2014 Volume 62 Issue 3 Pages 274-280
2014 Volume 62 Issue 3 Pages 274-280
To achieve efficient skin delivery of polyphenols, we prepared a novel oil-in-water (o/w)-type microemulsion (MESL) using sucrose laurate as a surfactant and ethanol, isopropyl myristate and water as other components. We examined its usefulness by in vitro studies on skin delivery of chlorogenic acid and resveratrol as hydrophilic and hydrophobic polyphenols using Yucatan micropig skin, and also examined the difference in the distribution of these polyphenols in skin. MESL significantly improved skin incorporation of these polyphenols at all time points examined (6, 20, 40 h) in the epidermis and at 20 and 40 h in the dermis, compared with the microemulsion using Tween 80 as a surfactant component (MEK), although the solubilization capacity of MESL was lower than that of MEK. Using MESL, the incorporation amount in the dermis of each polyphenol increased with time, while the amount in the epidermis was almost constant during the time examined. Incorporation efficiencies into skin of chlorogenic acid and resveratrol induced by MESL at 40 h after application were about 6-fold and 19-fold higher in the epidermis and 3.5-fold and 15-fold higher in the dermis, respectively, than those by MEK. The increase was more prominent for resveratrol. Hydrophilic chlorogenic acid was distributed slightly more in the epidermis, while hydrophobic and smaller-molecular-weight resveratrol was mainly distributed in the dermis. These findings suggest that MESL could be a promising vehicle for the efficient skin delivery of chlorogenic acid and resveratrol, especially for resveratrol to the dermis.
Polyphenols are well-known antioxidants and have been shown to have a wide range of therapeutic potential, such as via anti-inflammatory and anticancer activities, as well as protective effects against cardiovascular and neurodegenerative diseases.1,2) Polyphenols can also be applied to skin for topical effects, including protection against UV-induced oxidative damage (photoaging), skin cancer prevention and skin care3,4); however, the penetration of polyphenols into skin is often limited due to their poor solubility in both aqueous and organic solvents as well as relatively high molecular weight. There is thus a need for enhancement systems to be introduced for their topical application.
Microemulsions consist of an aqueous phase, an oil phase, a surfactant and a co-surfactant component, which are thermodynamically stable and have been shown to have high solubilization capacity and to facilitate the skin incorporation of both hydrophilic and lipophilic drugs.5,6) In previous studies, we revealed that microemulsions using polyoxyethylene sorbitan monooleate (Tween 80) as a surfactant component are useful for the intradermal delivery of polyphenols, such as quercetin, genistein and chlorogenic acid.7–9) However, the incorporation efficiencies of these polyphenols from the vehicle into skin were low. In another study, we also revealed that an anionic surfactant component, Aerosol OT, was incorporated into skin during Aerosol OT microemulsion-enhanced intradermal delivery of polyphenols.10) Although microemulsions have many advantages for topical formulations, the microemulsion components incorporated into skin, especially a surfactant and a co-surfactant component, have the possibility of inducing local irritancy. Thus, we have to choose microemulsion components and composition carefully, in terms of having high drug delivery potential and, at the same time, being mild on skin and resulting in no adverse effects.
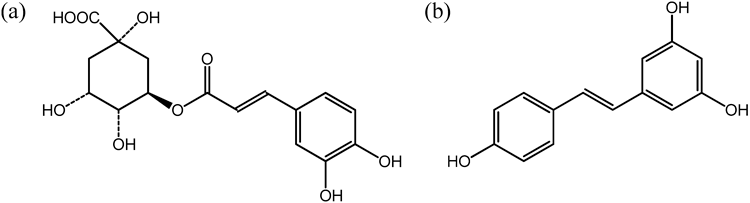
Sucrose fatty acid esters are well known as biodegradable nonionic surfactants, which are non-toxic, non-sensitizing, cause no skin irritation and have been applied in many fields, such as the food, cosmetic and pharmaceutical industries.11) Using sucrose laurate as a surfactant component, we attempted to prepare a novel oil-in-water (o/w)-type microemulsion and, thereby, to achieve the efficient skin delivery of polyphenols in vitro using Yucatan micropig (YMP) skin. As polyphenols, we selected chlorogenic acid and resveratrol. Chlorogenic acid (Fig. 1a) is an abundant ingredient in coffee and is also found in many types of food, such as sweet potatoes and apples.12) Like other polyphenols, it has been suggested to have anti-inflammatory and anticancer activities and also to be effective for skin photoprotection.9,13) Its molecular weight is 354.31 and it is relatively hydrophilic (log P=0.370). Resveratrol (trans-3,5,4′-trihydroxystilbene, Fig. 1b) is a naturally occurring polyphenolic phytoalexin synthesized by a wide variety of plant species, including grapes, berries and peanuts.14) Resveratrol also has many benefits for the skin, for example, antiproliferative and chemopreventive effects on skin carcinogenesis and prevention against skin damage from UV exposure.15,16) Its molecular weight is 228.24 and it is hydrophobic (log P=3.024).
In this study, we first attempted to determine the composition of the microemulsion using sucrose laurate, designated this composition of the microemulsion as MESL, and characterized its physicochemical properties. Next, we examined the time course of skin incorporation and permeation of chlorogenic acid and resveratrol induced by MESL and also another microemulsion using Tween 80 as a surfactant component (MEK), whose usefulness we previously reported, as a comparison.9) We further examined the difference in distribution between chlorogenic acid and resveratrol in the epidermis and dermis of YMP skin because the distribution in skin is directly involved in the exhibition of physiological activities.
Sucrose laurate (Ryoto Sugar Ester® L-1695) was kindly provided by Mitsubishi-Kagaku Foods Co. (Tokyo, Japan). Tween 80 and isopropyl myristate (IPM) were purchased from Nacalai Tesque, Inc. (Kyoto, Japan). Resveratrol and trans-ferulic acid were obtained from Tokyo Chemical Industry Co., Ltd. (Tokyo, Japan). Methylene blue was purchased from Waldeck GmbH & Co., KG (Münster, Germany). Chlorogenic acid, ethanol and all other reagents were purchased from Wako Pure Chemical Industries, Ltd. (Osaka, Japan). Yucatan micropig skin (YMP skin set) was purchased from Charles River Laboratories Japan, Inc. (Yokohama, Japan) and stored at −80°C until use. Fat and subdermal tissues were removed following the method of Fujii et al.17) Ultrapure water was obtained using a water purification system, Direct-Q UV (Millipore Co., Billerica, U.S.A.), and used throughout the experiments.
Pseudo-Ternary Phase Diagrams ConstructionIn order to determine the composition of the microemulsion using sucrose laurate, pseudo-ternary phase diagrams were constructed at room temperature. Water was used as an aqueous phase, IPM as an oil phase, sucrose laurate as a surfactant component and ethanol as a co-surfactant component. Ethanol also works as an aqueous phase ingredient in the system. Microemulsion-forming domain boundaries were defined at different mixing weight ratios of sucrose laurate to ethanol, 1 : 1, 2 : 1, 2.5 : 1, 3 : 1 and 4 : 1. At each sucrose laurate to ethanol ratio, 1 g of water/sucrose laurate/ethanol mixture was prepared at weight ratios varying from 1 : 9 to 9 : 1 ((sucrose laurate/ethanol) : water), and then titrated with 10 µL of IPM, mixed using a vortex mixer and left until the system reached equilibrium. This procedure was repeated until a transparent dispersion converted to a turbid mixture, which indicated phase separation. The weight ratio at which the mixture became turbid was plotted on the phase diagrams and the boundary of microemulsion-forming domain was determined.
Characterization of MicroemulsionsMean particle diameter and viscosity of the selected microemulsion were measured at 25°C using a particle analyzer, FPAR-1000 (Otsuka Electronics Co., Ltd., Osaka, Japan), and a viscometer, TVE-22L, equipped with a 1°34′×R24 cone rotor (Toki Sangyo Co., Ltd., Tokyo, Japan), respectively. The microstructure of the system (water-in-oil (w/o), o/w or bicontinuous) was assessed using a hydrophilic and a lipophilic dye. Namely, methylene blue and sudan III was added to water and IPM, respectively, at equal concentration; then, sucrose laurate, water, ethanol and IPM were mixed and the color of the prepared microemulsion was observed. Electrical conductivity of the system was also measured at 25°C using a conductivity meter, ES-51 (Horiba, Ltd., Kyoto, Japan), to assess the microstructure. The value was continuously measured with increasing water content of the system along the dilution line shown in Fig. 2c. To validate the features of the electrical conductivity curve, 150 mM NaCl solution was chosen as an aqueous phase component.

The line shown in (c) was used in electrical conductivity measurement.
The solubility of chlorogenic acid and resveratrol was measured after incubation of an excess amount of these polyphenols in the microemulsions at 37°C for about 20 h. After rapid centrifugation at 12000×g for 1 min, the concentration of the supernatant was determined using an HPLC system (LaChrom Elite) equipped with an L-2420 UV-VIS detector (Hitachi High-Technologies Co., Tokyo, Japan). The HPLC analyses of these polyphenols were carried out as described previously.9,10) Separation was performed on a reversed-phase column (Mightysil RP-18 GP, 3.0 mm i.d., 150 mm, Kanto Chemical Co., Inc.) at 40°C using a mobile phase consisting of methanol, water and phosphoric acid at volume ratios of 60 : 140 : 1 for chlorogenic acid and 70 : 130 : 1 for resveratrol. The detection wavelengths were 327 nm for chlorogenic acid and 305 nm for resveratrol. trans-Ferulic acid was used as the internal standard for both polyphenols.
In Vitro Study on Skin Delivery of Chlorogenic Acid and ResveratrolIn vitro studies on the intradermal and transdermal delivery of chlorogenic acid and resveratrol were performed as described previously.10) YMP skin was mounted in a Franz-type diffusion cell with a water jacket (37°C). The available diffusion area was approximately 0.83 cm2 and the receptor cell had a volume of about 5.0 mL. After 2 h of pretreatment of the skin with 150 mM NaCl solution, both donor and receptor compartments were washed and 1 mL of vehicle containing chlorogenic acid or resveratrol was added to the donor compartment at 100 mM for chlorogenic acid in both microemulsions and resveratrol in MEK or at a saturated concentration for resveratrol in MESL under solubilized conditions. Phosphate-buffered saline (PBS (pH 7.4)) was added to the receptor compartment. After 6, 20 and 40 h of treatment, the skin was removed from the cell, and the treated skin area was punched out and washed with ice-cold methanol. After the separation of the epidermis from the dermis by a heat separation technique,18) each skin sample was minced and placed in 10 mL of methanol, and then homogenized using a tissue homogenizer, Polytron PT3100 (Kinematica AG, Lucerne, Switzerland), at 5000 rpm for 1 min. The samples were then centrifuged and the supernatant layer was used for evaluation by HPLC as described above. The receptor solution was also collected after treatment to determine the cumulative amount of polyphenols that permeated through skin by HPLC. As for each polyphenol, we determined the incorporation efficiency, which means the relative incorporation amount into skin per applied dose. We further determined the relative permeation ratio from the vehicle into the receptor compartment and distribution ratio between the dermis and epidermis.
Statistical AnalysisThe statistical significance of differences in the incorporation amount of polyphenols between MESL and MEK was analyzed using unpaired t-test. Differences were regarded as significant at p<0.05.
To determine the composition of the microemulsion using sucrose laurate, pseudo-ternary phase diagrams were constructed at different mixing weight ratios of sucrose laurate to ethanol, 1 : 1, 2 : 1, 2.5 : 1, 3 : 1 and 4 : 1. As shown in Fig. 2, each diagram had a single phase domain of almost the same area (about 27% of the total area). Hence, the ratio of sucrose laurate to ethanol had little effect on the area of the single phase domain. However, the bottom-left on the phase diagrams, which represents the domain containing a large amount of water, slightly increased with variation of the ratio from 1 : 1 to 2.5 : 1, and decreased from 2.5 : 1 to 4 : 1. Thus, we determined the composition of the microemulsion to be sucrose laurate : ethanol : IPM : water=25 : 10 : 5 : 60, from the region containing low surfactant/co-surfactant content as well as high water content. We designated this composition of the microemulsion as MESL. MEK, o/w-type microemulsion that we previously used (Tween 80 : ethanol : IPM : aqueous phase=30 : 15 : 4 : 51),9) was also studied for comparison in the experiments hereafter.
Physicochemical Properties of MicroemulsionsMean particle diameter and viscosity of microemulsions are shown in Table 1. Mean particle diameter had little difference between microemulsions, whereas the viscosity of MESL was approximately 17-fold lower than that of MEK.
| Vehicle | Particle diameter (nm) | Viscosity (mPa·s) |
|---|---|---|
| MESL | 11.5±4.1 | 7.75±0.14 |
| MEK | 12.3±4.8a) | 128.9±1.1 |
Data are expressed as the mean±S.D. of three values. a) Mean particle diameter of MEK was previously reported.9)
The appearance of MESL with the addition of methylene blue and sudan III is shown in Fig. 3. As shown in this figure, MESL was transparent and looked blue and almost the same color as water dyed with methylene blue. This indicates that oily droplets were finely dispersed in water; in other words, the system formed an o/w-type microstructure.
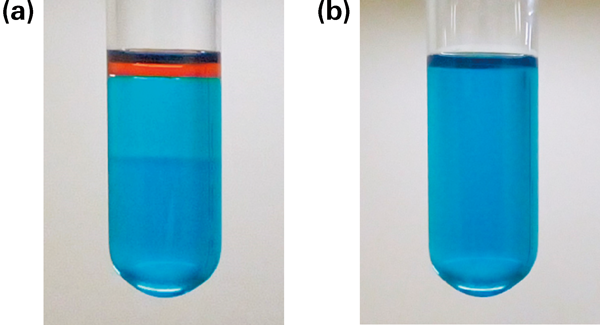
Before (a) and after mixing (b). Water was dyed blue by methylene blue and IPM was dyed red by sudan III.
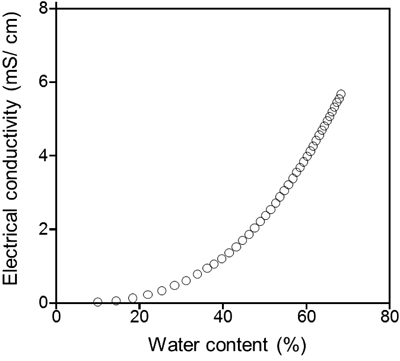
The result of the electrical conductivity measurement is shown in Fig. 4. According to the addition of water content to the system of the defined composition of sucrose laurate : ethanol : IPM=25 : 10 : 5, from 10 to 30% water content, the electrical conductivity of the system gradually increased with increasing water content, indicating that w/o microemulsion was formed.19) On the other hand, above 50% water content, electrical conductivity sharply increased, indicating that o/w microemulsion was formed.19) Thus, MESL, which had 60% water content, was estimated to be o/w microemulsion.
We next examined the solubilizing capacity of microemulsions for chlorogenic acid and resveratrol. As shown in Table 2, the solubility of each polyphenol in MESL was lower than that in MEK: about one-seventh for chlorogenic acid and one-eighteenth for resveratrol, but still higher than that in 150 mM NaCl solution or IPM alone (solubility in each vehicle was nearly 50 mM and 0.01 mM for chlorogenic acid and nearly 0.3 mM and 1.2 mM for resveratrol).9,10) Chlorogenic acid was more soluble than resveratrol in both o/w-type microemulsions, owing to its hydrophilicity.
| Vehicle | Solubility (mM) | |
|---|---|---|
| Chlorogenic acid | Resveratrol | |
| MESL | 116.0±6.3a) | 14.4±0.7 |
| MEK | 797.6±6.5 | 260.7±2.6 |
Data are expressed as the mean±S.D. of four values. a) Concentration limit at which microemulsion did not break down.
In vitro studies on the intradermal and transdermal delivery of chlorogenic acid and resveratrol were performed. Figure 5 shows the incorporation amounts of chlorogenic acid (Fig. 5A) and resveratrol (Fig. 5B) in the epidermis (a) and dermis (b) at 6, 20 and 40 h after application using each microemulsion. For both polyphenols, the incorporation amounts induced by MESL in the dermis increased in a time-dependent manner, while the amounts in the epidermis were approximately constant at 6–40 h. The amounts by MEK, on the other hand, had a tendency to increase slightly with time in both the epidermis and the dermis. As shown in Fig. 5A, MESL significantly increased the incorporation amounts of chlorogenic acid in the epidermis at all time points examined and in the dermis at 20 and 40 h compared with MEK. Especially in the epidermis, the incorporation amounts by MESL drastically increased in a short period of time. Similar to the results for chlorogenic acid, as shown in Fig. 5B, MESL significantly increased the incorporation amounts of resveratrol in the epidermis at all time points and in the dermis at 20 and 40 h compared with MEK.

Data are the means±S.D. of eight values. * p<0.05, significantly different from the value in MEK at the same time point.
As for chlorogenic acid, incorporation efficiencies induced by MESL at 40 h were about 0.25% in the epidermis and 0.12% in the dermis, which were about 6-fold higher and 3.5-fold higher than by MEK and, as for resveratrol, 0.19% in the epidermis and 0.45% in the dermis, which were about 19-fold higher and 15-fold higher than by MEK. Using MESL, both polyphenols were efficiently incorporated into skin. The increase was more prominent for resveratrol, which is hydrophobic and has a smaller molecular weight.
The permeation ratios through skin of chlorogenic acid and resveratrol induced by MESL at 40 h were 0.081% and 0.018%, respectively. The permeation ratios by MESL at 6 and 20 h were low and even negligible. The permeation ratios of these polyphenols by MEK were still lower than those by MESL at all time points examined. Resveratrol hardly permeated through skin, even at 40 h, although it penetrated into the dermis.
Distribution ratios between the dermis and epidermis of the polyphenols were calculated and the time courses of their logarithm values are shown in Fig. 6. As shown in this figure, the ratio of each polyphenol by MESL increased with time, while that by MEK had a tendency to decrease slightly after 20 h; however, both of them seemed to reach a constant value. Hydrophilic chlorogenic acid was distributed slightly more in the epidermis, whereas hydrophobic and smaller-molecular-weight resveratrol was mainly distributed in the dermis for both microemulsions at 40 h.
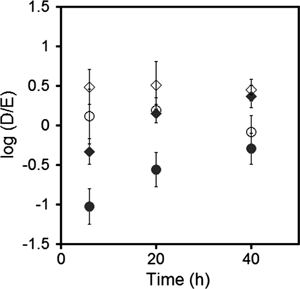
MESL (●), MEK (○) for chlorogenic acid and MESL (◆), MEK (◇) for resveratrol. Data are the means±S.D. of eight values.
Microemulsions offer several advantages for pharmaceutical use, such as long-term stability, high solubilization capacity for both hydrophilic and lipophilic drugs, and improved intradermal and/or transdermal delivery.6) Sucrose fatty acid esters have also been shown to work as percutaneous penetration enhancers for some drugs, such as lidocaine hydrochloride,20) lidocaine and ketoprofen,21) and ibuprofen.22) However, skin penetration studies using sucrose fatty acid ester microemulsions have yet to be performed adequately. In this study, their effectiveness for the skin delivery of polyphenols was examined. The increase of the incorporation efficiency can reduce the applied dose of polyphenols required to exert pharmacologic effects in skin. The findings obtained in this study demonstrated that, by using MESL, an o/w-type microemulsion with sucrose laurate as a surfactant component, efficient skin incorporation was obtained not only for the relatively hydrophilic polyphenol, chlorogenic acid, but also for hydrophobic polyphenol, resveratrol, although the solubilizing capacity of these polyphenols in MESL was lower than that in MEK. MESL therefore enabled efficient skin delivery of these polyphenols.
Although the reasons for the high incorporation efficiencies of these polyphenols by MESL are not clear, some possibilities are as follows. First, thermodynamic activity of the polyphenols in MESL may play an important role in their efficient skin incorporation. Thermodynamic activity of a solute in the vehicle is a significant driving force for its release and distribution into skin and thermodynamic activity reaches a maximum in its saturated solution.6) As for MESL, chlorogenic acid was applied near the saturated concentration and resveratrol was at saturated concentration; therefore, the thermodynamic activity of these polyphenols in MESL is assumed to be higher than that in MEK, which leads to increased skin incorporation. Second, the low viscosity of MESL could facilitate solute diffusion in the vehicle and access onto the skin surface.23) This should improve the release of a solute from the vehicle and penetration into skin. Furthermore, some sucrose laurate in the microemulsion might penetrate into skin and interact directly with corneocytes or lipid components in the stratum corneum,20,24) increasing fluidity, thereby enhancing skin incorporation and the diffusion of polyphenols. Sucrose moiety seemed to contribute to the hydration of the stratum corneum by MESL,25) which induced the reduction of its barrier function. Microemulsions are dynamic systems in which the interfacial film consisting of both surfactant and co-surfactant component is continuously and spontaneously fluctuating6); therefore, higher levels of free surfactant monomers might be available to penetrate into skin with increased incorporation. These factors overall seemed to contribute to the enhanced skin penetration of the polyphenols by MESL, although further study is necessary to clarify which of them contributed the most.
It was also clarified in this study that both polyphenols penetrated the dermis and, in particular, resveratrol, which has a smaller molecular weight, was distributed in the dermis at a higher ratio. These results are consistent with previous findings using Aerosol OT microemulsion10) and are favorable for modifying the activities of matrix metalloproteinases, which are involved in skin photoaging.26) However, the transdermal delivery of resveratrol was very limited under the conditions in this study, possibly due to the binding to albumin,27) which is present in large quantities in the dermis.28)
The time courses of the distribution ratio between the dermis and epidermis of the polyphenols differed between MESL and MEK; rapid distribution from the vehicle to the epidermis but slower distribution from the epidermis to the dermis were observed in MESL, while both distribution processes seemed to be slow in MEK. Although it was not elucidated, hydration of the stratum corneum as mentioned above and the dermal-epidermal basement membrane zone barrier, such as basement membrane, might be responsible for this difference in distribution.25,29) Further study of many solutes in the presence and absence of an enhancement system is necessary to clarify the cause of this difference in distribution profile, which may help in the elucidation of factors regulating the distribution of solutes in the skin.
MESL, consisting of sucrose laurate, ethanol, IPM and water, which had an o/w-type microstructure, significantly improved the efficiency of skin delivery of resveratrol as well as chlorogenic acid compared with MEK, although the solubility of these polyphenols in MESL was lower. The improvement was especially large for resveratrol. Hydrophilic chlorogenic acid was distributed slightly more in the epidermis, while hydrophobic and smaller-molecular-weight resveratrol was mainly distributed in the dermis. These findings suggest the potential usefulness of MESL for the efficient skin delivery of chlorogenic acid and resveratrol, especially for resveratrol to the dermis.