield 68%, mp 210–213°C. IR (KBr) cm−1: 1137, 1327 (SO2), 1572 (C=N), 1628 (C=C), 3255 (NH). 1H-NMR (DMSO-d6) δ: 4.38 (s, 2H, CH2), 7.76 (d, 1H, HB, J=14.7 Hz), 7.90 (d, 1H, HA, J=14.7 Hz), 7.28–7.88 (m, 9H, Ar-H), 8.49 (br s, 1H, SO2–NH). 13C-NMR (DMSO-d6) δ: 48.3 (CH2), 129.1 (C-HB), 139.7 (C-HA), 170.4 (C-2), 181.6 (C-5), 126.8, 131.4, 132.0, 133.4, 134.0, 141.4, 147.1, 149.4 (aromatic carbons). MS m/z: 391.89 (M+). Anal. Calcd for C17H14ClN3O2S2: C, 52.10; H, 3.60; N, 10.72. Found: C, 52.18; H, 3.61; N, 10.79.
General Procedure for the Synthesis of 2-(Arylsulfonylaminomethyl)-5-(4-phenyl-4,5-dihydro-1H-pyrazol-3-yl)-1,3,4-oxadiazole (5a–c)/2-(Arylsulfonylaminomethyl)-5-(4-phenyl-4,5-dihydro-1H-pyrazol-3-yl)-1,3,4-thiadiazole (6a–c)To a cooled solution of compound 3/4 (5 mmol) in dichloromethane (20 mL), an ethereal solution of diazomethane (40 mL, 0.4 M) and triethylamine (5 mmol) were added and kept at −20 to −15°C for 48 h. The solvent was removed and the resultant residue was purified by column chromatography (silica gel, 60–120 mesh) using hexane–ethyl acetate (3 : 1) as eluent.
2-(Phenylsulfonylaminomethyl)-5-(4-phenyl-4,5-dihydro-1H-pyrazol-3-yl)-1,3,4-oxadiazole (5a): White solid, yield 71%, mp 158–160°C. IR (KBr) cm−1: 1132, 1324 (SO2), 1559 (C=N), 3250 (NH). 1H-NMR (DMSO-d6) δ: 3.60 (dd, 1H, HX, JAX=6.5 Hz, JMX=10.6 Hz), 4.23 (dd, 1H, HM, JAM=12.4 Hz), 4.27 (s, 2H, CH2), 4.59 (dd, 1H, HA), 7.17–7.76 (m, 10H, Ar-H), 8.51 (br s, 1H, SO2–NH), 10.02 (br s, 1H, NH). 13C-NMR (DMSO-d6) δ: 45.4 (CH2), 53.9 (C-5′), 66.5 (C-4′), 152.9 (C-3′), 158.9 (C-2), 168.0 (C-5), 127.7, 128.4, 129.7, 130.6, 131.2, 132.5, 133.1, 135.6 (aromatic carbons). MS m/z: 383.42 (M+). Anal. Calcd for C18H17N5O3S: C, 56.38; H, 4.47; N, 18.27. Found: C, 56.34; H, 4.44; N, 18.36.
2-(p-Methylphenylsulfonylaminomethyl)-5-(4-phenyl-4,5-dihydro-1H-pyrazol-3-yl)-1,3,4-oxadiazole (5b): White solid, yield 73%, mp 149–151°C. IR (KBr) cm−1: 1129, 1321 (SO2), 1561 (C=N), 3254 (NH). 1H-NMR (DMSO-d6) δ: 2.30 (s, 3H, Ar-CH3), 3.56 (dd, 1H, HX, JAX=6.2 Hz, JMX=10.4 Hz), 4.14 (dd, 1H, HM, JAM=12.2 Hz), 4.23 (s, 2H, CH2), 4.52 (dd, 1H, HA), 7.14–7.67 (m, 9H, Ar-H), 8.44 (br s, 1H, SO2–NH), 9.93 (br s, 1H, NH). 13C-NMR (DMSO-d6) δ: 21.9 (Ar-CH3), 44.9 (CH2), 53.0 (C-5′), 65.9 (C-4′), 151.5 (C-3′), 158.6 (C-2), 167.2 (C-5), 125.8, 126.6, 127.3, 128.4, 129.5, 130.3, 131.2, 134.8 (aromatic carbons). MS m/z: 397.45 (M+). Anal. Calcd for C19H19N5O3S: C, 57.42; H, 4.82; N, 17.62. Found: C, 57.51; H, 4.86; N, 17.76.
2-(p-Chlorophenylsulfonylaminomethyl)-5-(4-phenyl-4,5-dihydro-1H-pyrazol-3-yl)-1,3,4-oxadiazole (5c): White solid, yield 76%, mp 169–171°C. IR (KBr) cm−1: 1140, 1330 (SO2), 1567 (C=N), 3267 (NH). 1H-NMR (DMSO-d6) δ: 3.65 (dd, 1H, HX, JAX=6.8 Hz, JMX=10.8 Hz), 4.26 (dd, 1H, HM, JAM=12.8 Hz), 4.28 (s, 2H, CH2), 4.61 (dd, 1H, HA), 7.21–7.87 (m, 9H, Ar-H), 8.57 (br s, 1H, SO2–NH), 10.14 (br s, 1H, NH). 13C-NMR (DMSO-d6) δ: 46.3 (CH2), 54.1 (C-5′), 66.9 (C-4′), 153.1 (C-3′), 160.2 (C-2), 169.2 (C-5), 126.7, 127.4, 128.3, 129.0, 130.1, 131.4, 133.7, 136.1 (aromatic carbons). MS m/z: 417.87 (M+). Anal. Calcd for C18H16ClN5O3S: C, 51.74; H, 3.86; N, 16.76. Found: C, 51.87; H, 3.84; N, 16.87.
2-(Phenylsulfonylaminomethyl)-5-(4-phenyl-4,5-dihydro-1H-pyrazol-3-yl)-1,3,4-thiadiazole (6a): Pale yellow solid, yield 66%, mp 215–217°C. IR (KBr) cm−1: 1136, 1332 (SO2), 1581 (C=N), 3250 (NH). 1H-NMR (DMSO-d6) δ: 3.79 (dd, 1H, HX, JAX=5.9 Hz, JMX=9.8 Hz), 4.30 (s, 2H, CH2), 4.40 (dd, 1H, HM, JAM=11.8 Hz), 4.75 (dd, 1H, HA), 7.35–7.97 (m, 10H, Ar-H), 8.69 (br s, 1H, SO2–NH), 10.16 (br s, 1H, NH). 13C-NMR (DMSO-d6) δ: 46.6 (CH2), 55.8 (C-5′), 68.3 (C-4′), 154.8 (C-3′), 160.3 (C-2), 170.0 (C-5), 129.5, 130.3, 131.8, 132.8, 133.1, 134.2, 135.0, 136.9 (aromatic carbons). MS m/z: 399.49 (M+). Anal. Calcd for C18H17N5O2S2: C, 54.12; H, 4.29; N, 17.53. Found: C, 54.23; H, 4.34; N, 17.65.
2-(p-Methylphenylsulfonylaminomethyl)-5-(4-phenyl-4,5-dihydro-1H-pyrazol-3-yl)-1,3,4-thiadiazole (6b): Pale yellow solid, yield 64%, mp 195–196°C. IR (KBr) cm−1: 1134, 1327 (SO2), 1577 (C=N), 3252 (NH). 1H-NMR (DMSO-d6) δ: 2.45 (s, 3H, Ar-CH3), 3.77 (dd, 1H, HX, JAX=5.7 Hz, JMX=9.6 Hz), 4.28 (s, 2H, CH2), 4.44 (dd, 1H, HM, JAM=11.7 Hz), 4.70 (dd, 1H, HA), 7.30–7.85 (m, 9H, Ar-H), 8.65 (br s, 1H, SO2–NH), 10.02 (br s, 1H, NH). 13C-NMR (DMSO-d6) δ: 23.6 (Ar-CH3), 46.0 (CH2), 55.1 (C-5′), 67.8 (C-4′), 153.6 (C-3′), 160.7 (C-2), 168.8 (C-5), 127.9, 128.5, 129.2, 130.8, 131.5, 132.5, 133.0, 136.5 (aromatic carbons). MS m/z: 413.52 (M+). Anal. Calcd for C19H19N5O2S2: C, 55.19; H, 4.63; N, 16.94. Found: C, 55.25; H, 4.65; N, 16.88.
2-(p-Chlorophenylsulfonylaminomethyl)-5-(4-phenyl-4,5-dihydro-1H-pyrazol-3-yl)-1,3,4-thiadiazole (6c): Pale yellow solid, yield 72%, mp 219–221°C. IR (KBr) cm−1: 1142, 1336 (SO2), 1585 (C=N), 3265 (NH). 1H-NMR (DMSO-d6) δ: 3.88 (dd, 1H, HX, JAX=6.2 Hz, JMX=10.1 Hz), 4.39 (s, 2H, CH2), 4.46 (dd, 1H, HM, JAM=12.0 Hz), 4.83 (dd, 1H, HA), 7.42–7.99 (m, 9H, Ar-H), 8.75 (br s, 1H, SO2–NH), 10.26 (br s, 1H, NH). 13C-NMR (DMSO-d6) δ: 47.4 (CH2), 56.0 (C-5′), 68.8 (C-4′), 155.4 (C-3′), 162.5 (C-2), 171.4 (C-5), 128.3, 129.5, 130.4, 131.2, 132.7, 133.9, 134.3, 138.1 (aromatic carbons). MS m/z: 433.93 (M+). Anal. Calcd for C18H16ClN5O2S2: C, 49.82; H, 3.72; N, 16.14. Found: C, 49.92; H, 3.76; N, 16.20.
General Procedure for the Synthesis of 2-(Arylsulfonylaminomethyl)-5-(4-phenyl-1H-pyrazol-3-yl)-1,3,4-oxadiazole (7a–c)/2-(Arylsulfonylaminomethyl)-5-(4-phenyl-1H-pyrazol-3-yl)-1,3,4-thiadiazole (8a–c)A mixture of 5/6 (1 mmol) and chloranil (1.4 mmol) in xylene (10 mL) was refluxed for 20–22 h and treated with a 5% NaOH solution. The organic layer was separated, washed with water and dried over anhydrous Na2SO4. The solvent was removed and the resultant solid was recrystallized from methanol.
2-(Phenylsulfonylaminomethyl)-5-(4-phenyl-1H-pyrazol-3-yl)-1,3,4-oxadiazole (7a): White solid, yield 70%, mp 198–200°C. IR (KBr) cm−1: 1138, 1325 (SO2), 1592 (C=N), 1634 (C=C), 3241 (NH). 1H-NMR (DMSO-d6) δ: 4.21 (s, 2H, CH2), 6.50 (br s, 1H, NH), 6.84–7.77 (m, 11H, C5′-H, Ar-H), 8.37 (br s, 1H, SO2–NH). 13C-NMR (DMSO-d6) δ: 46.0 (CH2), 133.9 (C-4′), 138.5 (C-5′), 150.1 (C-3′), 159.0 (C-2), 174.0 (C-5), 126.9, 127.4, 128.6, 129.4, 131.8, 132.8, 134.2, 138.5 (aromatic carbons). MS m/z: 381.41 (M+). Anal. Calcd for C18H15N5O3S: C, 56.68; H, 3.96; N, 18.36. Found: C, 56.77; H, 3.95; N, 18.27.
2-(p-Methylphenylsulfonylaminomethyl)-5-(4-phenyl-1H-pyrazol-3-yl)-1,3,4-oxadiazole (7b): White solid, yield 68%, mp 166–168°C. IR (KBr) cm−1: 1133, 1322 (SO2), 1583 (C=N), 1622 (C=C), 3237 (NH). 1H-NMR (DMSO-d6) δ: 2.35 (s, 3H, Ar-CH3), 4.18 (s, 2H, CH2), 6.45 (br s, 1H, NH), 6.82–7.72 (m, 10H, C5'-H, Ar-H), 8.25 (br s, 1H, SO2–NH). 13C-NMR (DMSO-d6) δ: 21.8 (Ar-CH3), 45.1 (CH2), 133.2 (C-4′), 137.8 (C-5′), 151.3 (C-3′), 162.5 (C-2), 173.2 (C-5), 126.3, 127.5, 128.6, 129.8, 131.4, 133.4, 134.3, 136.0 (aromatic carbons). MS m/z: 395.43 (M+). Anal. Calcd for C19H17N5O3S: C, 57.71; H, 4.33; N, 17.71. Found: C, 57.66; H, 4.38; N, 17.85.
2-(p-Chlorophenylsulfonylaminomethyl)-5-(4-phenyl-1H-pyrazol-3-yl)-1,3,4-oxadiazole (7c): White solid, yield 74%, mp 201–203°C. IR (KBr) cm−1: 1143, 1335 (SO2), 1594 (C=N), 1630 (C=C), 3245 (NH). 1H-NMR (DMSO-d6) δ: 4.29 (s, 2H, CH2), 6.56 (br s, 1H, NH), 6.91–7.81 (m, 10H, C5'-H, Ar-H), 8.42 (br s, 1H, SO2–NH). 13C-NMR (DMSO-d6) δ: 46.7 (CH2), 134.3 (C-4′), 139.0 (C-5′), 152.5 (C-3′), 164.3 (C-2), 174.5 (C-5), 126.9, 127.8, 129.3, 130.4, 131.9, 134.1, 136.5, 139.1 (aromatic carbons). MS m/z: 415.85 (M+). Anal. Calcd for C18H14ClN5O3S: C, 51.99; H, 3.39; N, 16.84. Found: C, 52.03; H, 3.41; N, 16.93.
2-(Phenylsulfonylaminomethyl)-5-(4-phenyl-1H-pyrazol-3-yl)-1,3,4-thiadiazole (8a): White solid, yield 61%, mp 190–192°C. IR (KBr) cm−1: 1135, 1328 (SO2), 1596 (C=N), 1632 (C=C), 3268 (NH). 1H-NMR (DMSO-d6) δ: 4.40 (s, 2H, CH2), 6.71 (br s, 1H, NH), 6.99–7.91 (m, 11H, C5′-H, Ar-H), 8.53 (br s, 1H, SO2-NH). 13C-NMR (DMSO-d6) δ: 47.2 (CH2), 135.7 (C-4′), 140.3 (C-5′), 149.9 (C-3′), 161.2 (C-2), 175.1 (C-5), 128.8, 129.5, 130.3, 131.5, 133.7, 134.7, 136.4, 140.3 (aromatic carbons). MS m/z: 397.47 (M+). Anal. Calcd for C18H15N5O2S2: C, 54.39; H, 3.80; N, 17.62. Found: C, 54.46; H, 3.82; N, 17.75.
2-(p-Methylphenylsulfonylaminomethyl)-5-(4-phenyl-1H-pyrazol-3-yl)-1,3,4-thiadiazole (8b): White solid, yield 72%, mp 165–167°C. IR (KBr) cm−1: 1132, 1323 (SO2), 1590 (C=N), 1623 (C=C), 3260 (NH). 1H-NMR (DMSO-d6) δ: 2.47 (s, 3H, Ar-CH3), 4.31 (s, 2H, CH2), 6.65 (br s, 1H, NH), 6.91–7.85 (m, 10H, C5′-H, Ar-H), 8.48 (br s, 1H, SO2–NH). 13C-NMR (DMSO-d6) δ: 23.6 (Ar-CH3), 46.4 (CH2), 135.3 (C-4′), 139.9 (C-5′), 154.8 (C-3′), 160.7 (C-2), 174.6 (C-5), 128.0, 129.2, 130.3, 131.5, 133.6, 135.4, 136.6, 138.1 (aromatic carbons). MS m/z: 411.50 (M+). Anal. Calcd for C19H17N5O2S2: C, 55.46; H, 4.16; N, 17.02. Found: C, 55.65; H, 4.14; N, 17.10.
2-(p-Chlorophenylsulfonylaminomethyl)-5-(4-phenyl-1H-pyrazol-3-yl)-1,3,4-thiadiazole (8c): White solid, yield 75%, mp 202–204°C. IR (KBr) cm−1: 1139, 1334 (SO2), 1598 (C=N), 1621 (C=C), 3272 (NH). 1H-NMR (DMSO-d6) δ: 4.43 (s, 2H, CH2), 6.77 (br s, 1H, NH), 7.14–7.92 (m, 10H, C5′-H, Ar-H), 8.60 (br s, 1H, SO2–NH). 13C-NMR (DMSO-d6) δ: 48.1 (CH2), 136.1 (C-4′), 140.9 (C-5′), 150.5 (C-3′), 161.5 (C-2), 176.0 (C-5), 128.9, 129.7, 131.5, 132.7, 133.6, 135.2, 136.9, 141.0 (aromatic carbons). MS m/z: 431.92 (M+). Anal. Calcd for C18H14ClN5O2S2: C, 50.05; H, 3.27; N, 16.21. Found: C, 50.11; H, 3.32; N, 16.31.
General Procedure for the Synthesis of 2-(Arylsulfonylaminomethyl)-5-(4-phenyl-1H-pyrrole-3-yl)-1,3,4-oxadiazole (9a–c)/2-(Arylsulfonylaminomethyl)-5-(4-phenyl-1H-pyrrole-3-yl)-1,3,4-thiadiazole (10a–c)The compound 3/4 (1 mmol) and TosMIC (1 mmol) in Et2O–DMSO (2 : 1) was added dropwise under stirring to a suspension of NaH (50 mg) in Et2O (20 mL) at room temperature and stirring was continued for 6–8 h. Then, water was added and the reaction mass was extracted with Et2O. The solvent was removed and the resultant residue was purified by column chromatography (silicagel, 60–120 mesh) using hexane–ethyl acetate (3 : 1) as eluent.
2-(Phenylsulfonylaminomethyl)-5-(4-phenyl-1H-pyrrole-3-yl)-1,3,4-oxadiazole (9a): White solid, yield 65%, mp 182–184°C. IR (KBr) cm−1: 1141, 1340 (SO2), 1586 (C=N), 1618 (C=C), 3253 (NH). 1H-NMR (DMSO-d6) δ: 4.12 (s, 2H, CH2), 6.63 (s, 1H, C5'-H), 7.21–7.84 (m, 11H, C2′-H, Ar-H), 8.31 (br s, 1H, SO2–NH), 9.12 (br s, 1H, NH). 13C-NMR (DMSO-d6) δ: 47.1 (CH2), 102.8 (C-4′), 110.2 (C-3′), 117.5 (C-5′), 121.5 (C-2′), 165.4 (C-2), 170.9 (C-5), 124.8, 126.2, 127.6, 128.5, 129.1, 130.4, 132.6, 138.2 (aromatic carbons). MS m/z: 380.42 (M+). Anal. Calcd for C19H16N4O3S: C, 59.99; H, 4.24; N, 14.84. Found: C, 59.95; H, 4.25; N, 14.83.
2-(p-Methylphenylsulfonylaminomethyl)-5-(4-phenyl-1H-pyrrole-3-yl)-1,3,4-oxadiazole (9b): White solid, yield 67%, mp 176–178°C. IR (KBr) cm−1: 1136, 1331 (SO2), 1584 (C=N), 1620 (C=C), 3256 (NH). 1H-NMR (DMSO-d6) δ: 2.39 (s, 3H, Ar-CH3), 4.09 (s, 2H, CH2), 6.53 (s, 1H, C5′-H), 7.18–7.88 (m, 10H, C2′-H, Ar-H), 8.37 (br s, 1H, SO2–NH), 9.01 (br s, 1H, NH). 13C-NMR (DMSO-d6) δ: 21.0 (Ar-CH3), 46.2 (CH2), 103.5 (C-4′), 109.4 (C-3′), 116.8 (C-5′), 120.7 (C-2′), 164.7 (C-2), 170.2 (C-5), 125.7, 126.5, 127.2, 128.6, 129.3, 130.1, 131.0, 138.8 (aromatic carbons). MS m/z: 394.45 (M+). Anal. Calcd for C20H18N4O3S: C, 60.90; H, 4.60; N, 14.20. Found: C, 60.98; H, 4.63; N, 14.12.
2-(p-Chlorophenylsulfonylaminomethyl)-5-(4-phenyl-1H-pyrrole-3-yl)-1,3,4-oxadiazole (9c): White solid, yield 71%, mp 192–194°C. IR (KBr) cm−1: 1145, 1342 (SO2), 1588 (C=N), 1626 (C=C), 3264 (NH). 1H-NMR (DMSO-d6) δ: 4.17 (s, 2H, CH2), 6.67 (s, 1H, C5′-H), 7.25–7.74 (m, 10H, C2′-H, Ar-H), 8.46 (br s, 1H, SO2–NH), 9.17 (br s, 1H, NH). 13C-NMR (DMSO-d6) δ: 48.0 (CH2), 104.2 (C-4′), 110.6 (C-3′), 118.0 (C-5′), 121.9 (C-2′), 166.9 (C-2), 171.8 (C-5), 126.7, 127.4, 128.8, 130.2, 131.5, 132.4, 134.6, 139.5 (aromatic carbons). MS m/z: 414.87 (M+). Anal. Calcd for C19H15ClN4O3S: C, 55.01; H, 3.64; N, 13.50. Found: C, 55.09; H, 3.68; N, 13.58.
2-(Phenylsulfonylaminomethyl)-5-(4-phenyl-1H-pyrrole-3-yl)-1,3,4-thiadiazole (10a): White solid, yield 63%, mp 171–173°C. IR (KBr) cm−1: 1138, 1329 (SO2), 1562 (C=N), 1631 (C=C), 3270 (NH). 1H-NMR (DMSO-d6) δ: 4.25 (s, 2H, CH2), 6.85 (s, 1H, C5′-H), 7.39–7.99 (m, 11H, C2′-H, Ar-H), 8.58 (br s, 1H, SO2–NH), 9.29 (br s, 1H, NH). 13C-NMR (DMSO-d6) δ: 48.1 (CH2), 105.9 (C-4′), 112.3 (C-3′), 119.2 (C-5′), 123.8 (C-2′), 167.3 (C-2), 172.1 (C-5), 126.6, 128.1, 129.4, 130.9, 131.5, 132.5, 134.9, 140.3 (aromatic carbons). MS m/z: 396.49 (M+). Anal. Calcd for C19H16N4O2S2: C, 57.56; H, 4.07; N, 14.13. Found: C, 57.52; H, 4.06; N, 14.28.
2-(p-Methylphenylsulfonylaminomethyl)-5-(4-phenyl-1H-pyrrole-3-yl)-1,3,4-thiadiazole (10b): White solid, yield 77%, mp 165–168°C. IR (KBr) cm−1: 1130, 1326 (SO2), 1574 (C=N), 1628 (C=C), 3262 (NH). 1H-NMR (DMSO-d6) δ: 2.40 (s, 3H, Ar-CH3), 4.18 (s, 2H, CH2), 6.74 (s, 1H, C5′-H), 7.39–7.98 (m, 10H, C2′-H, Ar-H), 8.51 (br s, 1H, SO2–NH), 9.25 (br s, 1H, NH). 13C-NMR (DMSO-d6) δ: 22.8 (Ar-CH3), 47.5 (CH2), 104.6 (C-4′), 111.6 (C-3′), 118.3 (C-5′), 122.8 (C-2′), 165.6 (C-2), 171.3 (C-5), 127.8, 128.9, 129.5, 130.7, 131.5, 132.2, 133.5, 140.5 (aromatic carbons). MS m/z: 410.51 (M+). Anal. Calcd for C20H18N4O2S2: C, 58.52; H, 4.42; N, 13.65. Found: C, 58.60; H, 4.45; N, 13.74.
2-(p-Chlorophenylsulfonylaminomethyl)-5-(4-phenyl-1H-pyrrole-3-yl)-1,3,4-thiadiazole (10c): White solid, yield 79%, mp 186–188°C. IR (KBr) cm−1: 1147, 1334 (SO2), 1578 (C=N), 1635 (C=C), 3275 (NH). 1H-NMR (DMSO-d6) δ: 4.29 (s, 2H, CH2), 6.89 (s, 1H, C5′-H), 7.44–7.88 (m, 10H, C2′-H, Ar-H), 8.67 (br s, 1H, SO2–NH), 9.35 (br s, 1H, NH). 13C-NMR (DMSO-d6) δ: 49.1 (CH2), 106.2 (C-4′), 112.9 (C-3′), 119.8 (C-5′), 123.7 (C-2′), 169.1 (C-2), 173.4 (C-5), 128.2, 129.7, 130.2, 132.4, 135.7, 137.2, 139.1, 141.2 (aromatic carbons). MS m/z: 430.93 (M+). Anal. Calcd for C19H15ClN4O2S2: C, 52.96; H, 3.51; N, 13.00. Found: C, 52.91; H, 3.53; N, 13.12.
Antimicrobial TestingThe compounds 3–10 were dissolved in DMSO at different concentrations of 12.5, 25, 50 and 100 µg/well. Bacterial strains Staphylococcus aureus, Bacillus subtilis, Pseudomonas aeruginosa, Klebsiella pneumoniae and fungi Aspergillus niger, Penicillium chrysogenum were obtained from Department of Microbiology, S.V. University, Tirupati.
Antibacterial and Antifungal AssaysThe in vitro antimicrobial studies were carried out by agar well diffusion method against test organisms.45,46) Nutrient broth (NB) plates were swabbed with 24 h old broth culture (100 µL) of test bacteria. Using the sterile cork borer, wells (6 mM) were made into each petriplate. Various concentrations of DMSO dissolved compounds (12.5, 25, 50, 100 µg/well) were added into the wells by using sterile pipettes. Simultaneously the standard antibiotics, Chloramphenicol for antibacterial activity and Ketoconazole for antifungal activity (as positive control) were tested against the pathogens. The samples were dissolved in DMSO which showed no zone of inhibition acts as negative control. The plates were incubated at 37°C for 24 h for bacteria and at 28°C for 48 h for fungi. After appropriate incubation, the diameter of zone of inhibition of each well was measured. Duplicates were maintained and the average values were calculated for eventual antibacterial activity.
Broth dilution test is used to determine minimum inhibitory concentration (MIC) of the above mentioned samples.47,48) Freshly prepared nutrient broth was used as diluents. The 24 h old culture of the test bacteria Staphylococcus aureus, Bacillus subtilis, Pseudomonas aeruginosa and Klebsiella pneumoniae and the test fungi Aspergillus niger and Penicillium chrysogenum were diluted 100 folds in nutrient broth (100 µL bacterial cultures in 10 mL NB). Increasing concentrations of the test samples (1.25, 2.5, 5, 10, 20, 40 µL of stock solution contains 6.25, 12.5, 25, 50, 100, 200 µg of the compounds) were added to the test tubes containing the bacterial and fungal cultures. All the tubes were incubated at 37°C for 24 h for bacteria and at 28°C for 48 h for fungi. The tubes were examined for visible turbidity and using NB as control. Control without test samples and with solvent was assayed simultaneously. The lowest concentration that inhibited visible growth of the tested organisms was recorded as MIC.
To determine the minimum bactericidal concentration (MBC)49) and minimum fungicidal concentration (MFC)50) for each set of test tubes in the MIC determination, a loopful of broth was collected from those tubes which did not show any growth and inoculated on sterile nutrient broth (for bacteria) and potato dextrose agar (PDA) (for fungi) by streaking. Plates inoculated with bacteria and fungi were incubated at 37°C for 24 h and at 28°C for 48 h, respectively. After incubation, the lowest concentration that kills the tested organisms was noted as MBC (for bacteria) or MFC (for fungi).
MTT Assay for Cell ViabilityThe cytotoxicity of the compounds was tested using A549 lung carcinoma cells. 5×104 cells were plated in each well of a 96-well tissue culture cluster (Nunc Inc., Germany) and incubated at 37°C in a medium containing Dulbecco’s modified Eagle’s medium (DMEM), 10% fetal bovine serum and antibiotics (Invitrogen, U.S.A.), in 5% CO2 atmosphere.51,52) After attachment of the cells (usually 3–4 h), different concentrations of the compound were added and incubated for 72 h. MTT solution (20 µL of 5 mg/mL) was added to each well and the incubation continued for additional 3 h. The dark blue formazan crystals formed within the healthy cells were solubilized with DMSO and the absorbance was estimated in enzyme-linked immunosorbent assay (ELISA) plate reader (7520 Microplate reader, Cambridge technologies, Inc.) at 550 nM and the absorbance was correlated with the cell number. Experiments were performed in triplicates and the values are the average of three (n=3) independent experiments. The inhibitory concentration (IC50) of the compound was assessed by Graph Pad Prism software.
The Department of Science and Technology (DST), New Delhi, India is gratefully acknowledged for its financial support.