2014 年 62 巻 11 号 p. 1119-1124
2014 年 62 巻 11 号 p. 1119-1124
2H-[1,2,3]Triazolo[4,5-g]isoquinoline-4,9-diones and 2H-[1,2,3]triazolo[4,5-g]quinoline-4,9-diones were synthesized and tested for in vitro antifungal activity against pathogenic fungi. Many of those synthesized showed potent antifungal activity. Compounds 3a, 3b, 3g, and 3h completely inhibited the growth of all fungal species tested at the MIC level of 0.8–12.5 µg/mL. The results suggest that 2H-[1,2,3]triazolo[4,5-g]isoquinoline-4,9-diones could be antifungal agents.
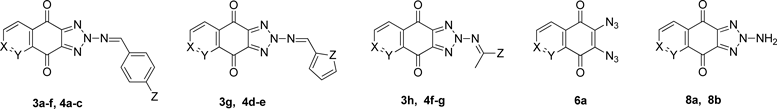
The increase of fungal infections, among patients undergoing anticancer chemotherapy and AIDS patients, has generated a renewed interest in antifungal drugs, including development of new antifungal agents in the development of resistance to drugs.1,2) Heterocyclic quinonoid compounds represent an attractive class of biologically active molecules.3) In our previous reports,4,5) quinonoid compounds such as 5,8-quinolinediones 14) and 1H-benzo[d]imidazole-4,7-diones 25) have demonstrated potent antifungal activity against pathogenic fungi (Fig. 1). To identify novel antifungal agents, we focused on developing quinonoid 2H-[1,2,3]triazolo[4,5-g]isoquinoline-4,9-diones 3 and 2H-[1,2,3]triazolo[4,5-g]quinoline-4,9-diones 4. Structure–activity relationship (SAR) studies from heterocyclic quinonoid compounds indicated that the ring number and the position of hetero atoms in the heterocyclic ring with various substituents were considerably important factors to affect the biological activities.5–9) We speculated that incorporation of a 2H-[1,2,3]triazole moiety into the 5,8-quinolinedione or 5,8-isoquinolinedione skeleton would change the physicochemical properties and lead to a new pharmacophore with a different biological profile from quinonoid compounds 1 and 2. Based on this information, 2H-[1,2,3]triazolo[4,5-g]isoquinoline-4,9-diones 3 and 2H-[1,2,3]triazolo[4,5-g]quinoline-4,9-diones 4 were synthesized and evaluated for their antifungal activity. The synthesis and biological activity of 2H-[1,2,3]triazole compounds 3 and 4 have not been reported to the best of our knowledge. Therefore, 2H-[1,2,3]triazolo[4,5-g]isoquinoline-4,9-dione scaffold 3 and 2H-[1,2,3]triazolo[4,5-g]quinoline-4,9-dione scaffold 4 with various substituents were designed and synthesized to elucidate their contribution to the antifungal activity (Chart 1).


Reagents and conditions: (a) NaN3/methanol/r.t./2 h/95–97%; (b) PPh3/methanol/reflux/24 h/85–92%; (c) Aryl aldehyde/CH2Cl2/HCOOH/reflux/24–72 h/44–82%; (d) Furan-2-carbaldehyde or thiophene-2-carbaldehyde/CH2Cl2/HCOOH/reflux/48 h/30–74%; (e) HCl/CH2Cl2/reflux/4 h/58–62%; (f) Acetone or butan-2-one/HCOOH/reflux/ 24 h/52–75%.
We describe herein our results on the synthesis of compounds 3 and 4 with their antifungal activity on the pathogenic fungal strains. The in vitro antifungal activity of new compounds 3a–h, 4a–g, 6a and 8a, b was determined by the twofold broth dilution method. Additional data for antifungal activity are provided.
A efficient method for synthesis and structures of 2H-[1,2,3]triazolo[4,5-g]isoquinoline-4,9-diones 3a–h and 2H-[1,2,3]triazolo[4,5-g]quinoline-4,9-diones 4a–g are shown in Chart 1 and Table 1. 6,7-Dichloro-5,8-isoquinolinedione (5a) was prepared by chloro-oxidation from commercially available isoquinolin-5-ol according to the known method.10,11) 6,7-Diazidoisoquinoline-5,8-dione (6a) was synthesized by nucleophilic substitution of compound 5a with sodium azide. To 1 eq of the compound 5a in MeOH, 2 eq of sodium azide was added and the mixture was stirred for 2 h at room temperature to give compound 6a. 2-((Triphenylphosphoranylidene)amino)-2H-[1,2,3]triazolo[4,5-g]-isoquinoline-4,9-dione (7a) was obtained in almost quantitative yield by cyclization of the compound 6a with triphenylphosphine. The compound 6a reacted with 1 eq of triphenylphosphine in MeOH to form compound 7a. The triphenylphosphine probably adds to the end nitrogen of azide via a Staudinger-like reaction.12) Consequently, compounds 3a–f were synthesized by substitution of the compound 7a with appropriate aryl aldehyde. To the compound 7a in CH2Cl2 in presence of catalytic amount of formic acid, the aryl aldehyde was added and then refluxed for 72 h to give compounds 3a–f. The compound 7a underwent aza-Wittig reaction12) with aryl aldehydes, producing compounds 3a–f, which could be obtained by reaction of aryl aldehydes with 2-amino-2H-[1,2,3]triazolo[4,5-g]isoquinoline-4,9-dione (8a). Also, compound 3g was synthesized by aza-Wittig reaction of the compound 7a with furan-2-carbaldehyde in CH2Cl2. Most of the reactions went as expected and had overall high yields. The compound 7a was hydrolyzed by HCl to obtain compound 8a. To the compound 7a in CH2Cl2, 6 M HCl solution was added and the mixture was refluxed for 4 h to afford the compound 8a. The compound 8a reacted with acetone to synthesize compound 3h. The compound 8a in acetone with a trace of formic acid as catalyst was refluxed for 24 h to form compound 3h. Most of these reactions went as expected and had overall high yields.
 | |||||||||
|---|---|---|---|---|---|---|---|---|---|
| Compound | X | Y | Z | MICa) (µg/mL) | |||||
| C. albicansb) | C. tropicalis | C. krusei | C. neoformans | A. niger | A. flavus | ||||
| 3a | N | CH | H | 1.6 | 0.8 | 12.5 | 12.5 | 1.6 | 1.6 |
| 3b | N | CH | F | 1.6 | 3.2 | 6.3 | 6.3 | 0.8 | 0.8 |
| 3c | N | CH | Cl | 12.5 | 6.3 | 12.5 | 100.0 | 12.5 | 6.3 |
| 3d | N | CH | Br | 12.5 | 3.2 | 12.5 | 100.0 | 12.5 | 12.5 |
| 3e | N | CH | CF3 | 3.2 | 3.2 | 12.5 | 100.0 | 12.5 | 6.3 |
| 3f | N | CH | 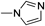 | 3.2 | 6.3 | 6.3 | 1.6 | 12.5 | 6.3 |
| 3g | N | CH | O | 1.6 | 0.8 | 1.6 | 6.3 | 0.8 | 0.8 |
| 3h | N | CH | Me | 1.6 | 0.8 | 6.3 | 6.3 | 1.6 | 1.6 |
| 4a | CH | N | H | 25.0 | 50.0 | 12.5 | 6.3 | 25.0 | 50.0 |
| 4b | CH | N | F | 50.0 | 50.0 | 12.5 | 25.0 | 50.0 | >100.0 |
| 4c | CH | N | CF3 | 25.0 | 50.0 | 12.5 | 12.5 | 12.5 | 25.0 |
| 4d | CH | N | O | 6.3 | 12.5 | 3.2 | 1.6 | 3.2 | 6.3 |
| 4e | CH | N | S | 25.0 | 50.0 | 25.0 | 25.0 | 50.0 | 100.0 |
| 4f | CH | N | Me | 25.0 | 50.0 | 12.5 | 12.5 | 12.5 | 25.0 |
| 4g | CH | N | Et | 50.0 | >100.0 | 25.0 | 6.3 | 50.0 | 50.0 |
| 6a | N | H | — | 50.0 | 100.0 | 50.0 | 100.0 | 50.0 | 100.0 |
| 8a | N | H | — | 12.5 | 6.3 | 6.3 | 100.0 | 6.3 | 3.2 |
| 8b | H | N | — | 50.0 | 100.0 | 6.3 | 3.2 | 50.0 | 100.0 |
| Fluconazole | — | — | — | 50.0 | 12.5 | 12.5 | 3.2 | 50.0 | 25.0 |
| 5-FCc) | — | — | — | 3.2 | 6.3 | 6.3 | 6.3 | 6.3 | 25.0 |
a) The MIC value is defined as lowest concentration of the antifungal agent exhibiting no fungal growth. MIC values were read after 1 d for Candida species and C. neoformans, and 2 d for A. niger, A. flavus in 37°C. The inoculum sizes contained approximately 1×105cells/mL. Culture media tested were the modified Sabouraud dextrose broth (Difco Lab.). The final concentration of antifungal agents was between 0.2 and 100 µg/mL. b) Fungi tested: Candida albicans Berkout KCCM 50235, C. tropicalis Berkout KCCM 50662, C. krusei Berkout KCCM 11655, Cryptococcus neoformans KCCM 50564, Aspergillus niger KCTC 1231, and A. flavus KCCM 11899. c) 5-FC: 5-Fluorocytosine.
In a similar manner, 2H-[1,2,3]triazolo[4,5-g]quinoline-4,9-diones 4a–g were synthesized (Chart 1 and Table 1). 6,7-Dichloro-5,8-quinolinedione (5b) was prepared by chloro-oxidation from 5-aminoquinolin-8-ol.10,11) 6,7-Diazidoisoquinoline-5,8-dione (6b) was synthesized by nucleophilic substitution of compound 5b with sodium azide. 2-((Triphenylphosphoranylidene)amino)-2H-[1,2,3]triazolo[4,5-g]-quinoline-4,9-dione (7b) was obtained by cyclization of compound 6b with triphenylphosphine. Consequently, compounds 4a–c were synthesized by substitution of compound 7b with appropriate aryl aldehyde in CH2Cl2 in presence of formic acid. Also, compounds 4d, e were synthesized by aza-Wittig reaction of the compound 7b with furan-2-carbaldehyde or thiophene-2-carbaldehyde in CH2Cl2. The compound 7b was hydrolyzed by HCl to obtain 2-amino-2H-[1,2,3]triazolo[4,5-g]quinoline-4,9-dione (8b). Compound 8b reacted with appropriate ketone to synthesize compounds 4f, g. The compound 8b in acetone or butan-2-one with a trace of formic acid was refluxed for 24 h to form compounds 4f, g. Most of these reactions went as expected and had overall high yields.
Antifungal EvaluationThe synthesized compounds 3a–h, 4a–g, 6a, and 8a, b were tested in vitro for their growth inhibitory activity against Candida, Aspergillus species and Cryptococcus neoformans using the standard twofold broth dilution method.13) The minimum inhibitory concentration (MIC) values were determined by a comparison with fluconazole and 5-fluorocytosine as standard agents.13) As indicated in Table 1, among synthesized compounds 3a–h, 4a–g, 6a, and 8a, b, many of compounds generally showed potent antifungal activity against all pathogenic fungal strains tested. Actually, the activity of compounds 3a, b, g, and h was superior or comparable to that of 5-fluorocytosine against tested fungi. The compounds 3a, b, g, and h completely inhibited the growth of all fungal species tested at the MIC level of 0.8–12.5 µg/mL. The activity of many of compounds tested was comparable to that of 5-fluorocytosine against some strain of fungi. Actually, the activity of compound 3e and 4d was superior or comparable to that of 5-fluorocytosine against Candida species and Aspergillus species. Most of the compounds among them tested also were superior or comparable to that of fluconazole against Candida species, Cryptococcus neoformans, and Aspergillus species.
In terms of SAR, the 2H-[1,2,3]triazolo[4,5-g]isoquinoline-4,9-diones 3a–h showed, in general, more potent antifungal activity than other 2H-[1,2,3]triazolo[4,5-g]quinoline-4,9-diones 4a–g. The activity of 2H-[1,2,3]triazolo[4,5-g]isoquinoline-4,9-diones 3a–h was better than that of 2H-[1,2,3]triazolo[4,5-g]quinoline-4,9-diones 4a–g. The 2H-[1,2,3]triazolo[4,5-g]isoquinoline-4,9-dione scaffold exhibited good activity, indicating a correlation that may offer insight into the mode of action of these compounds. The position of nitrogen atom in isoquinoline ring of compounds 3a–h was considerably important factors to affect the antifungal activity. In addition, 6,7-diazidoisoquinoline-5,8-dione (6a) exhibited no or poor, if any, antifungal activity. In contrast, many 2H-[1,2,3]triazole compounds 3a–h, 4a–g, and 8a, b showed more potent antifungal activity than compound 6a. The 2H-[1,2,3]triazole moiety of compounds 3a–h, 4a–g, and 8a, b should be impotent for the activity, for example, as non-2H-[1,2,3]triazole compound 6a lost the activity, indicating a correlation that may offer insight into the mode of action of these triazole compounds. The 2-(benzylideneamino)-, 2-((furan-2-ylmethylene)amino)- or 2-(propan-2-ylideneamino)-moiety for the compounds 3a–h, 4a–g, and 8a, b may contribute partially toward biological potency. Thus, the moiety appears to be a factor to affect their antifungal activity. However, the substituents (Z: H, X, Me, etc.) for compounds 3a–f and 4a–c may not contribute partially toward biological potency.
Compounds 7a, b were synthesized from dichloroquinones 5a, b via formation of bis-diazides 6a, b followed by reaction with triphenylphosphine. The compounds 7a, b underwent aza-Wittig reaction with appropriate aldehyde, producing compounds 3a–g and 4a–e. Compounds 8a, b were prepared by hydrolysis of compounds 7a, b with HCl. The compounds 8a, b reacted with appropriate ketone to synthesize compounds 3h and 4f, g. Most of these reactions went as expected and had overall high yields. Synthesized new compounds were tested in vitro for their growth inhibitory activity against pathogenic fungi. Among them tested, many of new 2H-[1,2,3]triazolo compounds 3a–h showed potent antifungal activity. The compounds 3a, 3b, 3g, and 3h completely inhibited the growth of all fungal species tested at the MIC level of 0.8–12.5 µg/mL. The results suggest that 2H-[1,2,3]triazolo[4,5-g]isoquinoline-4,9-dione scaffold could be promising leads for the development of antifungal agents.
All melting points were measured with Büchi melting point B-545 and were uncorrected. 1H-NMR spectra, 13C-NMR and 19F-NMR spectra were recorded on Varian Unity INOVA 400 MHz FT-NMR spectrometer with tetramethylsilane (TMS) or CFCl3. High resolution (HR)-MS spectra were recorded with a Agilent 6220 Accurate-mass time-of-flight (TOF)/LC-MS equipped with an electrospray ionisation ion source used. Mass spectra were taken with JEOL JMS AX505 WA. The IR spectra were taken from PerkinElmer, Inc. 1420r IR spectrometer with KBr pellets or Nujol. Elemental analyses were performed by CE instruments EA1110 with sulfanilamide as standard material, and analytical results for C, H, and N were within ±0.4% of theoretical values.
The 6,7-dichloro-5,8-isoquinolinedione (5a) and 6,7-dichloro-5,8-quinolinedione (5b) were prepared by chloro-oxidation from commercially available isoquinolin-5-ol and 5-aminoquinolin-8-ol according to the known method.10,11)
The products were separated by silica gel column chromatography. Purity of products was determined by both TLC and HPLC. The results showed that a single compound was contained in each product. TLC was performed on precoated silica gel (60G 254, Merck) using n-hexane–ethyl acetate for solvent. The compounds were detected under UV light (254 nm).
2-((Triphenylphosphoranylidene)amino)-2H-[1,2,3]triazolo[4,5-g]isoquinoline-4,9-dione (7a)To a solution of 6,7-dichloroisoquinoline-5,8-dione (5a, 2.0 mmol) in 50 mL of MeOH, sodium azide (7.0 mmol) was added portionwise. The mixture was stirred at room temperature for 2 h with the yellow slurry and it turned into bright red to form compound 6a. To this reaction mixture, tripheylphosphine (0.38 g, 1.5 mmol) was added slowly and the mixture was then refluxed for 24 h. The solvent was removed in vacuo. The precipitate was filtered, washed two times with 150 mL portion of water, and dried in vacuum to afford compound 7a. Dark red powder (92%). mp 245–247°C. IR (KBr) cm−1: 1689 (s), 1534 (s), 1346 (s), 1221 (s), 1111 (s). 1H-NMR (CDCl3, 400 MHz) δ: 9.38 (s, 1H), 9.01 (d, 1H, J=4.8 Hz), 7.90 (d, 1H, J=4.8 Hz), 7.90 (m, 6H), 7.58 (m, 9H). 13C-NMR (CDCl3, 100 MHz) δ: 178.2, 176.6, 155.7, 147.6, 139.0, 133.4 (2C), 133.0 (6C), 132.9 (6C), 129.4 (3C), 129.2 (3C), 124.8, 118.7. HR-MS m/z: 476.1275 [(M+H)+] (Calcd for C27H19N5O2P 476.1276).
General Procedure for Synthesis of 2H-[1,2,3]Triazolo[4,5-g]isoquinoline-4,9-diones (3a–g)To a mixture of compound 7a (0.5 mmol) with benzyl aldehyde derivatives or furan-2-carbaldehyde (1.5 mmol) in 50 mL of CH2Cl2, 3 mL of formic acid was added dropwise. The mixture was refluxed for 2–3 d and concentrated in vacuo. The precipitate was filtered and washed three times with 50 mL portion of methanol and crystalized from 95% ethanol to afford compounds 3a–g.
2-(Benzylideneamino)-2H-[1,2,3]triazolo[4,5-g]isoquinoline-4,9-dione (3a)Peach powder (73%). mp 266–268°C. IR (KBr) cm−1: 1695 (s), 1598 (s), 1407 (s), 1235 (s). 1H-NMR (DMSO-d6, 400 MHz) δ: 9.71 (s, 1H), 9.40 (s, 1H), 9.20 (s, 1H), 8.16 (d, 1H, J=7.2 Hz), 7.83 (m, 1H), 7.71 (m, 2H), 7.63 (m, 2H). 13C-NMR (CDCl3, 100 MHz) δ: 176.7, 176.4, 160.3, 156.2, 148.3, 139.6, 132.6 (2C), 131.8 (2C), 127.8 (2C), 126.8, 121.4, 119.1, 119.0. HR-MS m/z: 304.0828 [(M+H)+] (Calcd for C16H10N5O2 304.0834). Anal. Calcd for C16H9N5O2: C, 63.37; H, 2.99; N, 23.09; Found: C, 63.39; H, 3.00; N, 23.04.
2-((4-Fluorobenzylidene)amino)-2H-[1,2,3]triazolo[4,5-g]isoquinoline-4,9-dione (3b)Dark yellow powder (60%). mp 270–272°C. IR (KBr) cm−1: 1685 (s), 1572 (s), 1559(s), 1389 (s), 1229 (s). 1H-NMR (DMSO-d6, 400 MHz) δ: 9.39 (s, 1H), 9.19 (d, 1H, J=4.8 Hz), 8.48 (s, 1H), 8.24 (m, 2H), 8.07 (d, 1H, J=4.8 Hz), 7.48 (m, 2H). 13C-NMR (CDCl3, 100 MHz) δ: 176.6, 176.3, 164.1, 160.1, 156.1, 155.9, 139.2, 132.9, 132.8 (2C), 127.7, 126.8, 119.0, 118.9, 116.8, 116.6. 19F-NMR (DMSO-d6, 376.3 MHz) δ: −116.6. HR-MS m/z: 322.0735 [(M+H)+] (Calcd for C16H9FN5O2 322.0740). Anal. Calcd for C16H8FN5O2: C, 59.82; H, 2.51; N, 21.80; Found: C, 59.84; H, 2.52; N, 21.81.
2-((4-Chlorobenzylidene)amino)-2H-[1,2,3]triazolo[4,5-g]isoquinoline-4,9-dione (3c)Yellow green powder (44%). mp 324–325°C. IR (KBr) cm−1: 1681 (s), 1577 (s), 1562, 1390 (s), 1225 (s), 1180. 1H-NMR (DMSO-d6, 400 MHz) δ: 9.72 (s, 1H), 9.39 (s, 1H), 9.19 (d, 1H, J=4.8 Hz), 8.17 (d, 2H, J=8.0 Hz), 8.01 (d, 1H, J=4.8 Hz), 7.71 (d, 2H, J=8.0 Hz). 13C-NMR (CDCl3, 100 MHz) δ: 176.7, 176.4, 160.2, 156.2, 148.3, 143.9, 139.6, 138.6 (2C), 131.7 (2C), 130.0 (2C), 129.6, 119.1, 119.0. HR-MS m/z: 338.0439 [(M+H)+] (Calcd for C16H9ClN5O2 338.0445). Anal. Calcd for C16H8ClN5O2: C, 56.90; H, 2.39; N, 20.74; Found: C, 56.92; H, 2.40; N, 20.73.
2-((4-Bromobenzylidene)amino)-2H-[1,2,3]triazolo[4,5-g]isoquinoline-4,9-dione (3d)Bright brown powder (46%). mp 328–329°C. IR (KBr) cm−1: 1698 (s), 1571 (s), 1556, 1393 (s), 1221 (s), 1182 (s). 1H-NMR (DMSO-d6, 400 MHz) δ: 9.70 (s, 1H), 9.39 (s, 1H), 9.19 (d, 1H, J=4.8 Hz), 8.09 (d, 1H, J=4.8 Hz), 8.04 (d, 2H, J=8.4 Hz), 7.85 (d, 2H, J=8.4 Hz). 13C-NMR (CDCl3, 100 MHz) δ: 176.7, 176.4, 160.3, 156.2, 148.3, 139.6, 132.6 (2C), 131.8 (2C), 130.3, 127.8 (2C), 126.8, 119.1, 119.0. HR-MS m/z: 381.9936 [(M+H)+] (Calcd for C16H9BrN5O2 381.9940). Anal. Calcd for C16H8BrN5O2: C, 50.28; H, 2.11; N, 18.33; Found: C, 50.25; H, 2.12; N, 18.32.
2-((4-(Trifluoromethyl)benzylidene)amino)-2H-[1,2,3]triazolo[4,5-g]isoquinoline-4,9-dione (3e)Dark ivory powder (20%). mp 231–233°C. IR (KBr) cm−1: 1692 (s), 1589 (s), 1402 (s), 1230 (s). 1H-NMR (DMSO-d6, 400 MHz) δ: 9.83 (s, 1H), 9.40 (d, 1H, J=4.8 Hz), 9.19 (d, 1H, J=4.8 Hz), 8.36 (d, 2H, J=8.4 Hz), 8.08 (m, 1H), 7.96 (d, 2H, J=8.4 Hz). 13C-NMR (CDCl3, 100 MHz) δ: 176.6, 176.3, 159.9, 156.1, 155.8, 139.1, 134.8, 132.9, 132.6, 130.6 (2C), 126.2, 126.1 (2C), 122.4, 119.0, 118.9. HR-MS m/z: 372.0701 [(M+H)+] (Calcd for C17H9F3N5O2 372.0708). Anal. Calcd for C17H8F3N5O2: C, 55.00; H, 2.17; N, 18.86; Found: C, 55.04; H, 2.18; N, 18.83.
2-((4-(1H-Imidazol-1-yl)benzylidene)amino)-2H-[1,2,3]triazolo[4,5-g]isoquinoline-4,9-dione (3f)Bright brown powder (82%). mp 282–284°C. IR (KBr) cm−1: 1690 (s), 1608 (s), 1405 (s), 1240 (s). 1H-NMR (DMSO-d6, 400 MHz) δ: 9.80 (s, 1H), 9.41 (s, 1H), 9.21 (d, 1H, J=5.2), 8.38 (dd, 2H, J=6.8 Hz, J=2.0 Hz), 8.15 (d, 1H, J=5.2 Hz), 8.09 (dd, 2H, J=6.8 Hz, J=2.0 Hz), 8.01 (d, 1H, J=5.4 Hz), 8.04 (d, 1H, J=5.4 Hz), 7.72 (s, 1H). 13C-NMR (CDCl3, 100 MHz) δ: 176.7, 176.4, 159.9, 156.2, 148.3, 135.6, 138.7, 139.6 (2C), 135.3, 131.7 (2C), 131.6 (2C), 125.7, 122.4, 120.3, 119.1, 118.3. HR-MS m/z: C19H12N7O2 370.1058 [(M+H)+] (Calcd for C19H12N7O2 370.1052). Anal. Calcd for C19H11N7O2: C, 61.79; H, 3.00; N, 26.55; Found: C, 61.60; H, 3.01; N, 26.53.
2-((Furan-2-ylmethylene)amino)-2H-[1,2,3]triazolo[4,5-g]isoquinoline-4,9-dione (3g)1688 (s), 1591 (s), 1404 (s), 1237 (s). 1H-NMR (DMSO-d6, 100 MHz) δ: 9.52 (s, 1H), 9.38 (d, 1H, J=4.8 Hz), 9.18 (d, 1H, J=4.8 Hz), 8.64 (d, 1H, J=3.6 Hz), 8.06 (d, 1H, J=4.8 Hz), 7.99 (d, 1H, J=4.8 Hz), 6.86 (t, 1H, J=3.6 Hz). 13C-NMR (CDCl3, 100 MHz) δ: 177.0, 175.2, 154.6, 150.2, 149.8, 148.3, 147.0, 144.8, 143.9, 135.1, 131.8, 129.0, 125.5, 114.2. HR-MS m/z: 294.0621 [(M+H)+] (Calcd for C14H8N5O3 294.0627). Anal. Calcd for C14H7N5O3: C, 57.34; H, 2.41; N, 23.88; Found: C, 57.35; H, 2.41; N, 23.85.
2-Amino-2H-[1,2,3]triazolo[4,5-g]isoquinoline-4,9-dione (8a)To a suspension of 7a (5 mmol) in 200 mL of CH2Cl2, 150 mL of 6 M HCl aqueous solution was added and the mixture was refluxed for 4 h. After cooling, the mixture was extracted three times with 150 mL of CH2Cl2, and the solvent was evaporated off. The residue was recrystallized with methanol to afford compound 8a. Yellow powder (58%). mp 245–247°C. IR (KBr) cm−1: 3350 (m), 3255 (m), 1687 (s), 1669 (s), 1586 (s), 1223 (s). 1H-NMR (DMSO-d6, 400 MHz) δ: 9.30 (s, 1H), 9.14 (d, 1H, J=5.2 Hz), 8.87 (s, 2H, NH2), 8.00 (d, 1H, J=5.2 Hz).13C-NMR (DMSO-d6, 100 MHz) δ: 175.4, 174.9, 154.2, 149.1, 143.4, 142.6, 135.1, 130.4, 127.3. HR-MS m/z: 216.1759 [(M+H)+] (Calcd for C9H6N5O2 216.1762).
2-(Propan-2-ylideneamino)-2H-[1,2,3]triazolo[4,5-g]isoquinoline-4,9-dione (3h)To a mixture of compound 8a (1 mmol) in 50 mL of acetone, 1 mL of formic acid was added dropwise. The mixture was refluxed for 2 d and concentrated in vacuo. The precipitate was filtered and washed three times with 5 mL of methanol and crystalized from 95% ethanol to afford compound 3h. Yellow powder (52%). mp 246–248°C. IR (KBr) cm−1: 1699 (s), 1605 (s), 1395 (s), 1242 (s). 1H-NMR (DMSO-d6, 400 MHz) δ: 9.35 (s, 1H), 9.17 (d, 1H, J=4.8 Hz), 8.04 (d, 1H, J=4.8 Hz,), 2.37 (s, 3H, CH3), 2.25 (s, 3H, CH3). 13C-NMR (DMSO-d6, 100 MHz) δ: 184.8, 176.1, 175.2, 153.6, 149.9, 143.7, 135.6, 135.3, 131.5, 128.7, 21.2, 19.2. HR-MS m/z: 256.0828 [(M+H)+] (Calcd for C12H10N5O2 256.0834). Anal. Calcd for C12H9N5O2: C, 56.47; H, 3.55; N, 27.44; Found: C, 56.45; H, 3.57; N, 27.43.
2-((Triphenylphosphoranylidene)amino)-2H-[1,2,3]triazolo[4,5-g]quinoline-4,9-dione (7b)To a solution of 6,7-dichloroquinoline-5,8-dione (5b, 2.0 mmol) in 50 mL of MeOH, sodium azide (7.0 mmol) was added portionwise. The mixture was stirred at room temperature for 2 h to form compound 6b. To this reaction mixture, tripheylphosphine (0.38 g, 1.5 mmol) was added slowly and the mixture was then stirred for 24 h. The solvent was removed in vacuo. The precipitate was filtered, washed two times with 150 mL portion of water, and dried in vacuum to afford compound 7b. Red powder (85%). mp 210–212°C. IR (KBr) cm−1: 1695 (s), 1547 (s), 1342 (s), 1225 (s), 1125 (s). 1H-NMR (DMSO-d6, 400 MHz) δ: 8.94 (dd, 1H, J=1.6 Hz, J=4.8 Hz), 8.37 (dd, 1H, J=1.6 Hz, J=4.8 Hz), 8.33 (s, 1H), 7.70–7.89 (m, 6H), 7.61–7.65 (m, 9H). 13C-NMR (DMSO-d6, 100 MHz) δ: 175.3, 174.1, 153.5, 149.1, 134.4, 133.5 (2C), 133.3 (6C), 133.0, 130.6 (6C), 129.3, 129.2 (3C), 127.6, 126.0, 125.0. HR-MS m/z: 476.1273 [(M+H)+] (Calcd for C27H19N5O2P 476.1276).
General Procedure for Synthesis of 2H-[1,2,3]Triazolo[4,5-g]quinoline-4,9-diones 4a–eTo a mixture of compound 7b (0.5 mmol) with benzyl aldehyde derivatives, furan-2-carbaldehyde or thiophene-2-carbaldehyde (1.5 mmol) in 50 mL of CH2Cl2, 0.5 mL of formic acid was added dropwise. The mixture was refluxed for 24 h and concentrated in vacuo. The precipitate was filtered and washed three times with 50 mL of methanol and crystalized from 95% ethanol to afford compounds 4a–e.
2-(Benzylideneamino)-2H-[1,2,3]triazolo[4,5-g]quinoline-4,9-dione (4a)Pale yellow powder (67%). mp 310–312°C. IR (KBr) cm−1: 1691 (s), 1601 (s), 1401 (s), 1229 (s). 1H-NMR (DMSO-d6, 400 MHz) δ: 9.70 (s, 1H), 9.40 (s, 1H), 9.10 (d, 1H, J=8.0 Hz), 8.50 (d, 1H, J=8.0 Hz), 7.88 (m, 2H), 7.58 (m, 3H). 13C-NMR (DMSO-d6, 100 MHz) δ: 176.4, 175.9, 161.0, 153.9, 149.2, 143.0, 142.2, 135.0, 134.8, 131.0, 130.8, 130.0, 129.4, 128.1 (2C), 30.7. HR-MS m/z: 304.0835 [(M+H)+] (Calcd for C16H10N5O2 304.0834). Anal. Calcd for C16H9N5O2: C, 63.37; H, 2.99; N, 23.09; Found: C, 63.38; H, 2.30; N, 23.06.
2-((4-Fluorobenzylidene)amino)-2H-[1,2,3]triazolo[4,5-g]quinoline-4,9-dione (4b)Yellow powder (73%). mp 282–285°C. IR (KBr) cm−1: 1689 (s), 1575 (s), 1560 (s), 1397 (s), 1232 (s). 1H-NMR (DMSO-d6, 400 MHz) δ: 9.50 (s, 1H), 9.09 (m, 1H), 8.70 (s, 1H), 8.55 (m, 2H), 8.12 (m, 1H), 7.85 (m, 2H). 13C-NMR (CDCl3, 100 MHz) δ: 177.2, 176.7, 163.8, 162.4, 158.4, 155.3, 140.1, 133.4, 132.5 (2C), 128.1, 126.5, 119.2, 118.2, 116.9, 115.8. 19F-NMR (DMSO-d6, 376.3 MHz) δ: −116.4. HR-MS m/z: 322.0735 [(M+H)+] (Calcd for C16H9FN5O2 322.0740). Anal. Calcd for C16H8FN5O2: C, 59.82; H, 2.51; N, 21.80; Found: C, 59.85; H, 2.50; N, 21.82.
2-((4-(Trifluoromethyl)benzylidene)amino)-2H-[1,2,3]triazolo[4,5-g]quinoline-4,9-dione (4c)Gold glitter powder (81%). mp 319–321°C. IR (KBr) cm−1: 1688 (s), 1597 (s), 1399 (s), 1232 (s). 1H-NMR (DMSO-d6, 400 MHz) δ: 9.53 (s, 1H), 9.04 (dd, 1H, J=1.6 Hz, J=4.4 Hz), 8.80 (s, 1H), 8.50 (dd, 1H, J=1.6 Hz, J=8.0 Hz), 8.00 (d, 2H, J=8.0 Hz), 7.95 (dd, 1H, J=4.4 Hz, J=8.0 Hz),7.88 (dd, 1H, J=4.4 Hz, J=8.0 Hz). 13C-NMR (DMSO-d6, 100 MHz) δ: 176.4, 175.9, 159.7, 154.3, 153.9, 149.7, 149.3, 135.1, 131.5 (2C), 130.9, 130.7 (2C), 128.2, 127.9, 126.3, 126.2. HR-MS m/z: 372.0703 [(M+H)+] (Calcd for C17H9F3N5O2 372.0708). Anal. Calcd for C17H8F3N5O2: C, 55.00; H, 2.17; N, 18.86; Found: C, 55.03; H, 2.15; N, 18.84.
2-((Furan-2-ylmethylene)amino)-2H-[1,2,3]triazolo[4,5-g]quinoline-4,9-dione (4d)Dark yellow powder (74%). mp 341–344°C. IR (KBr) cm−1: 1690 (s), 1595 (s), 1402 (s), 1239 (s). 1H-NMR (DMSO-d6, 400 MHz) δ: 9.49 (s, 1H), 9.10 (dd, 1H, J=3.6 Hz, J=4.4 Hz), 8.58 (dd, 1H, J=1.6. Hz, J=8.0 Hz), 8.51 (t, 1H, J=8.0 Hz), 7.93 (dd, 1H, J=4.4 Hz, J=8.0 Hz), 7.60 (s, 1H), 6.88 (dd, 1H, J=1.6 Hz, J=3.6 Hz). 13C-NMR (DMSO-d6, 100 MHz) δ: 176.3, 174.8, 154.1, 149.7, 149.6, 148.1, 146.7, 144.3, 143.6, 134.7, 131.4, 128.1, 125.0, 113.8. HR-MS m/z: 294.0628 [(M+H)+] (Calcd for C14H8N5O3 294.0627). Anal. Calcd for C14H7N5O3: C, 57.34; H, 2.41; N, 23.88; Found: C, 57.32; H, 2.40; N, 23.89.
2-((Thiophen-2-ylmethylene)amino)-2H-[1,2,3]triazolo[4,5-g]quinoline-4,9-dione (4e)Green yellow powder (73%). mp 339–342°C. IR (KBr) cm−1: 1698 (s), 1601 (s), 1401 (s), 1234 (s). 1H-NMR (DMSO-d6, 400 MHz) δ: 9.10 (m, 1H), 8.60 (m, 1H), 8.13 (m, 1H), 7.91–7.84 (m, 2H), 7.62 (s, 1H), 7.37 (m, 1H). 13C-NMR (DMSO-d6, 100 MHz) δ: 176.3, 174.8, 155.0, 154.2, 149.6, 144.3, 143.6, 139.5, 135.5, 135.2, 135.0, 131.4, 129.2, 128.1. HR-MS m/z: 310.0397 [(M+H)+] (Calcd for C14H8N5O2S 310.0399). Anal. Calcd for C14H7N5O2S: C, 54.36; H, 2.28; N, 22.64; Found: C, 54.35; H, 2.27; N, 22.66.
2-Amino-2H-[1,2,3]triazolo[4,5-g]quinoline-4,9-dione (8b)To a suspension of compound 7a (5 mmol) in 200 mL of CH2Cl2, 150 mL of 6 M HCl aqueous solution was added and the mixture was refluxed for 4 h. After cooling, the mixture was extracted three times with 150 mL of CH2Cl2, and the solvent was evaporated off. The residue was recrystallized with methanol to afford compound 8b. Yellow powder (62%). mp 308–310°C. IR (KBr) cm−1: 3343 (m), 3260 (m), 1691 (s), 1662 (s), 1590 (s), 1229 (s). 1H-NMR (DMSO-d6, 400 MHz) δ: 9.04 (d, 1H, J=4.8 Hz), 8.50 (d, 1H, J=8.0 Hz), 7.88 (dd, 1H, J=4.8 Hz, J=8.0), 3.41 (s, 2H, NH2). 13C-NMR (DMSO-d6, 100 MHz) δ: 175.9, 174.6, 153.9, 149.2, 143.0, 142.2, 134.8, 130.8, 127.8. HR-MS m/z: 216.0519 [(M+H)+] (Calcd for C9H6N5O2 216.0521).
General Procedure of Synthesis of 2H-[1,2,3]Triazolo[4,5-g]quinoline-4,9-diones 4f, gTo a mixture of compound 8b (1 mmol) in 50 mL of acetone or butan-2-one, 1 mL of formic acid was added dropwise. The mixture was refluxed for 2 d and concentrated in vacuo. The precipitate was filtered and washed three times with 5 mL of MeOH and crystalized from 95% EtOH to afford compounds 4f, g.
2-(Propan-2-ylideneamino)-2H-[1,2,3]triazolo[4,5-g]quinoline-4,9-dione (4f)Pale yellow powder (75%). mp 229–332°C. IR (KBr) cm−1: 1690 (s), 1595 (s), 1408 (s), 1229 (s). 1H-NMR (DMSO-d6, 400 MHz) δ: 9.03 (dd, 1H, J=1.6 Hz, J=4.8 Hz), 8.50 (dd, 1H, J=1.6 Hz, J=8.0 Hz), 7.87 (ddd, 1H, J=1.6 Hz, J=4.8 Hz, J=8.0 Hz), 3.37 (s, 6H, methyl). 13C-NMR (DMSO-d6, 100 MHz) δ: 184.3, 176.3, 174.9, 154.1, 149.4, 144.1, 135.0, 134.8, 131.1, 128.1, 20.9, 19.4. HR-MS m/z: 256.0832 [(M+H)+] (Calcd for C12H10N5O2 256.0834). Anal. Calcd for C12H9N5O2: C, 56.47; H, 3.55; N, 27.44; Found: C, 56.43; H, 3.58; N, 27.42.
2-(Butan-2-ylideneamino)-2H-[1,2,3]triazolo[4,5-g]quinoline-4,9-dione (4g)Yellow powder (66%). mp 289–291°C. IR (KBr) cm−1: 1686 (s), 1588 (s), 1397 (s), 1247 (s). 1H-NMR (DMSO-d6, 400 MHz) δ: 9.05 (dd, 1H, J=1.6 Hz, J=4.9 Hz), 8.51 (dd, 1H, J=1.6 Hz, J=8.0 Hz), 7.88 (ddd, 1H, J=1.6 Hz, J=4.9 Hz, J=8.0 Hz), 3.33 (s, 3H, methyl), 2.50 (q, 2H, J=7.2 Hz), 2.08 (t, 3H, J=7.2 Hz). 13C-NMR (DMSO-d6, 100 MHz) δ: 175.9, 174.6, 153.9, 149.2, 143.0, 142.2, 134.8 (2C), 130.8, 127.9, 30.7, 17.5, 15.3. HR-MS m/z: 270.0987 [(M+H)+] (Calcd for C13H12N5O2 270.0991). Anal. Calcd for C13H11N5O2 : C, 57.99; H, 4.12; N, 26.01; Found: C, 57.97; H, 4.13; N, 26.03.
Antifungal in Vitro Susceptibility TestingThe MIC values of compounds 3a–h, 4a–g, 6a, and 8a, b were determined by the standard broth dilution method.13 The antifungal activities were tested in modified Sabouraud dextrose broth against the following fungal strains: Candida albicans ATCC 10231, C. glabrata ATCC 2001, C. krusei ATCC 749, C tropicalis ATCC 28775, and Aspergillus niger KCTC 1231. Fluconazole and 5-fluorocytosine as standard agents were used. The compounds were tested in the 0.1–100 µg/mL range. That was added to the modified Sabouraud dextrose broth (Difco Lab.) for fungi over a final concentration range of 0.1 to 100 µg/mL. The inoculum sizes contained approximately 1×105 cfu/mL. They were incubated at 37°C for appropriate periods of time that sufficed to show clearly visible growth on drug-free control broths. The MIC value was defined, as the lowest concentration of the antifungal agent at which there showed optically clear. MIC values were read after 1 d for Candida species and 2 d for A. niger in 37°C.
This study was supported by a Grant of the Korea Healthcare Technology R&D Project, Ministry for Health, Welfare and Family Affairs, Republic of Korea (A08-0414-AA1723-08N1-00010A).