2014 Volume 62 Issue 6 Pages 538-544
2014 Volume 62 Issue 6 Pages 538-544
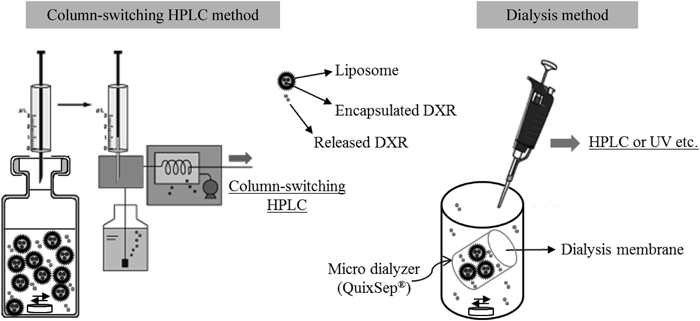
A novel in vitro release test methodology for a liposome formulation was developed using a column-switching high-performance liquid chromatography (HPLC) system. Doxorubicin (DXR) liposome formulations were used as a model. A DXR liposome formulation was dispersed into a release medium, and the dispersion fluid was directly injected at predetermined time points into the column-switching HPLC system. To evaluate the release profile, this system can be used for determining the released and encapsulated DXR in the liposome formulation separately. Comparison with a conventional in vitro release test methodology by dialysis revealed that the methodology developed by column-switching HPLC had no rate-limiting process of membrane permeation of the drug (which is occasionally observed in the dialysis method). The in vitro release profiles of DXR liposome formulations were well characterized using the method developed by column-switching HPLC, and different in vitro release characteristics were revealed. The developed method did not require a large amount of sample or a complicated pretreatment. In addition, the developed column-switching HPLC system was applicable for characterization of the encapsulation profile of liposome formulations.
Several studies of pharmaceutical formulations for drug-delivery systems (DDS), such as liposomes, microspheres, and nanosuspensions, have been conducted. Their advantages, such as targeted/controlled delivery, enhanced efficacy, and reduced toxicity, have been verified.1–4) However, the number of approved and marketed pharmaceutical products with DDS technology is limited. The reasons for this are thought to be the difficulty of robust mass production as well as the lack of sophisticated quality control accompanied by a deep understanding of in vitro and in vivo characteristics. With regard to liposome formulations, the enhanced permeability and retention (EPR) effect of polyethylene glycol-conjugated liposomes has been demonstrated in several studies, and are very attractive as passive targeting methods of antitumor agents.5–9) Considerable efforts are being made for the development of several administration routes for liposomes, such as inhalational, transdermal, and ocular in addition to injection.10–14) In contrast, analytical technology for liposome formulations may not be sufficiently mature to assure efficacy and safe performance as well as to direct the optimization of formulations or improvements in manufacturing processes. Drug release characteristics are considered to be one of the important properties to assure the performance of liposome formulations.15) These characteristics may change depending on the lot-to-lot variation of ingredients and/or changes in manufacturing processes such as scale-up. The importance of evaluation of the in vitro release characteristics is increasing and it is one of the regulatory requirements.16–19) Although in vitro release characteristics are conventionally evaluated by the dialysis method, Franz diffusion cell method, or dispersion method accompanied by centrifugation or filtration after sampling,20–23) a standard procedure is lacking. In the dialysis method or Franz diffusion cell method, it is difficult to completely deny the rate limiting of membrane permeation of the drug if the measurement conditions are not selected adequately. In the dispersion method, pretreatments such as centrifugation or filtration must be conducted to separate the released and encapsulated drugs in the dispersion fluid after sampling and the instabilities of the sample, such as chemical degradation or progress of release during pretreatment cannot be ignored. Although new methodologies of in vitro release test using USP apparatus 4 or two-stage reverse dialysis have been reported,24,25) there is a compelling need for sophisticated analytical techniques to evaluate in vitro drug release characteristics from liposome formulations.
We developed a novel in vitro release test methodology for a liposome formulation using a column-switching high-performance liquid chromatography (HPLC) system; a doxorubicin (DXR) liposome formulation was selected as the model. The developed in vitro release test methodology mainly comprises the column-switching HPLC system, which can evaluate free (released) and encapsulated DXR in the liposome formulation separately.26) The DXR liposome formulation was dispersed into a release medium in an HPLC glass vial, and the dispersion fluid was injected directly into the column-switching HPLC at predetermined time points. On comparison with the dialysis method, it was demonstrated that the developed methodology did not have a rate-limiting process, such as the drug permeation across the membrane other than the drug release from liposomes. Furthermore, this methodology did not essentially affect the stability of the sample during pretreatment, and a large amount of sample or pretreatment of the sample was not required. Moreover, the developed column-switching HPLC system could be used for evaluation of the characteristics of drug encapsulation into the liposome formulation. This report suggests the concept of a novel in vitro release test methodology and highlights the advantage, and describes the in vitro characterization of liposome formulations containing DXR by the developed method.
DXR hydrochloride was purchased from Sicor (Milan, Italy). Hydrogenated soy phosphatidylcholine (HSPC) was obtained from Lipoid (Steinhausen, Switzerland). Egg phosphatidylcholine (EPC) was obtained from Nippon Oil and Fats (Tokyo, Japan). Cholesterol was obtained from Sigma-Aldrich (St. Louis, MO, U.S.A.). Two brands of N-(carbonyl-methoxypolyethylene glycol 2000)-1,2-distearoyl-sn-glycero-3-phosphoethanolamine sodium, MPEG2000-DSPE and DSPE-020CN, were purchased from Corden Pharma (Liestal, Switzerland) and Nippon Oil and Fats, respectively. Ammonium sulfate, sucrose, and l-histidine were purchased from Wako Pure Chemical Industries, Ltd. (Osaka, Japan). Doxil® (DXR hydrochloride liposome injection; 2 mg/mL DXR) was obtained from Janssen Pharmaceuticals (Tokyo, Japan). Phosphate-buffered saline (PBS; 137 mmol/L NaCl, 8.1 mmol/L Na2HPO4, 2.68 mmol/L KCl, and 1.47 mmol/L KH2PO4, pH 7.4) and 0.1 mol/L hydrochloric acid (HCl) were purchased from Wako Pure Chemical Industries, Ltd. Water was deionized and purified by a Milli-Q® TOC purification system (Millipore, Bedford, MA, U.S.A.). All other chemicals were commercially available products of reagent grade. All reagents were used without further purification.
Two types of 28 µm thick dialysis membranes, CelluSep® F1 and F3, with molecular weight cut-offs (MWCOs) of 3.5 kDa and 12–14 kDa, respectively, were purchased from Membrane Filtration Products, Inc. (Seguin, TX, U.S.A.).
Preparation of DXR LiposomesA solution comprising 9% sucrose and 10 mm l-histidine was used as a diluent. A total of 5 mg/mL DXR dissolved in the diluent was used as a DXR stock solution. The DXR stock solution was diluted to 2 mg/mL with the diluent and used as the DXR solution. This solution was subsequently used as the DXR standard solution after dilution to the prescribed concentration with the release medium for the in vitro release test.
Liposomes were prepared using the lipid film method. The phospholipids shown in Table 1 were co-dissolved in a mixture of chloroform and methanol, which was removed by reduced pressure evaporation until a lipid film was obtained. The lipid film was dispersed in 250-mm ammonium sulfate solution (the pH was adjusted to 5.5 by the addition of citric acid and sodium citrate) maintained at 40°C and extruded several times through a polycarbonate membrane (pore size, 100 nm) to adjust the diameter. The obtained liposome underwent ultracentrifugation at 452000×g using CP-80WX (Hitachi Koki, Tokyo, Japan) for 60 min. After the precipitate was adequately dispersed in the diluent, the obtained suspension was used as the stock liposome (total lipid concentration: 40 mg/mL).
| Components | Formulation | ||||
|---|---|---|---|---|---|
| EPC | EPC+HSPC | Doxil® | HSPC stock liposome | ||
| Doxorubicin HCl (mg/mL) | 2.0 | 2.0 | 2.0 | — | |
| Phospholipid | HSPC (mg/mL) | — | 7.6 | 9.6 | 24 |
| EPC (mg/mL) | 9.6 | 2.0 | — | — | |
| Cholesterol (mg/mL) | 3.2 | 3.2 | 3.2 | 8 | |
| MPEG2000-DSPE (mg/mL) | — | — | 3.2 | 8 | |
| DSPE-020CN (mg/mL) | 3.2 | 3.2 | — | — | |
| Inner phase | Ammonium sulfate (mm) | 250 | 250 | 250 | 250 |
| Citric acid/sodium citrate | q.s. | q.s. | q.s. | q.s. | |
| pH | 5.5 | 5.5 | 5.5 | 5.5 | |
| Outer phase | Histidine (mm) | 10 | 10 | 10 | 10 |
| Sucrose (mg/mL) | 9 | 9 | 9 | 9 | |
| pH | 7.5 | 7.5 | 7.5 | 7.5 | |
| Encapsulation efficiency (%)a) | 97.4±0.06 | 94.3±0.54 | 97.7±0.49 | — | |
| Mean diameter (nm)a) (polydispersity index) | in 0.1 mol/L HCl | 98.4±0.46 | 104.6±1.01 | 83.4±4.50 | — |
| (0.077±0.011) | (0.066±0.021) | (0.116±0.037) | |||
| in PBS | 137.7±1.54 | 138.9±1.99 | 136.6±4.69 | — | |
| (0.272±0.019) | (0.253±0.003) | (0.267±0.017) | — | ||
| Zeta potential (mV)a) in PBS | −5.2±1.6 | −5.6±1.3 | −7.9±4.2 | — | |
a) Results are represented as mean±S.D. of three experiments.
DXR was encapsulated into the liposome using a remote loading method.27–30) The DXR stock solution (5 mg/mL) was mixed with the stock liposome at the ratio of 1 : 1 (v/v), and diluted with the diluent to obtain 2 mg/mL of DXR. The obtained suspension was annealed with occasional shaking in a water bath maintained at 60°C for 5 min. This was used as the DXR liposome after cooling to room temperature. Doxil® was used as received.
Encapsulation Efficiency (EE)The DXR liposome was diluted to 0.2 mg/mL of DXR with water and placed at room temperature in the column-switching HPLC system (which can evaluate free and encapsulated DXR in the liposome formulation separately). We previously reported the methodology of the column-switching HPLC system in detail.26) A schematic representation of the column-switching HPLC system is shown in Fig. A1. In brief, after sample injection by Pump B1 (water), the released DXR was trapped on the separation column (Inertsil Diol, 10 mm×4.0 mm i.d., 5 µm; GL Sciences, Tokyo, Japan) (Diol SPE), whereas the encapsulated DXR in the inner phase of the liposome was eluted out. The eluted encapsulated DXR was trapped on the extraction column (YMC-Pack Pro C18, 23 mm×4.0 mm i.d., 3 µm; YMC, Kyoto, Japan) (ODS SPE) after being extracted from the inner phase of the liposome by mixing with an organic solvent by Pump B2 (a mixture of acetonitrile, water, and ammonium acetate, 800 : 200 : 1, v/v/w). The released and encapsulated DXR were trapped on the separation column and extraction column, respectively, and sequentially eluted and analyzed on the analytical column (YMC-Pack Pro C18, 75 mm×4.6 mm i.d., 3 µm; YMC, Kyoto, Japan) using a gradient HPLC, by pump A1 (water) and A2 (a mixture of acetonitrile, water, and ammonium acetate, 800 : 200 : 1, v/v/w). DXR was monitored at a UV wavelength of 235 nm. The analytical conditions are summarized in Supplementary Fig. A1. All column-switching HPLC equipment was obtained from Shimadzu (Kyoto, Japan).
Using the obtained free and encapsulated DXR, the EE was calculated according to the following formula:
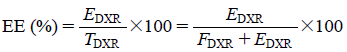 |
where, TDXR: total amount of DXR (mg/mL), FDXR: free DXR in the liposome formulation (mg/mL), EDXR: encapsulated DXR in the liposome formulation (mg/mL).
Particle Size and Zeta PotentialThe DXR liposome was dispersed into the test fluid to avoid multi-scattering. The particle size and zeta potential were measured using dynamic light scattering and laser Doppler electrophoresis, respectively (ELS-8000, Otsuka Electronics, Osaka, Japan). The particle size was measured thrice by data accumulation of 100 runs in 0.1 mol/L HCl or PBS, and the mean diameter was determined by cumulant analysis. Measurements of zeta potential were performed thrice in PBS.
Solubility of DXRDXR was added to 0.1 mol/L HCl or PBS. More than 20 mg/mL of DXR dissolved completely in 0.1 mol/L HCl. On the other hand, the suspension of DXR in PBS was stirred for 24 h at 37°C and filtered using a 0.2-µm filter (GL chromatodisc 4A; GL Sciences, Tokyo, Japan). After one-tenth dilution with water, DXR in the filtrate was quantified by comparison with the peak area obtained from the DXR standard solution (0.2 mg/mL) by HPLC. The solubility of DXR in PBS was estimated to be 17.3 mg/mL (average, n=3).
In Vitro Release Study by Column-Switching HPLCFigure 1 illustrates a schematic overview of the developed in vitro release test methodology by column-switching HPLC. The developed in vitro release method for liposome formulations mainly comprises the column-switching HPLC system used for EE determination (Supplementary Fig. A1). Ten microliters of the DXR liposome was dispersed into an HPLC glass vial containing 90 µL of the release medium (theoretical concentration of DXR: 0.2 mg/mL). The HPLC glass vial was maintained at 37°C and occasionally stirred gently. The sink condition for DXR was maintained throughout the experiment. The solubility of DXR in 0.1 mol/L HCl and in PBS was >20 mg/mL and >15 mg/mL, respectively. To determine the ratio of DXR released into the release medium and DXR encapsulated in the inner phase of the liposome, 5 µL of the dispersion was automatically withdrawn at predetermined time points and directly injected into the column-switching HPLC system. The percentage of the released DXR was calculated using the following formula:
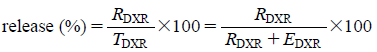 |
where, TDXR: total amount of DXR (mg/mL), RDXR: released DXR into the release medium (mg/mL), EDXR: encapsulated DXR in the inner phase of the liposome (mg/mL).
In Vitro Release Study by DialysisFigure 1 illustrates a schematic overview of the in vitro release test methodology by dialysis. A dialysis membrane was rinsed by soaking in distilled water for 20 min and maintained overnight in the release medium to equilibrate before use. One hundred microliters of the DXR liposome was transferred into a QuixSep® microdialyzer (Membrane Filtration Products, TX, U.S.A.) and wrapped with the dialysis membrane. The permeable area of the dialysis membrane in the microdialyzer was approximately 0.8 cm2. The microdialyzer was soaked in a glass vessel containing 50 mL of the release medium. The release medium was maintained at 37°C and stirred at 350 rpm using a magnetic stirrer. The sink condition for DXR was maintained throughout the experiment. One milliliter of the release medium was withdrawn at predetermined time points. One hundred microliters of the sample and the corresponding DXR standard solution were analyzed by HPLC to determine the amount of released DXR. The operating conditions for HPLC were identical to those of linear gradient analyses in the column-switching HPLC.
Encapsulation Characteristics by Column-Switching HPLCThe HSPC stock liposome (total lipid concentration, 40 mg/mL) was mixed with the DXR stock solution (5 mg/mL) at the ratio of 1 : 1 (v/v). The mixture was dispersed into the diluent to create a DXR concentration of 0.2 mg/mL. The obtained suspension was statically annealed in an HPLC glass vial maintained at various temperatures. Five microliters of the dispersion was injected automatically into the column-switching HPLC system at predetermined time points to determine the amount of DXR encapsulated into the inner phase of the liposome and free (not encapsulated) DXR. Encapsulation was calculated according to the formula used to calculate EE.
The column-switching HPLC method has been validated previously.26) However, method verification was additionally conducted with respect to recovery of DXR from EPC and EPC+HSPC liposome formulations because the detailed lipid components were different from the previous one. Recovery of DXR from Doxil® was validated in the previous study.
Recoveries were evaluated by comparing the peak area of DXR obtained from sample suspensions of DXR liposome formulations with that obtained from the corresponding DXR standard solution (0.2 mg/mL).
The obtained recoveries (average±S.D., n=3) for EPC and EPC+HSPC liposome formulations were 100.2±1.5% and 101.2±2.0%, respectively. These results indicated that the column-switching HPLC method was suitable and applicable for the evaluation of free and encapsulated DXR in the liposome formulations used in the present study.
On the other hand, the limit of quantitation (LOQ) and limit of detection (LOD) of DXR in this column-switching HPLC method were defined as 1 µg/mL and 0.1 µg/mL, respectively, in the previous study.
Characterization of DXR Liposome FormulationsThe component compositions, EEs, particle sizes, and zeta potentials of the prepared DXR liposome formulations and Doxil® are shown in Table 1. The lipid component determines the permeability (or rigidity) of the liposome bilayer, which results in a difference in the drug release characteristics. For instance, naturally derived unsaturated phosphatidylcholines such as EPC produce a much more permeable and less stable bilayer. In contrast, saturated phospholipids with long acyl chains such as HSPC form a rigid impermeable bilayer, and combination with cholesterol stabilizes the liposome bilayer. The lipid components of the prepared liposome formulations were selected to produce diversity in the release characteristics, whereas the particle sizes and zeta potentials presented no significant difference.
The particle size in PBS grew slightly larger than that in 0.1 mol/L HCl. This increase in size was considered to be caused by formation a complex with PO43−.31,32) Furthermore, a change in the appearance to a viscous dispersion was observed when the prepared liposome formulations were dispersed in PBS at a high concentration. All EEs were >90% and suitable for the in vitro release study.
In Vitro Release StudyThree DXR liposome formulations (EPC, EPC+HSPC, and Doxil®) were subjected to the in vitro release test by dialysis (CelluSep F1; MWCO, 3.5 kDa) and column-switching HPLC in 0.1 mol/L HCl and PBS. In addition, the DXR solution (2 mg/mL) was subjected to the in vitro release test by dialysis to investigate the permeability of DXR through the dialysis membrane. Figure 2 summarizes the obtained release profiles.
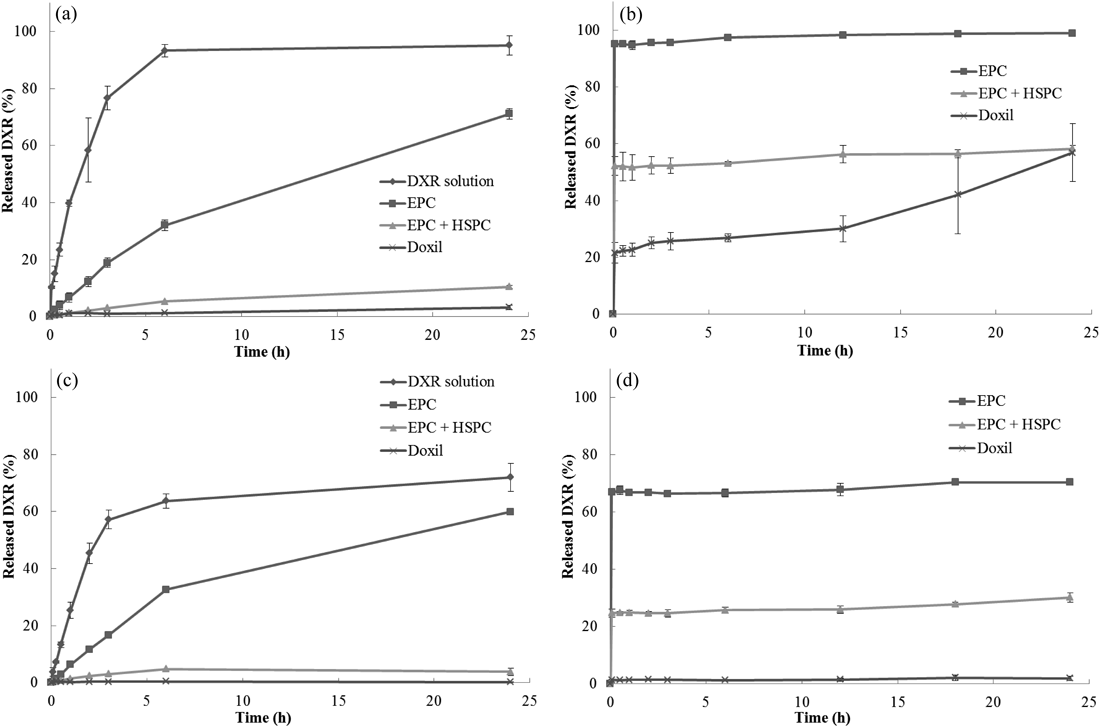
CelluSep F1 (MWCO: 3.5 kDa) was used as the dialysis membrane. Data are represented as mean±S.D. of three experiments.
In the in vitro release test by dialysis (Figs. 2a, c), the release rates were EPC>EPC+HSPC>Doxil® in both 0.1 mol/L HCl and PBS. As a result, the release rates of the three DXR liposome formulations reflected the permeability (or rigidity) of the lipid components in the two media, and increased as the liposome bilayer became fragile. The release rates in 0.1 mol/L HCl were higher than those in PBS. However, it took approximately 6 h even for the DXR solution to achieve complete drug leakage through the dialysis membrane in 0.1 mol/L HCl or to plateau in PBS. The dialysis of a drug occurs at the membrane surface and is dependent on the concentration difference of the two solutions on either side of the membrane. This process is affected by the temperature, viscosity, and mixing rate of a solution. Considering that DXR became a viscous dispersion in PBS, these slow and non-complete releases observed in PBS were assumed to be caused by the low fluidity (insufficient diffusion) of DXR in PBS, and not only by the lower solubility of DXR in PBS than that in 0.1 mol/L HCl. To test this hypothesis, the influence of the agitation speed was investigated. The in vitro release profiles of the DXR solution by dialysis were evaluated by agitation speeds of 200, 350, and 800 rpm (Fig. 3). Complete release in PBS was not achieved even when the agitation speed was 800 rpm. Although the in vitro release profile at 200 rpm showed a slow release, no significant difference was observed between those at 350 and 800 rpm. Therefore, it was considered that the low fluidity of DXR within the dialysis membrane was the main cause of the slow and incomplete releases. On the other hand, a large variation was observed in the release profile at 800 rpm. This may have been caused because of the air bubbles generated by vigorous agitation. Therefore, it was considered that an agitation speed of 350 rpm, as used in the comparison study (Fig. 2), was suitable for evaluation of the in vitro release profiles of the DXR liposome formulations.
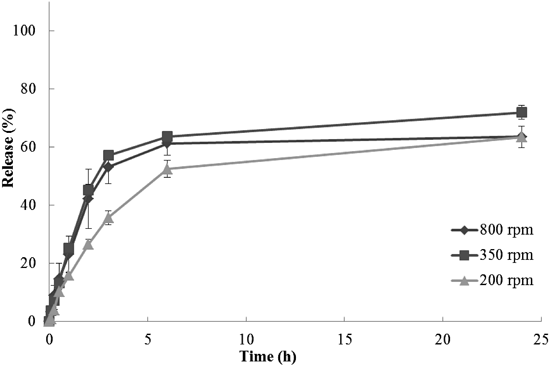
CelluSep F1 (MWCO: 3.5 kDa) was used as the dialysis membrane. Data are represented as mean±S.D. of three experiments.
Considering these results, the delay of drug leakage through the dialysis membrane of the DXR solution suggested that the release profiles obtained by dialysis comprises drug release from the liposome and drug permeation through the dialysis membrane. This means that, understanding the drug release characteristics of a liposome formulation only by the dialysis method can be difficult. This is particularly true if the drug release from the liposome is much faster than drug permeation through the dialysis membrane, thereby resulting in rate-limiting of membrane permeation.
Additionally, drug permeation through a dialysis membrane should be maximized when applying dialysis to an in vitro release test for a liposome formulation, whereas the released drug is adequately separated from the encapsulated drug in a liposome. Although the permeation rate and size of a solute are only two of the several interacting factors, the permeation rate is, in general, inversely related to the molecular weight because the molecular size is highly correlated with molecular weight. As the molecular size approaches and exceeds the size of the membrane pore (MWCO), the passage of solutes will be completely or partially prevented. The MWCO of a dialysis membrane can be selected and/or optimized during method development of in vitro drug release by dialysis. Influence of the MWCO on the membrane permeation of DXR was investigated. The in vitro release profiles of DXR solution and Doxil® were obtained using two types of the dialysis membrane, CelluSep F1 (MWCO: 3.5 kDa) and F2 (MWCO: 12–14 kDa) and are shown in Fig. 4. In 0.1 mol/L HCl (Fig. 4a), releases from Doxil® were ≤5% even after 24 h, whereas leakage from the DXR solution took approximately 6 h to complete regardless of the MWCO. No significant influence of the MWCO of the dialysis membranes was observed on the release profiles. In PBS (Fig. 4b), although a slight difference was observed in the amount of DXR released by 24 h, the time required for DXR solution to achieve the plateau leakage was approximately 6 h regardless of the MWCO. Furthermore, no significant difference was observed in the release profiles of Doxil® in PBS. In accordance, it was concluded that 3.5 kDa MWCO, used in the comparison study (Fig. 2), was acceptable and suitable as a dialysis membrane for evaluation of the in vitro release profiles of DXR liposome formulations in the range we could accomplish.

Data are represented as mean±S.D. of three experiments.
In contrast, in the developed in vitro release test by column-switching HPLC (Figs. 2b, d), the releases of all three DXR liposomes were extremely rapid and achieved plateaus in ≤5 min. The degree of plateau releases was dependent upon the permeability (or inversely proportional to rigidity) of the lipid components of the liposomes (EPC>EPC+HSPC>Doxil®), and those in 0.1 mol/L HCl were larger than those in PBS. Although the relative orders of the releases by dialysis and column-switching HPLC methods were in agreement, a difference between the in vitro release profiles obtained by both methods was apparent. The in vitro release method by column-switching HPLC does not essentially produce a rate-limiting step of membrane permeation because no membrane permeation process exists. Therefore, it was considered that the column-switching HPLC method revealed different in vitro release characteristics of DXR liposome formulations. This method also did not present any significant difference of the release speed observed between the in vitro release profiles in 0.1 mol/L HCl and PBS, but the degrees of the plateau releases were obviously different from each other. Therefore, it was considered that the difference in the release profiles between 0.1 mol/L HCl and PBS observed in the dialysis method was caused mainly by the difference in the degree of the plateau releases (equilibrium) in the media with different pH values and not only by the difference in solubility.
In addition, there were distinct differences between the release values after 24 h (equilibrium values), obtained by the dialysis and column-switching HPLC methods (Figs. 2a–d). We consider that there were several reasons for this discrepancy. The limited permeable area of the dialysis membrane and the low fluidity of the DXR liposomal suspensions inside the dialysis membrane may prevent the diffusion of DXR through the dialysis membrane. These might be imperfections of dialysis method.
Encapsulation Characteristics by Column-Switching HPLCThe developed column-switching HPLC system was considered to have a potential application in evaluating the characteristics of drug encapsulation into liposome formulations. The temperature dependency of the encapsulation characteristics of DXR into the inner phase of the liposome (HSPC stock liposome) was evaluated. The encapsulation rate increased as the incubation temperature increased (Fig. 5). Complete encapsulation was achieved in <4 h in static annealing at 50°C, but complete encapsulation was not achieved even after 24 h at 30°C. This dependency was considered to exist because the lipid membrane of the liposome became fragile and had high drug permeability at higher temperatures than the phase transition temperature. This characterization of temperature-dependent encapsulation by the developed method will be useful for development of the formulation and manufacturing processes of liposome formulations by remote drug loading.

Data for 30°C are the mean±S.D. of six experiments. Data for 40°C and 50°C are represented as mean±S.D. of three experiments.
The developed novel in vitro release test methodology by column-switching HPLC had no rate-limiting process such as drug permeation across the membrane because no membrane permeation process exists. In contrast, the conventional dialysis method was considered to present the in vitro release profiles attained by a combination of drug release from the liposome and drug permeation through a dialysis membrane. Therefore, the developed method was useful to understand the in vitro release characteristics of DXR liposome formulations, and complemented the results of the conventional in vitro release method by dialysis. In addition, automation did not require a large amount of sample. The developed column-switching HPLC system was also applicable for evaluation of the characteristics of drug encapsulation. The concept and application of this technique will be advantageous for the characterization, quality control, and/or further investigation of liposome formulations.
A schematic representation of the column-switching HPLC system, which is the main component of the developed novel in vitro release test methodology and the typical chromatograms are shown in Fig. A1.