2014 Volume 62 Issue 7 Pages 617-626
2014 Volume 62 Issue 7 Pages 617-626
The formulation characteristics of 6 brands of enteric-coated aspirin tablets under unpackaged conditions at 40°C and 60°C for 4 weeks were analyzed. Appearance, salicylic acid content, dissolution rates, and surface properties (by Raman microscopy) were evaluated to determine stability data, taking into account the clinical use of generic drugs. No change in appearance, decomposition, or dissolution rates was observed in unpackaged aspirin tablets stored at 40°C for 4 weeks. However, when stored at 60°C, tablets of 5 of the 6 brands showed whiskers on their surfaces along with an increase in salicylic acid content and a decrease in dissolution rate. Results of Raman mapping on the surface and cross sectional surface of the tablets with whiskers showed a salicylic acid peak associated with storage at 60°C for 4 weeks. However, for tablets from 1 of the 6 brands, no salicylic acid peaks were observed. For this tablet, Raman microscopy revealed 2 layers of film coating, and talc, which greatly affected the stability of the acetylsalicylic acid, was found only in the outer layer film. These results indicated that the protection of compatibility with talc is one of the important factors in enhancement of aspirin tablet stability in this tablet. We concluded that certification of the characteristics associated with stability and formulation is essential for generic drugs, which are not required to undergo stability testing under extreme storage conditions.
Generic drugs have significantly lower development costs than those associated with brand drugs, and thus have lower National Health Insurance (NHI) drug prices than those of the original formulations. Therefore, generic drugs are recommended for patient use since they help curb the national medical expenditure and ease patients’ financial burden. However, many medical institutions are cautious about using generic drugs in Japan. This is considered to be due in part to a lack of information about generic drugs compared to the extent of information available for original formulations.1) Therefore, it is necessary to acquire information on individual generic drugs in order to promote their safe use. Furthermore, although multiple generic drugs contain the same active ingredient, differences in quality and efficacy have reportedly been associated with different excipients and formulation methods.2–4) Hence, it is important to conduct a detailed assessment of differences between generic drugs.
Recently, comparative analyses of original formulations and generic drugs of various dosage forms have been carried out.5–7) Nakauchi et al. reported the comparability of brand and generic pravastatin by using the dissolution test.8) In fact, most studies have focused on dissolution of the principal ingredient, while few studies have investigated differences in formulation characteristics.
In present study, aspirin enteric tablets were used as model medication. Although low-dose aspirin has been widely used to prevent the onset of arterial thrombotic diseases, its use may cause gastrointestinal disorders because of an increase in the level of undissociated drug in the acidic milieu of the stomach.9,10) To circumvent this problem, aspirin tablets used for antiplatelet effects are often coated with enteric-coating films (hereinafter referred to as aspirin enteric tablets). Furthermore, temperature and humidity adversely affect uncoated tablets, and aspirin hydrolyzes to form needle crystals (whiskers).11) Film coating is thought to prevent the decrease in the stability of aspirin caused by these factors.
Film coating is a useful formulation technology, which stabilizes the principal ingredients in a tablet, while also improving formulation characteristics such as taste and odor and thereby patients’ compliance to treatment. However, even if a similar coating material is used, differences in the formulation or technology may affect the dissolution profile, consequently altering bioequivalence.12) Therefore, as the efficacy and safety of aspirin enteric tablets are controlled by their film, it is important to sufficiently study the various conditions that could influence characteristics.
When developing drugs, many tests are required to assure the quality, and stability tests are conducted in unpackaged condition as well as under more sever conditions than those used for the accelerated testing. However, for generic drugs such as aspirin enteric tablets, the basic accelerated test data may be filed as reference information on stability, which is required for marketing approval. Long-term prescription of aspirin enteric tablets has been facilitated, as tablets are often dispensed as one dose package to enhance patient compliance and to prevent accidental ingestion of press-through package (PTP) sheets.13) Dispensing methods such as one dose packages may vary, and the storage conditions after dispensing may differ depending on the patients receiving those drugs. The accelerated testing alone may not be sufficient to adequately provide stability-related information for clinical use. Storage condition of medication after dispensing is varied and medication might be stored in the serve condition. Therefore, stability information after storage in the serve condition is needed in the clinical side. There is little report about stability information of medication after storage in serve condition. Kodama et al. have reported the quality evaluation of ethyl icosapentaenoate products in stress condition (60°C, 30 d).14) And Nishimura et al. reported storage of insulin medication in serve condition.15)
The aim of this study was to evaluate six generic aspirin tablet after storage at 40 and 60°C for definite period by their appearance, salicylic acid content, dissolution rates, and surface properties (by Raman microscopy). According to the direction of Ministry of Health, Labour and Welfare in Japan and pharmaceutical company, there is not the original bland of the aspirin enteric tablets, and all of the aspirin enteric tablets are the generic medicines. Therefore, comparison of the original brand with generic medicines were not conducted in this study.
Raman spectroscopy is a spectrometric method involving laser irradiation of the sample with detection of the scattered light (Raman-scattered light) by using a spectroscope. This spectroscopic method can be used to analyze any sample (solid, liquid, or gas) in a nondestructive and noncontact manner. Aqueous solutions can be analyzed using this method because Raman scattering for water is relatively small and because a sharp band corresponding to the OH-group can be observed. Raman spectroscopy is used in the field of pharmaceutical sciences for molecular analyses that include distinction of crystal polymorphisms of principal ingredients in solid dosage forms as well as analysis of tablets components.16,17) Therefore, physical properties of aspirin and excipients were assessed by micro-Raman spectroscopy before and after long-term storage under accelerated or stress conditions.
One lot each of 6 brands of 100-mg aspirin enteric tablets was used in this study; all were marketed as of December 2008 (Table 1). Each test drug was randomly assigned and labeled as Tablets A to F. Information on excipients used in the formulation of each of the aspirin enteric tablets was obtained from the composition section of its package insert and interview form. All reagents used in this study were of analytical grade.
| Aspirin tablets | Excipientsa) |
|---|---|
| Tablet A | Partly pregelatinized starch, crystalline cellulose, sodium carboxymethyl starch, sodium lauryl sulfate, tartaric acid, talc, dried methacrylic acid copolymer LD, triethyl citrate, titanium oxide |
| Tablet B | Corn starch, carmellose, talc, stearic acid, light anhydrous silicic acid, methacrylic acid copolymer LD, sodium lauryl sulfate, polysorbate 80, macrogol 6000 |
| Tablet C | Corn starch, carmellose, talc, stearic acid, anhydrous silicic acid, methacrylic acid copolymer LD, sodium lauryl sulfate, polysorbate 80, macrogol |
| Tablet D | Corn starch, carmellose, talc, stearic acid, light anhydrous silicic acid, methacrylic acid copolymer LD, sodium lauryl sulfate, polysorbate 80, macrogol 6000 |
| Tablet E | Cellulose, corn starch, carmellose, methacrylic acid copolymer LD, triethyl citrate, talc |
| Tablet F | Powder cellulose, corn starch, methacrylic acid copolymer LD, sodium lauryl sulfate, polysorbate 80, talc, triethyl citrate |
a) All excipient information was collected from ethical drug package insert for each medication.
An equivalent amount of each of the aspirin enteric tablets in its unpackaged state was placed in an airtight polyethylene container (Shinko Chemical Co., Ltd., Kanazawa, Japan), and stored protected from light. One container was maintained at 40°C and the other at 60°C. Tests for appearance and various other characteristics were performed before storage as well as at 1, 2, and 4 weeks after storage.
Assay of Salicylic Acid in the TabletsThe stability of aspirin was established by quantifying the salicylic acid produced by hydrolysis.
Sample PreparationFive aspirin enteric tablets were powdered, and an amount of powder equivalent to 0.125 g of aspirin was added to 15 mL of extraction solution (a mixture of acetonitrile and phosphoric acid (500 : 1)). This solution was shaken for 20 min, and the volume was made up to 25 mL by using the extraction solution. The solution was subsequently passed through a 0.2-µm filter (MILLEX-LG; Nihon, Millipore K.K., Tokyo, Japan).
High-Performance Liquid Chromatography (HPLC) AnalysisThe HPLC system consisted of an LC-10AD pump, a CTO-10AC column oven, an SPD-10AV UV detector (all from Shimadzu Corporation), and NUCLEOSIL 100 C18 column (5 µm, 4.6 i.d.×150 mm, GL Science, Tokyo, Japan). The mobile phase consisted of 2.5 mM potassium dihydrogen phosphate and methanol (3 : 2). Column temperature, flow rate, and detection wavelength were 40°C, 1.1 mL/min, and 295 nm, respectively. For calibration curves of salicylic acid, response linearity was obtained in the range of 2.5 to 200 µg/mL.
Dissolution test was conducted on 6 aspirin enteric tablets according to the Paddle Method of Dissolution described in the Japanese Pharmacopoeia (JP), 16th Edition, by using a dissolution test apparatus (NTR-3000; Toyama Sangyo Co., Ltd., Tokyo, Japan) at a rotation speed of 50 rpm. Since aspirin enteric tablets are delayed-release dosage forms, 900 mL of the 2nd fluid for dissolution test (pH 6.8) was used as the dissolution medium, and the temperature was maintained at 37°C. Ten milliliters of medium were withdrawn before the start of the test and at 10, 15, 30, 60, 120, and 240 min after the start of the test. The amount of withdrawn dissolute was replaced with the dissolution fluid.
Assay for Aspirin in the MediumThe dissolution medium was passed through a 0.45-µm filter (MILLEX-HA; Nihon Millipore K.K., Tokyo, Japan). Three milliliters of 0.3 M sodium hydroxide TS were added to 1 mL of the filtrate, and the absorbance of this solution was measured at 298 nm with a UV detector (UVmini-1240; Shimadzu Corporation, Tokyo, Japan).
Statistical AnalysisDifferences compare to control (0 week) were tested by Dunnet’s test, with a significance level of 5%.
Scanning Electron Micrograph (SEM)The surfaces of tablets were observed using scanning electron microscopy (VE-7800, KEYENCE Corporation, Osaka, Japan) at an acceleration voltage of 1.7 kV.
Raman Microscopy and MappingOptical images were obtained using an Olympus BH-2 microscope (Olympus Corporation, Tokyo, Japan). Raman spectra were obtained with a Nicolet Almega XR (Thermo Fisher Scientific K.K., Yokohama, Japan) with a 532-nm laser, irradiating the laser for 3 s, and integrating 10 times. The inside of the blue square frame in the optical microscope image ((a) and (b) of Figs. 6, 8, respectively) showing the cross-section of each tablet was observed using an objective lens of 100× magnification, and was measured under the following conditions: exposure time, 6.0 s; frequency, 5 times; and stage step width, 75 µm. Raman mapping was used to obtain spectra by using the ratio of peak intensity, which corresponded to the peak intensity at 1634 cm−1 (derived from salicylic acid) divided by the peak intensity at 1605 cm−1 (derived from aspirin).
Excipients included in aspirin enteric tablet formulations are shown in Table 1. The film-coating agent methacrylic acid copolymer was common in all 6 brands. However, other excipients differed between the formulations.
When stored at 40°C for 4 weeks, no changes were observed in visual or microscopic appearance (no data were shown). Photographs of appearance and surface on the tablet in each of the aspirin enteric tablets before and after storage at 60°C for 4 weeks are shown in Fig. 1. In Tablets A, B, C, and D, whiskers appeared on the surface of the tablets within the first week. And smooth surface of tablets were observed in the electron micrographs of Tablets A, B, C and D after storage at 60°C for 4 weeks. No changes in the appearance and surface of the tablet state during the 4-week storage period were observed in Tablets E and F.
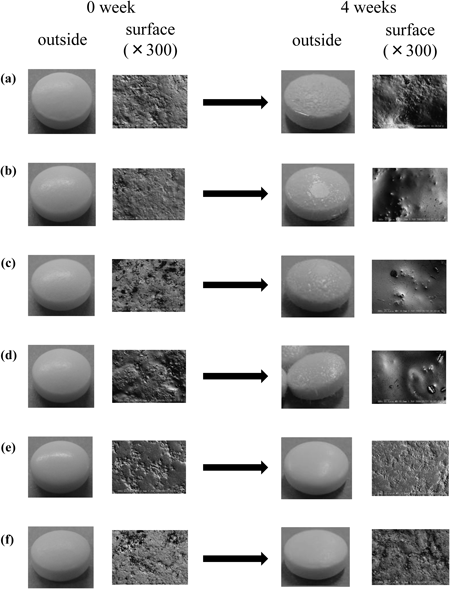
(a) Tablet A, (b) Tablet B, (c) Tablet C, (d) Tablet D, (e) Tablet E, and (f) Tablet F.
To investigate the effect of storage time and temperature on acetylsalicylic acid stability in aspirin tablets, changes in the salicylic acid content after storage at 40°C and 60°C for a fixed time were evaluated. The results are shown in Fig. 2. The amount of salicylic acid contained in each of the aspirin enteric tablets prior to testing was found to differ between the formulations: Tablets B, C, and D had higher values than those in Tablets A, E, and F. The amount of salicylic acid contained in these aspirin enteric tablets did not change after storage at 40°C for 4 weeks (Fig. 2a). As shown in Fig. 2b, when stored at 60°C, all brands except Tablet E showed a tendency toward an increase in the amount of salicylic acid. In particular, Tablets A, D, and F had a higher salicylic acid content after 4 weeks of storage (more than 3%) than that found in other preparations. Since the appearance of the aspirin tablets varied after storage at 40°C and 60°C for a fixed time, differential dissolution of acetylsalicylic acid was presumed to have occurred.
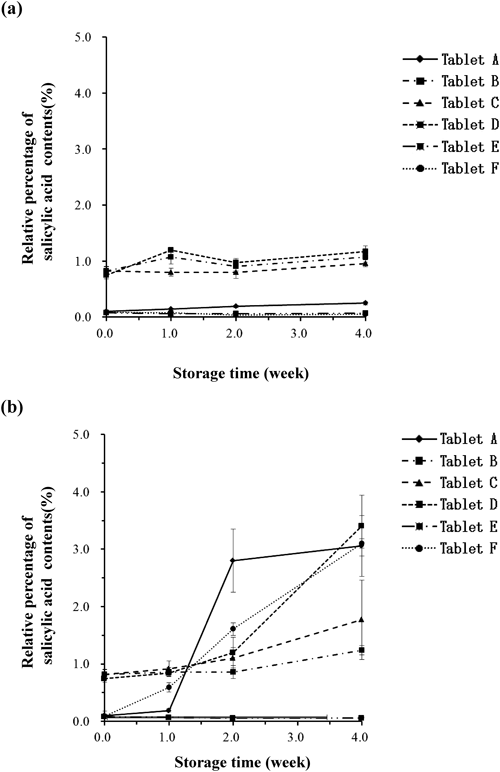
(a) 40°C, (b) 60°C. Each value represents the mean±S.D. (n=6).
Results of dissolution test on aspirin enteric tablets after storage at 40°C for a fixed duration are shown in Fig. 3. Tablets A, C, D, E, and F did not show any alterations in dissolution rate until week 4 of storage. For Tablet B, the dissolution rate at the 60-min interval decreased after 1 week of storage, while the dissolution rate decreased at the 120-min interval after 2-week and 3-week storage. However, the dissolution rate at the 240-min interval exceeded the criteria (85%).
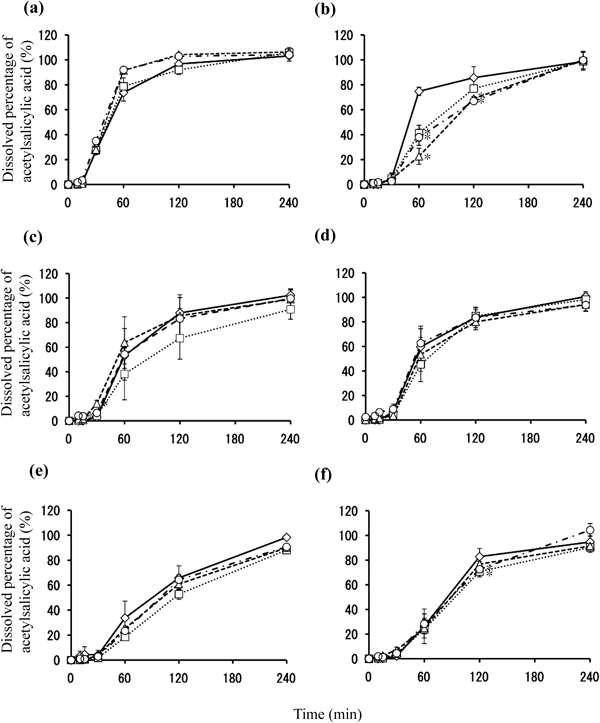
(a) Tablet A, (b) Tablet B, (c) Tablet C, (d) Tablet D, (e) Tablet E, and (f) Tablet F. Graph symbols indicate 0 (◇), 1 (□), 2 (△), and 4 (○) weeks of storage time. Each value represents the mean±S.D. (n=6). * p<0.05 compared to control (0 week).
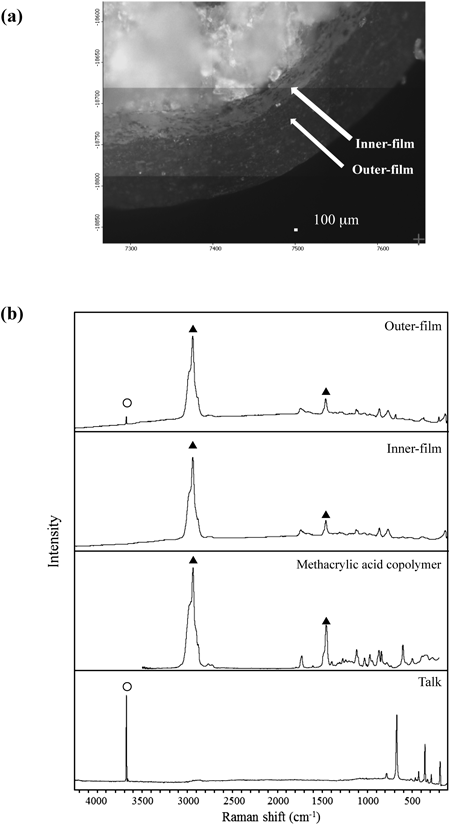
As shown in Fig. 4, when stored at 60°C, the dissolution rate in Tablet E decreased by around 10% after 4 weeks of storage. On the other hand, the dissolution rate of acetylsalicylic acid in Tablet A significantly decreased at the 60-min and 120-min interval after only 1 week of storage. The dissolution rate for Tablet A significantly decreased through the 240-min interval in the 2-week and 4-week storage periods. As for Tablets B, C, and D, the dissolution rate of acetylsalicylic acid decreased markedly after 1 week of storage, with the dissolution rate at the 240-min interval dropping below 5%. While no significant decrease in the dissolution rate of acetylsalicylic acid in Tablet F was observed through 1-week storage, the dissolution rate at the 60-min and 120-min intervals decreased after 2 weeks of storage, with the dissolution rate of acetylsalicylic acid at the 240-min interval decreasing by more than 10% after 4 weeks of storage. These results indicated that surface condition on the tablets after storage at 60°C affected acetylsalicylic acid release from the tablet. To evaluate the surface properties of each tablet after storage at 60°C, Raman microscopy was performed before and after storage of tablets. Figure 5 shows microphotographs of the tablet surfaces of Tablet A before and after storage at 60°C for 4 weeks, along with the Raman spectra of the surface. Tablet surfaces appeared glossy before storage (Fig. 5a). Furthermore, peaks corresponding to methacrylic acid copolymer (▲) and talc (○) were observed as shown in Fig. 5c. The microphotographs and spectra obtained after storage for 4 weeks were different from those before storage (Figs. 5b, d). Various crystalline materials were not observed in the microphotographs prior to storage, but were visible after 4 weeks of storage. A peak derived from salicylic acid (□) was seen in Raman spectra in the vicinity of these precipitates. This finding was consistent with that for appearance, as shown in Fig. 1. In order to further investigate the distribution of salicylic acid produced during storage, Raman mapping of the cross-section of Tablet A was performed, and visualized with the ratio of the peak intensity, which was the peak intensity at 1634 cm−1 (derived from salicylic acid) divided by the peak intensity at 1605 cm−1 (derived from aspirin). These results are shown in Fig. 6. While no region corresponding to salicylic acid was observed in the samples before storage, the salicylic acid-derived areas shown in green and red were observed after storage (Fig. 6d). For Tablet E, which showed little decline in the dissolution rate of aspirin when stored at 60°C, the results of micro-Raman spectroscopy indicated that no change in visual and microscopic appearance occurred when stored at 60°C for 4 weeks (Figs. 7a, c). The Raman spectra of the surfaces (Figs. 7b, d) as well as the cores (Figs. 8b, d) did not show any salicylic acid peak. In order to investigate the mechanism underlying the stabilization of aspirin in Tablet E, an optical microscopic image of the tablet cross-section was obtained (Fig. 9). Thus, it was clear that the film coating of this tablet consisted of 2 layers, an inner and an outer layer. Accordingly, a Raman spectrum of each layer was measured with results indicating that both the inner and outer layers had a peak derived from methacrylic acid copolymer but only the outer layer had a peak derived from talc at 3675 cm−1.
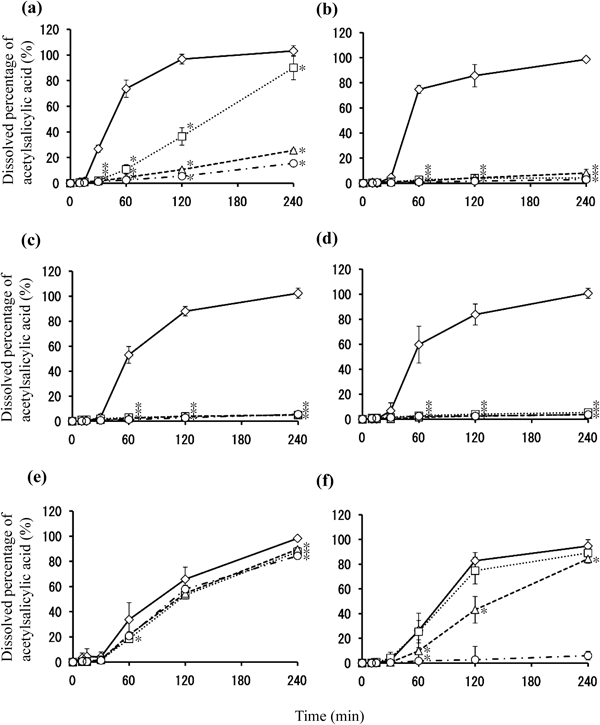
(a) Tablet A, (b) Tablet B, (c) Tablet C, (d) Tablet D, (e) Tablet E, and (f) Tablet F. Graph symbols indicate 0 (◇), 1 (□), 2 (△), and 4 (○) weeks of storage time. Each value represents the mean±S.D. (n=6). * p<0.05 compared to control (0 week).
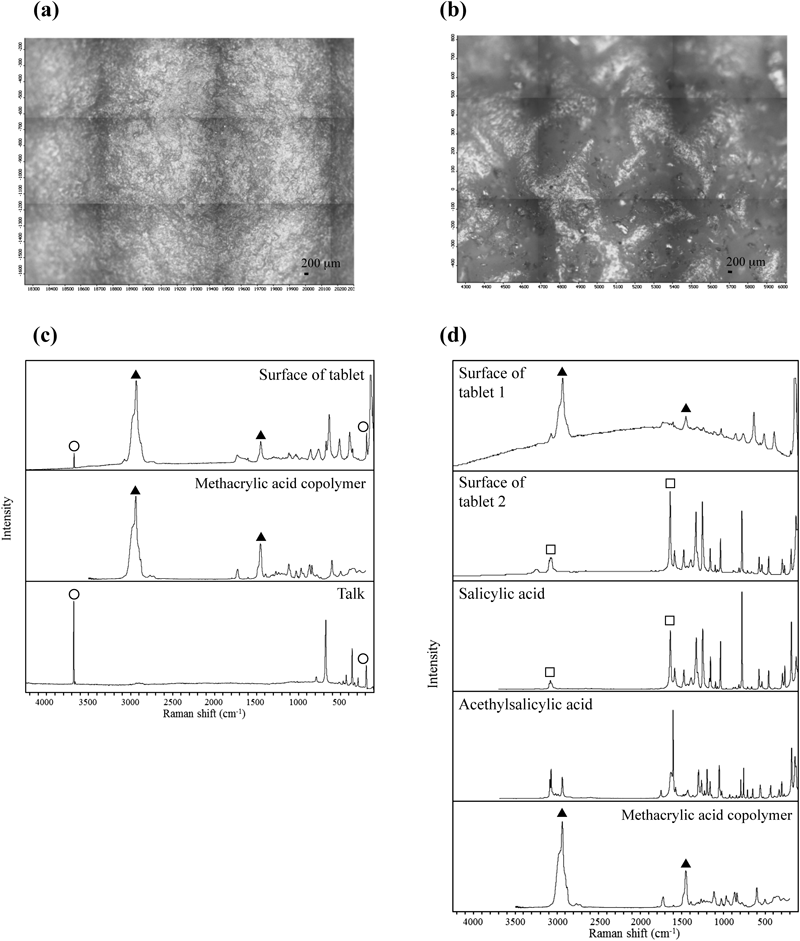
(a) and (c) show a microscopic picture and Raman spectra of Tablet A before storage, respectively. (b) and (d) show a microscopic picture and Raman spectra of Tablet A after storage at 60°C for 4 weeks, respectively. (▲) Methacrylic acid copolymer, (○) talc, and (□) salicylic acid.
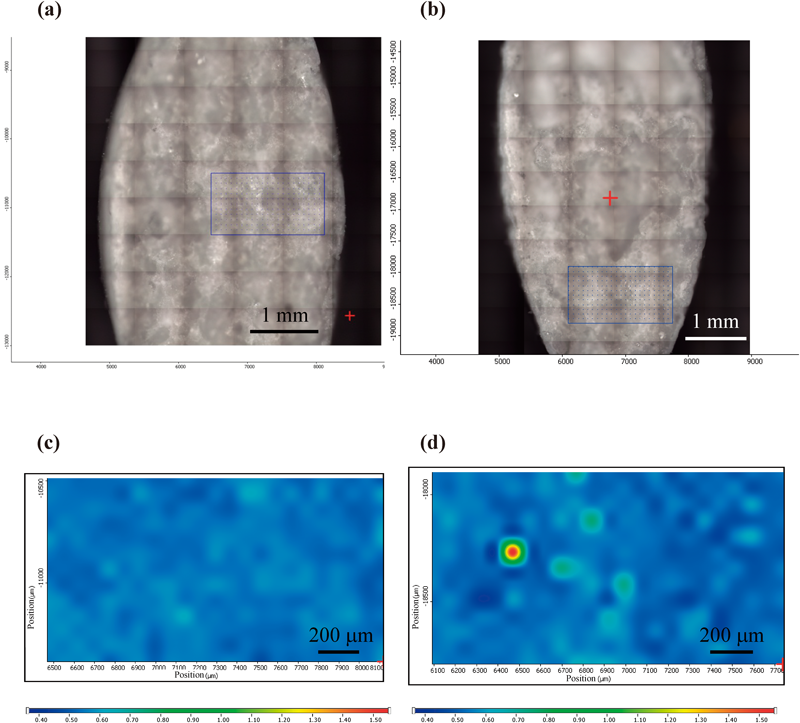
(a) and (c) show a microscopic picture and Raman mapping of Tablet A before storage, respectively. (b) and (d) show a microscopic picture and Raman mapping of Tablet A after storage at 60°C for 4 weeks, respectively.
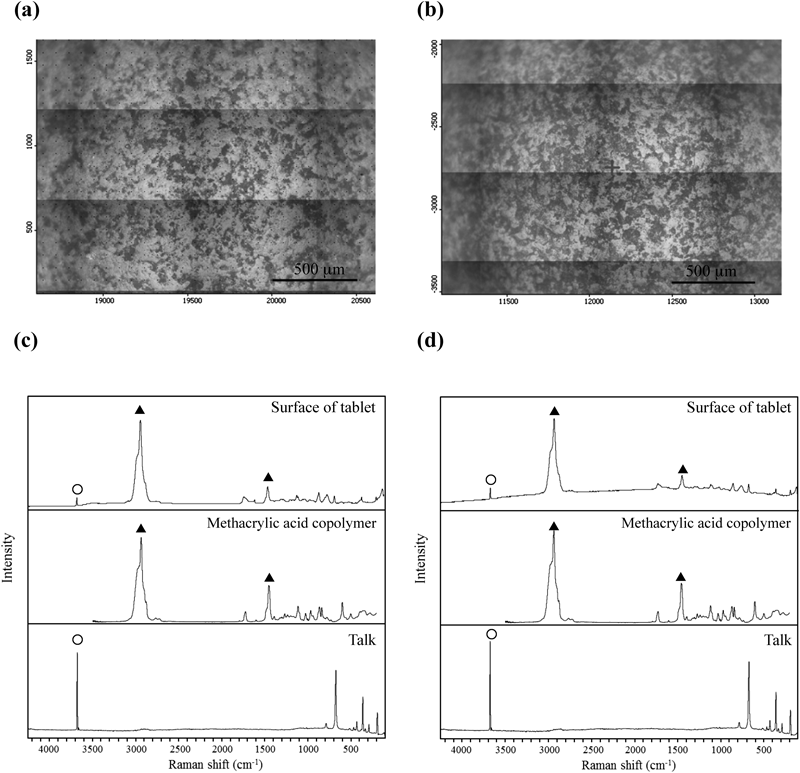
(a) and (c) show a microscopic picture and Raman spectra of A Tablet E before storage, respectively. (b) and (d) show a microscopic picture and Raman spectra of Tablet E after storage at 60°C for 4 weeks, respectively. (▲) Methacrylic acid copolymer and (○) talc.
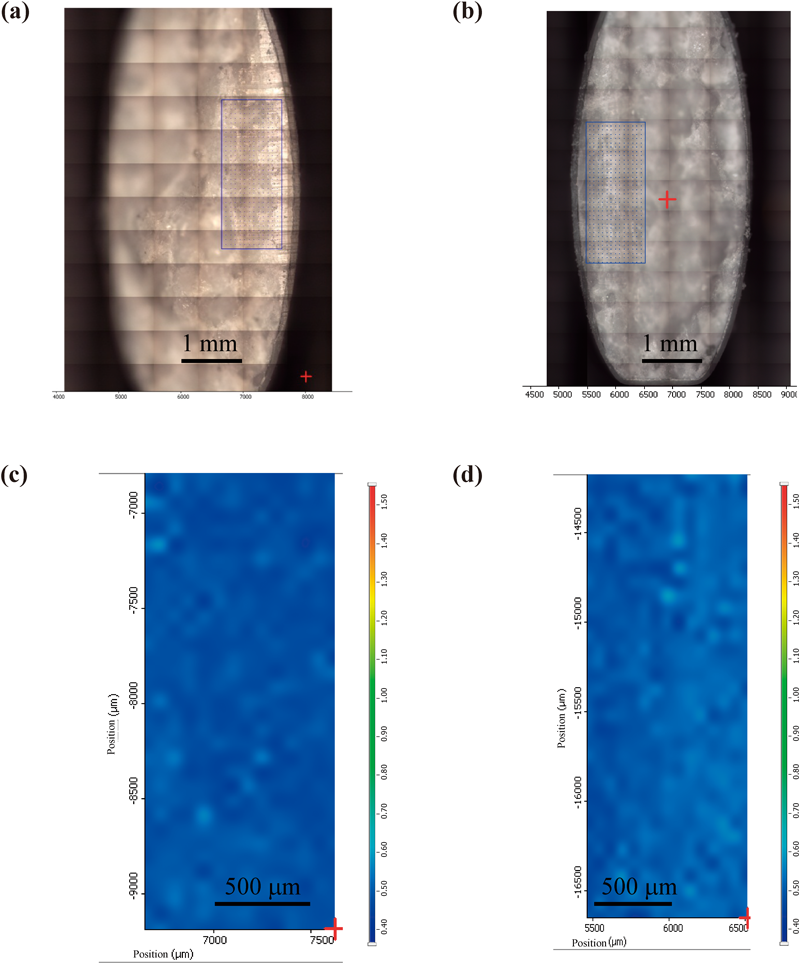
(a) and (c) show a microscopic picture and Raman mapping of Tablet E before storage, respectively. (b) and (d) show a microscopic picture and Raman mapping of Tablet E after storage at 60°C for 4 weeks, respectively.
(a) Microscopic picture. (b) Raman spectra. (▲) Methacrylic acid copolymer and (○) talc.
During drug development, many tests are required to assure the quality and stability. Stability tests are performed on unpackaged drugs and under more severe conditions than those used for accelerated testing. Generic drugs, on the other hand, are assessed for their stability by accelerated testing on fully packaged products. In the case of drugs used primarily for chronic illnesses in elderly people, such as aspirin enteric tablets, single dose packaging of these drugs has been predicted to avoid missed doses.13) Furthermore, since patients manage their medications for long period because of the revision of health insurance treatment fees, it might be needed information on the stability of the drug in an unpackaged state under more severe conditions.
In this study, aspirin enteric tablets were analyzed in order to obtain information on drug stability under extreme conditions (storage at 40°C and 60°C in the unpackaged state), taking into account the actual clinical use.
When each of the aspirin enteric tablets was stored at 40°C (a condition similar to that in the accelerated testing), no change in the appearance and content of salicylic acid in the formulations was observed for up to 4 weeks of storage.
However, when stored at 60°C (stress condition), whiskers appeared on the surface of the tablets; changes in the film were observed in 4 brands. In the dissolution test, no differences were observed between the preparations in terms of their dissolution profiles prior to storage, all preparations exhibited a dissolution rate of about 80% at the 120-min interval, and about 100% at the 240-min interval. When stored at 40°C, a delay in dissolution was observed only for Tablet B, therefore it was suggested that the dissolution profile of this drug might be subject to change. On the other hand, when stored at 60°C, a significant difference was observed between the preparations, and this difference was correlated with a change in the surface state, which was clearly observed on scanning electron micrographs (Fig. 1). An increase in the salicylic acid content was observed over time when the drugs were stored under extreme conditions (at 60°C). From the results of Fig. 5, it was suggested that storage under stressful conditions caused denaturation of the film and accelerated aspirin degradation. The relationship between a change in surface state and a change in dissolution profile was unknown. However, denaturation of the film may have affected the dissolution profile of aspirin.
Because a salicylic acid peak detected in Raman mapping was observed in the tablet core, aspirin degradation was suggested to proceed not only on the surface but also inside the tablet.
Stability of the active ingredient in the preparations may be susceptible to various factors such as temperature, humidity, and compatibility with excipients. Therefore, excipients included in the formulation of drug products have been chosen following a complete investigation of their influence on the active ingredient. For example, the preparation containing amlodipine besylate was developed after taking into account its incompatibility with excipients under stress conditions in the unpackaged state.18) With regard to aspirin, while absorption of moisture decreased the stability and an increase in temperature promoted hydrolysis, incompatibility with excipients possibly affected its stability. Concerning the incompatibility of aspirin with the excipients used in film-coated tablets, the possibility of an increase in the free salicylic acid content when compounded with macrogol19) and talc20) has been reported. As seen with the 6 brands tested in this study, talc was included as an excipient in all the brands, while macrogol was included in only 3 brands.
Tablet E, which did not show any change in salicylic acid content even under stressful conditions, did not contain macrogol as an excipient (according to information in the package insert). Results of micro-Raman spectroscopy of the tablet cross-section indicated that Tablet E had 2 layers of film. It was assumed that talc was present only in the outer layer of the 2-layer film coating, which did not come into direct contact with aspirin as the peak of talc was not observed in the inner layer. A decrease in the frequency of contact between aspirin and talc could represent an underlying cause of improved stability for Tablet E under stressful conditions. Incompatibility of aspirin with excipients in the other 5 brands may have been caused by an increase in salicylic acid content at 60°C.
In this study, the formulation characteristics of aspirin enteric tablets were assessed, with a focus on stability when stored under stressful conditions. Five out of 6 brands of tablets showed a change in appearance, an increase in salicylic acid content, and a significant change in the dissolution profile. These changes in the formulation characteristics were suggested to result from denaturation of the film as well as incompatibility with excipients such as talc. It was concluded that stability and formulation characteristics should be carefully investigated by taking into account factors such as the dispensing method and dosing period, in order to determine appropriate efficacy in the clinical use.