2014 Volume 62 Issue 1 Pages 84-87
2014 Volume 62 Issue 1 Pages 84-87
The [RhCl(CO)2]2-catalyzed [2+2+1] cycloaddition of bis(allene)s, which have a substituent at the allenic terminus, produced the 8-substituted bicyclo[5.3.0]deca-1(10),6-dien-9-one frameworks. The synthesis of 8,10-dimethylbicyclo[5.3.0]deca-1(10),6-dien-9-one could also be achieved from the bis(1,1,3-trisubstituted-allene).
Recent efforts from this laboratory disclosed that the Rh(I)-catalyzed carbonylative [2+2+1] cycloaddition (Pauson–Khand-type reaction, PKTR)1) of allenynes (alkyne/allene derivatives)2–6) or allenenes (alkene/allene)7,8) afforded the corresponding bicyclo[5.3.0] compounds in satisfactory yields, which could hardly be obtained by the classical Pauson–Khand reaction9,10) of enynes (alkyne–alkene).11–17) Furthermore, the PKTR of the bis(allene)s 1 (n=1,2) has become a powerful tool for constructing not only the bicyclo[5.3.0]decadienones 2 (n=1), but also the larger-sized bicyclo[6.3.0]undecadienone skeletons 2 (n=2) in satisfactory to high yields18–20) (Chart 1). This ring-closing reaction has exclusively occurred at both terminal double bonds of the allenic moieties of 1 (n=1, 2). We have now investigated the application of this Rh(I)-catalyzed PKTR to the tri- and tetrasubstituted bis(allene)s 3 (R1=substituent, R2 and/or R3=H or substituent) to determine its scope and limitations.

Our initial evaluation for the Rh(I)-catalyzed PKTR was carried out using bis(allene) 5a, which has a methyl group at one of the two allenic termini (Table 1). According to the conditions previously reported for the PKTR of substrates 1, a solution of 5a in toluene was heated at 80°C for 12 h in the presence of 5 mol% [RhCl(CO)dppp]2 under an atmosphere of CO to afford the desired 8-methylbicyclo[5.3.0]decadienone 6a (dr=3 : 2) in 38% yield (entry 1). An increase in the loading amounts of [RhCl(CO)dppp]2 from 5 to 10 mol% provided 6a (dr=1 : 1) in a lower yield (27%, entry 2). Another catalyst, [RhCl(CO)2]2 (5 or 10 mol%), also gave the ring-closed products 6a (dr=1 : 1) in rather low yields (13% or 18%, entries 3, 4). A significant improvement (6a, dr=1 : 1, 90%) was observed when the ring-closing reaction was carried out in the presence of 5 mol% [RhCl(CO)2]2 under a higher CO atmosphere (5 atm) (entry 5).21) The formation of 6a can be rationalized in terms of the intermediacy of the 8-methylbicyclo[5.3.0]deca-1,6-dien-9-one derivative 6a′, the β,γ-unsaturated ketone moiety of which should immediately isomerize to the α,β-unsaturated one.
 | |||||
|---|---|---|---|---|---|
| Entry | Catalyst (mol%) | CO (atm) | Time (h) | Yield (%) | dr |
| 1 | [RhCl(CO)dppp]2 (5) | 1 | 12 | 38 | 3 : 2 |
| 2 | [RhCl(CO)dppp]2 (10) | 1 | 4 | 27 | 1 : 1 |
| 3 | [RhCl(CO)2]2 (5) | 1 | 20 | 13 | 1 : 1 |
| 4 | [RhCl(CO)2]2 (10) | 1 | 22 | 18 | 1 : 1 |
| 5 | [RhCl(CO)2]2 (5) | 5 | 2 | 90 | 1 : 1 |

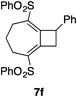
We next investigated the [RhCl(CO)2]2-catalyzed PKTR of several bis(allene) species 5b–f having trisubstituted-allenyl/disubstituted-allenyl moieties under 5 atm CO. These results are summarized in Table 2. The bis(allene)s 5b and c, having a heteroatom on the alkyl tether, consistently produced the corresponding bicyclic compounds 6b, c in good yields (6b: 69%, dr=3 : 1, 6c: 67%, dr=5 : 1, entries 1, 2). In the case of the malonate derivative 5d, the desired carbonylative [2+2+1] cycloaddition product 6d (dr=3 : 2) was formed in 33% yield as a minor product (entry 3). Instead, the corresponding [2+2] cycloaddition product 7d became the major product (63%). Introduction of the more hindered normal butyl group onto the allenyl moiety did not disturb this ring-closing reaction. Indeed, the ring-closing reaction of the bis(allene) derivative 5e having a normal butyl substituent produced the 8-butylbicyclo[5.3.0]decadienone derivative 6e in 75% yield (dr=9 : 5, entry 4). A better yield (86%, dr=9 : 5) was obtained when 5e was exposed to 10 atm CO conditions (entry 5). The phenylallenyl derivative 5f produced 6f in 56% yield (dr=10 : 7) along with the by-production of the [2+2] cycloadduct22) 7f in 32% yield (entry 6). The more preferential formation of 6f (73%) over 7f (17%) could be achieved when treated with 10 mol% [RhCl(CO)2]2 (entry 7). Thus, it became apparent that the bis(allene)s, possessing a substituent at one of the two allenic termini, can tolerate this ring-closing reaction and efficiently produce the 8-substituted bicyclo[5.3.0]decadienone skeletons.
Our next interest was directed toward the PKTR of two bis(allene) derivatives; one was the bis(trisubstituted-allene) derivative 5g and the other was the bis(allene) 5h having 1,1,3,3-tetrasubstituted-allenyl/1,1-disubstituted-allenyl moieties to confirm the limitations of this Rh(I)-catalyzed carbonylative ring-closing reaction. Upon exposure to the standard conditions, 5g underwent the ring-closing reaction to provide the desired 8,10-dimethylbicyclo[5.3.0]decadienone 6g in 56% yield (dr=3 : 2, Chart 2), although the prolonged reaction time (20 h) was necessary. On the other hand, 5h was found to be an inadequate substrate for our purpose; in fact, the undesired [2+2] cycloaddition product 7h was obtained in 25% yield as the sole isolatable product.

In conclusion, this investigation disclosed the following results. Namely, the Rh(I)-catalyzed PKTR of 1-substituted-3,7-bis(phenylsulfonyl)nona-1,2,7,8-tetraene derivatives consistently afforded the carbonylative bicyclo[5.3.0]decadienones, similar to those of the 1-unsubstituted ones.18,19) The 1,9-dimethyl congener also produced the corresponding carbonylative bicyclic compound in good yield. However, it was not the case when the 1,1-dimethyl-3,7-bis(phenylsulfonyl)nonatetraene derivative furnished the [2+2] cycloaddition product instead of the desired compound. Application of this procedure for the synthesis of the multisubstituted bicyclo[6.3.0]undecadienones is currently in progress.
Melting points are uncorrected. IR spectra were measured in CHCl3. 1H-NMR spectra were taken in CDCl3 unless otherwise indicated. CHCl3 (7.26 ppm) for silyl compounds and tetramethylsilane (0.00 ppm) for compounds without a silyl group were used as internal standards. 13C-NMR spectra were recorded in CDCl3 with CDCl3 (77.00 ppm) as an internal standard unless otherwise stated. All reactions were carried out under a nitrogen atmosphere unless otherwise stated. Silica gel (silica gel 60, 230–400 mesh) was used for chromatography. Organic extracts were dried over anhydrous Na2SO4.
General Procedure for Pauson–Khand-Type [2+2+1] Cycloaddition under an Atmosphere of COTo a solution of the bis(allene) 5a23) (0.10 mmol) in toluene (1.0 mL) was added Rh(I) catalyst. The reaction mixture was heated at 80°C under a CO atmosphere until the complete disappearance of the starting material (monitored by TLC). Toluene was evaporated off, and the residual oil was chromatographed with hexane-AcOEt to afford the cyclized product. Chemical yields are summarized in Table 1.
General Procedure for Pauson–Khand-Type [2+2+1] Cycloaddition under 5 atm COTo a solution of the bis(allene) 523) (0.10 mmol) in toluene (1.0 mL) was added 5 mol% [RhCl(CO)2]2. The reaction mixture was heated at 80°C under 5 atm CO pressure until the complete disappearance of the starting material (monitored by TLC). Toluene was evaporated off, and the residual oil was chromatographed with hexane–AcOEt to afford the cyclized product. Chemical yields are summarized in Tables 1, 2 and Chart 2.
| Entry | Bis(allene) | Time (h) | Result | |
|---|---|---|---|---|
| 1 |  | 2 |  | |
| 2 |  | 2 |  | |
| 3 |  | 2 |  |  |
 |  | |||
| 4 | 12 | 75%, dr=9:5 | ||
| 5b) | 2 | 86%, dr=9:5 | ||
 |  |  | ||
| 6 | 4.5 | 56%, dr=10:7 | 32% | |
| 7c) | 3 | 73%, dr=10:8 | 17% |
a) 5 was treated with 5 mol% of [RhCl(CO)2]2 in toluene under 5 atm of CO at 80°C. b) Reacted under 10 atm of CO. c) 10 mol% of Rh catalyst was used.
(2R*,8R*)- and (2R*,8S*)-8-Methyl-2,6-bis(phenylsulfonyl)bicyclo[5.3.0]deca-1(10),6-dien-9-one (6a): A mixture of diastereomer 6a in the ratio of 50 to 50 was obtained as a colorless oil; IR 1717, 1317, 1310, 1150 cm−1; 1H-NMR δ: 7.88–7.82 (m, 4H), 7.71–7.63 (m, 2H), 7.59–7.55 (m, 4H), 6.58 (s, 50/100×1H), 6.28 (s, 50/100×1H), 4.46 (t, 50/100×1H, J=6.4 Hz), 4.28 (t, 50/100×1H, J=7.3 Hz), 3.98 (q, 50/100×1H, J=7.3 Hz), 3.78 (q, 50/100×1H, J=7.3 Hz), 2.68–1.66 (m, 6H), 1.54 (d, 50/100×3H, J=7.3 Hz), 1.35 (d, 50/100×3H, J=7.3 Hz); 13C-NMR δ: 205.3, 160.63, 160.59, 151.4, 150.1, 139.8, 139.3, 139.1, 138.7, 137.2, 137.1, 137.0, 136.3, 134.52, 134.45, 133.9, 133.7, 129.49, 129.44, 129.41, 128.8, 128.6, 127.8, 127.5, 66.7, 64.7, 47.7, 47.2, 30.0, 27.9, 25.8, 25.0, 24.8, 24.0, 19.6, 19.1; FAB-MS m/z 443 (M+H, 2.4); FAB-high resolution (HR)-MS Calcd for C23H23O5S2 443.0987, Found 443.0986.
(2R*,8R*)- and (2R*,8S*)-N-(4-Methylbenzenesulfonyl)-8-methyl-2,6-bis(phenylsulfonyl)-4-azabicyclo[5.3.0]deca-1(10),6-dien-9-one (6b): A mixture of diastereomer 6b in the ratio of 76 to 24 was obtained as a colorless oil; IR 1720, 1325, 1310, 1153 cm−1; 1H-NMR δ: 8.00–7.99 (m, 24/100×2H), 7.96–7.94 (m, 76/100×2H), 7.79–7.54 (m, 76/100×8H, 24/100×8H), 7.34–7.33 (m, 76/100×2H, 24/100×2H), 7.13–7.11 (m, 76/100×2H, 24/100×2H), 6.20 (s, 24/100×1H), 5.68 (s, 76/100×1H), 4.85 (d, 76/100×1H, J=18.7 Hz), 4.79 (dd, 76/100×1H, J=9.0, 6.7 Hz), 4.60 (d, 24/100×1H, J=18.7 Hz), 4.44 (t, 24/100×1H, J=8.2 Hz), 4.30 (dd, 76/100×1H, J=15.6, 9.0 Hz), 4.18 (d, 24/100×1H, J=18.7 Hz), 4.14 (dd, 24/100×1H, J=13.7, 8.2 Hz), 4.07 (d, 76/100×1H, J=18.7 Hz), 3.93 (dd, 24/100×1H, J=13.7, 8.2 Hz), 3.77 (dd, 76/100×1H, J=15.6, 6.7 Hz), 3.58 (q, 76/100×1H, J=7.2 Hz), 3.35 (q, 24/100×1H, J=7.2 Hz), 2.34 (s, 76/100×3H, 24/100×3H), 1.47 (d, 76/100×3H, J=7.2 Hz), 1.24 (d, 24/100×3H, J=7.2 Hz); 13C-NMR δ: 203.47, 203.42, 157.0, 156.5, 151.6, 149.4, 144.7, 144.6, 140.9, 139.4, 138.7, 138.0, 136.33, 136.30, 136.0, 135.7, 135.5, 135.09, 135.02, 134.5, 134.3, 133.9, 129.8, 129.79, 129.75, 129.69, 129.67, 129.63, 128.8, 128.6, 128.4, 127.7, 127.0, 126.8, 67.9, 65.0, 50.7, 48.8, 47.0, 46.6, 45.47, 45.40, 21.4, 19.0, 18.9; MS m/z 597 (M+, 0.9); Anal. Calcd for C29H27NO7S3: C, 58.27; H, 4.55; N, 2.34, Found: C, 57.96; H, 4.59; N, 2.42.
(2R*,8R*)- and (2R*,8S*)-8-Methyl-2,6-bis(phenylsulfonyl)-4-oxabicyclo[5.3.0]deca-1(10),6-dien-9-one (6c): A mixture of diastereomer 6c in the ratio of 83 to 17 was obtained as a colorless oil; IR 1718, 1323, 1312, 1153 cm−1; 1H-NMR δ: 7.82–7.44 (m, 83/100×10H, 17/100×10H), 6.34 (s, 17/100×1H), 6.28 (s, 83/100×1H), 4.70 (dd, 83/100×1H, J=13.5, 3.7 Hz), 4.64–4.61 (m, 83/100×2H, 17/100×2H), 4.52 (d, 17/100×1H, J=17.7 Hz), 4.39 (d, 83/100×1H, J=17.1 Hz), 4.31 (d, 17/100×1H, J=17.7 Hz), 4.13 (dd, 17/100×1H, J=12.5, 4.9 Hz), 3.99–3.94 (m, 83/100×2H), 3.77 (q, 17/100×1H, J=7.3 Hz), 1.51 (d, 17/100×3H, J=7.3 Hz), 1.40 (d, 83/100×3H, J=7.3 Hz); 13C-NMR δ: 204.7, 204.6, 158.1, 157.7, 149.6, 149.3, 140.8, 139.9, 139.8, 139.3, 138.3, 137.0, 136.5, 135.6, 134.6, 134.14, 134.06, 129.6, 129.5, 129.3, 129.1, 129.0, 128.8, 127.7, 127.1, 77.5, 72.4, 70.3, 70.1, 69.4, 67.0, 47.0, 46.2, 19.7, 18.8; MS m/z 444 (M+, 8.8); Anal. Calcd for C22H20O6S2: C, 59.44; H, 4.53, Found: C, 59.28; H, 4.57.
(2R*,8R*)- and (2R*,8S*)-4,4-Bis(methoxycarbonyl)-8-methyl-2,6-bis(phenylsulfonyl)bicyclo[5.3.0]deca-1(10),6-dien-9-one (6d): A mixture of diastereomer 6d in the ratio of 60 to 40 was obtained as a colorless oil; IR 1734, 1717, 1317, 1310, 1150 cm−1; 1H-NMR δ: 7.96–7.93 (m, 40/100×2H), 7.88–7.86 (m, 60/100×2H), 7.82–7.80 (m, 60/100×2H), 7.74–7.55 (m, 60/100×6H, 40/100×8H), 7.01 (d, 60/100×1H, J=2.2 Hz), 5.85 (s, 40/100×1H), 4.49 (dd, 40/100×1H, J=7.3, 4.6 Hz), 4.29–4.26 (m, 60/100×1H), 3.83 (q, 40/100×1H, J=7.3 Hz), 3.80 (q, 60/100×1H, J=7.3 Hz), 3.78 (s, 60/100×3H), 3.66 (s, 40/100×3H), 3.64 (s, 40/100×3H), 3.36 (s, 60/100×3H), 3.32, 3.11 (ABq, 40/100×2H, J=17.8 Hz), 3.02, 2.95 (ABq, 60/100×2H, J=16.4 Hz), 2.94–2.92 (m, 40/100×2H), 2.75 (dd, 60/100×1H, J=15.4, 10.1 Hz), 2.58 (dd, 60/100×1H, J=15.4, 2.6 Hz), 1.47 (d, 60/100×3H, J=7.3 Hz), 1.41 (d, 40/100×3H, J=7.3 Hz); 13C-NMR δ: 204.9, 204.6, 169.5, 168.8, 160.4, 159.0, 153.2, 150.6, 139.5, 139.1, 138.6, 136.7, 136.5, 136.0, 134.9, 134.86, 134.83, 134.6, 134.04, 134.03, 130.5, 129.60, 129.59, 129.5, 129.4, 129.2, 129.0, 128.2, 127.82, 127.78, 64.7, 61.1, 55.9, 55.2, 53.6, 53.3, 53.2, 47.0, 45.5, 33.3, 31.9, 31.3, 30.1, 20.0, 19.1; FAB-MS m/z 559 (M+H, 6.1); FAB-HR-MS Calcd for C27H27O9S2 559.1096, Found 559.1094.
4,4-Bis(methoxycarbonyl)-8-methyl-2,6-bis(phenylsulfonyl)bicyclo[5.2.0]nona-1,6-diene (7d): Colorless oil; IR 1735, 1321, 1308, 1148 cm−1; 1H-NMR δ: 7.90–7.88 (m, 4H), 7.66–7.64 (m, 2H), 7.60–7.56 (m, 4H), 3.60 (s, 3H), 3.59–3.57 (m, 1H), 3.46 (s, 3H), 3.41 (ddt, 1H, J=18.0, 9.0, 2.6 Hz), 3.13–3.10 (m, 2H), 2.92–2.88 (m, 1H), 2.79–2.75 (m, 1H), 2.71 (ddt, 1H, J=12.6, 4.5, 2.6 Hz), 1.46 (d, 3H, J=7.1 Hz); 13C-NMR δ: 169.7, 168.4, 153.6, 148.2, 139.7, 139.5, 135.8, 134.5, 133.80, 133.77, 129.30, 129.26, 128.1, 127.8, 54.3, 53.3, 52.8, 38.5, 37.8, 35.8, 35.0, 21.1; MS m/z 530 (M+, 17.5); HR-MS Calcd for C26H26O8S2 530.1069, Found 530.1072.
(2R*,8R*)- and (2R*,8S*)-8-Butyl-2,6-bis(phenylsulfonyl)bicyclo[5.3.0]deca-1(10),6-dien-9-one (6e): A mixture of diastereomer 6e in the ratio of 64 to 36 was obtained as a yellow oil; IR 1713, 1319, 1310, 1150 cm−1; 1H-NMR δ: 7.86–7.82 (m, 64/100×4H, 36/100×4H), 7.70–7.63 (m, 64/100×2H, 36/100×2H), 7.58–7.54 (m, 64/100×4H, 36/100×4H), 6.62 (s, 64/100×1H), 6.43 (s, 36/100×1H), 4.41 (t, 36/100×1H, J=6.6 Hz), 4.28 (t, 64/100×1H, J=8.2 Hz), 3.93 (dd, 36/100×1H, J=9.0, 3.4 Hz), 3.81 (dd, 64/100×1H, J=7.9, 3.3 Hz), 2.71–2.64 (m, 64/100×1H, 36/100×1H), 2.49–2.38 (m, 64/100×1H, 36/100×1H), 2.33–2.23 (m, 64/100×2H, 36/100×2H), 2.18–2.13 (m, 36/100×2H), 2.05–1.92 (m, 64/100×2H, 36/100×2H), 1.72–1.64 (m, 64/100×2H), 1.54–0.92 (m, 64/100×4H, 36/100×4H), 0.89 (t, 36/100×3H, J=6.8 Hz), 0.78 (t, 64/100×3H, J=7.4 Hz); 13C-NMR δ: 204.9, 204.6, 161.2, 160.8, 150.8, 150.1, 139.4, 139.3, 138.2, 137.3, 137.2, 137.1, 136.1, 134.5, 134.4, 133.8, 133.7, 129.43, 129.41, 129.39, 128.9, 128.7, 127.7, 127.5, 66.0, 64.5, 52.1, 51.9, 33.6, 33.4, 29.6, 28.0, 27.7, 27.0, 25.7, 24.9, 24.7, 24.0, 22.6, 22.5, 13.9, 13.7; MS m/z 484 (M+, 16.2); HR-MS Calcd for C26H28O5S2 484.1378, Found 484.1378.
(2R*,8R*)- and (2R*,8S*)-8-Phenyl-2,6-bis(phenylsulfonyl)bicyclo[5.3.0]deca-1(10),6-dien-9-one (6f): A mixture of diastereomer 6f in the ratio of 56 to 44 was obtained as a yellow oil; IR 1724, 1308, 1151 cm−1; 1H-NMR δ: 7.93–7.84 (m, 56/100×2H, 44/100×2H), 7.72–7.50 (m, 56/100×4H, 44/100×4H), 7.38–7.13 (m, 56/100×8H, 44/100×8H), 6.85 (s, 44/100×1H), 6.79–6.77 (m, 56/100×1H, 44/100×1H), 6.37 (s, 56/100×1H), 5.26 (s, 56/100×1H), 5.06 (s, 44/100×1H), 4.55 (t, 56/100×1H, J=6.2 Hz) 4.40 (m, 44/100×1H), 3.04 (dd, 44/100×1H, J=17.4, 7.4 Hz), 2.92–2.78 (m, 56/100×1H, 44/100×1H), 2.56–2.37 (m, 56/100×2H, 44/100×2H), 2.28–2.19 (m, 56/100×1H), 2.15–2.06 (m, 44/100×1H), 2.04–1.94 (m, 56/100×1H), 1.93–1.87 (m, 56/100×1H), 1.83–1.78 (m, 44/100×1H); 13C-NMR δ: 201.0, 200.9, 163.1, 162.4, 148.7, 147.7, 139.7, 139.64, 139.60, 139.2, 138.8, 137.3, 137.0, 136.5, 134.5, 133.6, 133.5, 129.5, 129.1, 129.0, 128.8, 128.7, 127.91, 127.87, 127.8, 127.2, 127.12, 127.09, 66.2, 64.6, 57.0, 56.5, 27.5, 27.2, 25.7, 25.0, 24.8, 23.8; FAB-MS m/z 505 (M+H, 80.7); FAB-HR-MS Calcd for C28H25O5S2 505.1144, Found 505.1146.
8-Phenyl-2,6-bis(phenylsulfonyl)bicyclo[5.2.0]nona-1,6-diene (7f): Colorless powder; mp 175.0–177.0°C (AcOEt); IR 1308, 1151 cm−1; 1H-NMR δ: 7.89–7.86 (m, 2H), 7.68–7.64 (m, 1H), 7.59–7.55 (m, 2H), 7.48–7.44 (m, 1H), 7.35–7.22 (m, 7H), 7.01–6.99 (m, 2H), 4.78–4.75 (m, 1H), 3.81–3.73 (m, 1H), 3.29–3.22 (m, 1H), 2.71–2.64 (m, 2H), 2.51–2.37 (m, 2H), 1.99–1.91 (m, 1H), 1.76–1.67 (m, 1H); 13C-NMR δ: 151.5, 148.1, 143.4, 140.1, 139.4, 139.2, 139.1, 133.7, 133.3, 129.4, 128.9, 128.6, 127.9, 127.8, 127.6, 126.8, 46.8, 41.2, 30.9, 23.1; MS m/z 428 (M+, 37.8); HR-MS Calcd for C27H24O4S2 428.1116, Found 428.1115; Anal. Calcd for C27H24O4S2: C, 68.04; H, 5.08, Found: C, 67.65; H, 5.11.
(2R*,8R*)- and (2R*,8S*)-8,10-Dimethyl-2,6-bis(phenylsulfonyl)bicyclo[5.3.0]deca-1(10),6-dien-9-one (6g): A mixture of diastereomer 6g in the ratio of 61 to 39 was obtained as a colorless oil; IR 1713, 1317, 1307, 1150 cm−1; 1H-NMR δ: 7.97–7.96 (m, 61/100×1H, 39/100×1H), 7.91–7.90 (m, 61/100×1H, 39/100×1H), 7.83–7.81 (m, 61/100×1H, 39/100×1H), 7.72–7.51 (m, 61/100×7H, 39/100×7H), 4.68–4.66 (m, 61/100×1H), 4.56–4.54 (m, 39/100×1H), 4.06 (q, 61/100×1H, J=7.1 Hz), 3.94 (q, 39/100×1H, J=7.3 Hz), 2.69–1.67 (m, 61/100×6H, 39/100×6H), 1.58 (d, 61/100×3H, J=7.1 Hz), 1.55 (d, 39/100×3H, J=7.3 Hz), 1.39 (s, 61/100×3H), 1.21 (s, 39/100×3H); 13C-NMR δ: 205.8, 205.4, 152.9, 152.2, 149.4, 148.7, 148.6, 148.5, 140.3, 140.2, 139.6, 138.2, 137.3, 135.5, 134.44, 134.38, 133.6, 133.3, 129.6, 129.4, 129.3, 129.1, 128.7, 128.6, 128.3, 127.2, 65.3, 65.1, 46.6, 44.6, 30.8, 26.3, 25.6, 24.9, 24.3, 19.1, 18.9, 9.3, 9.2; MS m/z 456 (M+, 8.4); HR-MS Calcd for C24H24O5S2 456.1065, Found 456.1061.
8,8-Dimethyl-2,6-bis(phenylsulfonyl)bicyclo[5.2.0]nona-1,6-diene (7h): Colorless oil; IR 1315, 1308, 1150 cm−1; 1H-NMR δ: 7.85–7.83 (m, 4H), 7.65–7.60 (m, 2H), 7.57–7.52 (m, 4H), 3.06 (t, 2H, J=2.9 Hz), 2.47–2.45 (m, 2H), 2.36–2.34 (m, 2H), 1.72–1.68 (m, 2H), 1.63 (s, 6H); 13C-NMR δ: 156.5, 146.2, 140.3, 139.9, 139.8, 138.3, 133.6, 133.5, 129.30, 129.26, 127.9, 127.6, 46.1, 45.0, 31.5, 31.2, 27.1, 23.0; MS m/z 428 (M+, 37.8); HR-MS Calcd for C23H24O4S2 428.1116, Found 428.1115.
This work was supported in part by a Grant-in-Aid for Scientific Research from the Ministry of Education, Culture, Sports, Science and Technology of Japan, for which we are thankful.