Mercury is a reputable highly toxic element.1–3) Notably, World Health Organization (WHO), Environmental Protection Agency (EPA), and U.S. Food and Drug Administration (FDA) reported that certain kinds of fishes accumulate high level of mercury which is harmful enough to fetuses, babies and children.3,4) Such an accumulation of mercury in fishes occurred through a food chain.2,5) Based on these facts, the provisional tolerable weekly intake (PTWI) for methylmercury and the reference dose (RfD) for mercuric chloride have been set at 1.6 µg/kg/week and 0.3 µg/kg/d, respectively.3) In any case, mercury originates from natural sources (such as volcanoes) and human activities (such as industrial wastes). To reduce mercury contamination in the environment, it is necessary to reduce and remove mercury from environments (oceans, rivers, and lakes).
For this purpose, various techniques have been reported. These include bioremediation,5,6) precipitation,7) non-specific adsorption (using activated carbon,8,9) aerogels,10) and chitosan8,11) as sorbents), and metal chelations (using imidazoles,12) thiols,13) and DNA/nucleobases14–19)). However, these techniques generally trap several kinds of metals, resulting in the loss of mineral balance in the treated water. It is thus indispensable to develop methods for the selective removal of mercury.
For this purpose, thymine–Hg(II)–thymine (T–Hg(II)–T) base pairing system which was used in a DNA-based Hg2+-specific sensor by the Ono and Togashi20) gave us a hint, since thymine is a highly Hg(II)-specific ligand. The key structural element of the T–Hg(II)–T base pair was originally proposed by Katz,21) based on pre-existing studies on Hg2+–DNA interactions.22–25) New insights were recently reported by Ono and colleagues.20,26,27) Although extensive structural and theoretical studies on the structure of this base pair in a DNA oligomer have been performed,28–41) the experimental structure of the T–Hg(II)–T base pair from NMR and Raman spectroscopy was recently reported27,42) (see the chemical structure of the T–Hg(II)–T base pair for that shown within Fig. 1b). Thermal denaturation experiments43) showed that the T–Hg(II)–T base pair is as stable as the Watson–Crick A–T base pairs. More importantly, it was demonstrated that a T–T mismatch can specifically bind to Hg2+ without binding to other divalent metal cations.26) Thus, such Hg2+-specificity of thymine base is suitable to develop an Hg2+-specific trapping system. We thus prepared Hg2+-trapping beads based on this concept (Fig. 1), and examined their trapping efficiency.
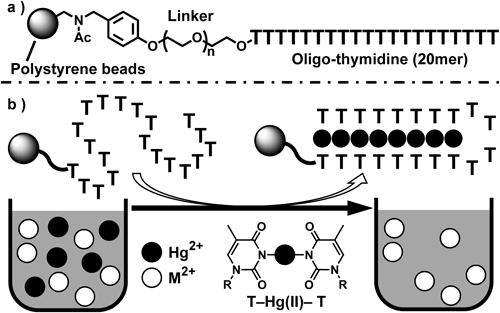
The Hg2+-trapping beads consist of oligo-thymidine (T20) attached onto the polystyrene (PS) beads (solid support), as shown in Fig. 1a. Following capture of the Hg2+ ions by T20, the Hg2+-trapped PS beads can be easily separated from the solution by filtration (Fig. 1b). To give mobility to T20, polyethylene glycol 400 (PEG 400) was used as a linker to attach T20 onto the PS beads, since PEG 400 is long enough to give attached molecules their mobility on the polymer surface.44) The detailed preparation is given in Chart S1 and Supplemental Methods in Supplemental Information.
Inductively coupled plasma atomic emission spectroscopy (ICP-AES) was used to assess the performance of the prepared Hg2+-trapping beads. This technique is suitable for the quantification of low levels of Hg2+ as well as the simultaneous determination of co-existing metal cations in solution.
Prior to conducting the Hg2+-trapping experiments, we confirmed the linearity between the Hg2+-concentration and the ICP-AES peak intensities, measured at the 253 nm and 194 nm mercury emissions (Hg2+ concentration range: 0.5–10 µM=0.1–2.0 ppm) (Fig. S1).
Based on the obtained linear range and signal to noise ratio, the Hg2+-trapping experiments were conducted at 50 µM (10 ppm) Hg2+, and the supernatant solutions after Hg2+ removal were diluted 10 times (maximum 5.0 µM Hg2+) for Hg2+ quantitation. The residual Hg2+ ratios (%), monitored at the 253 and 194 nm emissions, as calculated by Eq. 1 in Supplemental Methods in Supplemental Information, are presented in Fig. 2. A ca. 50% reduction in the residual Hg2+-concentration was observed for the Hg2+-trapping beads (white bars in Fig. 2). It should be also mentioned that capture of Hg2+ was negligible in the absence of the oligo-thymidine moieties (PS beads and PS beads+linker samples in Fig. 2 and Table S1). Thus, the Hg2+-trapping capabilities of the beads are exclusively reliant on the presence of oligo-thymidine moieties.
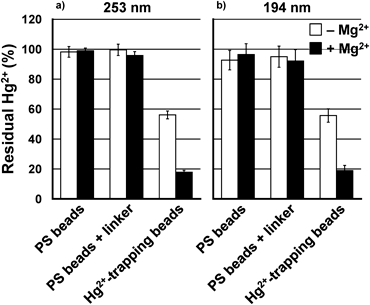
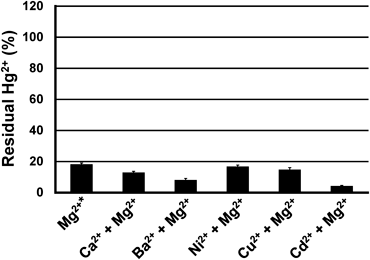
The Hg2+-selective trapping was initially examined in the presence of Mg2+ to simulate the natural conditions of seawater that contains minerals such as Na+, Mg2+, and Ca2+. Because the actual concentration of Na+ (481 mM) and possibly Mg2+ (54 mM) in seawater exceeds the upper limit of the ICP-AES measurements, the present experiments were conducted under a 5.0 mM Mg2+ solution. Unexpectedly, in the presence of Mg2+, the residual Hg2+ concentration decreased from ca. 50 to ca. 20% (Fig. 2, Table S1). The same trend was observed for both 253 nm and 194 nm mercury emissions (Fig. 2, Tables S1 and S2). Therefore, for the subsequent studies, only the results from the 253 nm emission spectra are presented as the 253 nm emission generated stronger signals.
As seawater includes several kinds of divalent metal cations, we investigated the performance of the beads to selectively trap Hg2+ over other divalent metal cations (Ca2+, Ba2+, Ni2+, Cu2+, Cd2+) in the presence of Mg2+ (Fig. 3, Fig. S2). The Hg2+-trapping efficiency of the beads, examined in the presence of co-existing metal cations (Ca2+, Ba2+, Ni2+, Cu2+, Cd2+), was similar or greater than that obtained in the presence of Mg2+ only (absence of the co-existing cations) (Fig. 3, Table S1). For the Hg2+-trapping efficiencies in the absence of Mg2+, see Fig. S2.
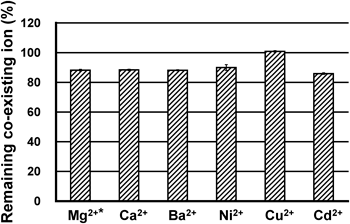
The non-specific binding of the co-existing metal cations by the Hg2+-trapping beads was then examined (Fig. 4, Figs. S3, S4, Tables S3, S4). The residual ratios (%) of the co-existing cations after Hg2+-trapping are shown in Fig. 4 and Table S3. As observed, the remaining percentages of all the co-existing metal cations in the corresponding supernatant solutions, after Hg2+-trapping, were very high.
The DNA-based Hg2+-trapping beads effectively removed mercury in both the presence of Mg2+ only, and several co-existing metal cations that included Mg2+ (Fig. 3). In contrast to this effective Hg2+-trapping, non-specific trapping of co-existing cations did not occur so much (Fig. 4). The results are consistent with our previous study that showed the specific binding of Hg2+ by thymine bases.26) Thus the herein developed Hg2+-trapping beads are suitable for use in natural conditions that involve a variety of mineral ingredients.
The enhanced performance of the Hg2+-trapping beads in the presence of co-existing metal cations is explained as follows. The formation of T–Hg(II)–T base pairs implies the formation of a DNA duplex. For the latter to form, electrostatic repulsion between the negatively-charged phosphates in the respective DNA strands needs to be suppressed. The Mg2+ and other co-existing cations thus suppress the electrostatic repulsion between the phosphate groups by neutralizing the negative charges (Fig. S5). Hence, in the absence of a charge compensation effect of the co-existing cations, formation of T–Hg(II)–T base pair and the resulting DNA duplex formation cannot occur efficiently. In addition, the competition between Mg2+ and Hg2+ during their phosphate-binding steps may assist preferential Hg2+-binding to thymine bases. This phenomenon was evident in the presence of Mg2+, as indicated by the considerable increase in the Hg2+-trapping efficiency (Figs. S2, S5c, d). A slight increase in the trapping efficiency was observed in the presence of the co-existing cations under Mg2+-depleted conditions (Figs. S2, S5e). These findings and some foregoing studies45,46) support our hypothesis that co-existing cations stabilize the formation of a DNA duplex following formation of T–Hg(II)–T base pair. The measured residual ratio of the co-existing metal cations after Hg2+-trapping (Fig. S3) further supported our hypothesis that co-existing cations served as counter ions for DNA, as explained: in the absence of Mg2+, the other co-existing cations were captured by the Hg2+-trapping beads, possibly as counter ions for the phosphate groups (Fig. S5e), hence other co-existing cations rarely remained in solution in the absence of Mg2+ (Fig. S3). The present experimental data indicate that the Hg2+-trapping beads show potential for their usage under natural environmental conditions, particularly in seawater with a high salt concentration.
The present findings were compared to those obtained by other reported methods (bioremediation,5,6) precipitation,7) non-specific adsorbents,8–10) and some metal chelators12,13)). In these studies, the Hg2+-specificity was not indicated nor was it expected on the basis of metal-specificities of the ligands.5–10,12,13) Only Hg2+-trapping beads, containing thymine moieties,14–19) showed good Hg2+-selectivity and these data are consistent with our previous results.26) As an exception, chitosan-based Hg2+-trapping beads showed Hg2+ selectivity,11) however, the origin of the Hg2+-specificity is unclear on the basis of an intrinsic metal-specificity of chitosan.
In conclusion, the prepared Hg2+-trapping beads can exclusively capture Hg2+ even in the presence of co-existing cations. More interestingly, the Hg2+-trapping efficiency of the beads improved in the presence of co-existing cations. The Hg2+-trapping beads are suitable for the elimination of Hg2+ from environmental water, especially seawater. As a practical usage of the Hg2+-trapping beads, the Hg2+-decontamination from industrial wastes would be possible.