2014 Volume 62 Issue 7 Pages 636-641
2014 Volume 62 Issue 7 Pages 636-641
A megamolecular polysaccharide sacran was newly extracted from cyanobacterium Aphanothece sacrum. Sacran has many preferable properties for transdermal application, e.g. a safe biomaterial, a high moisturizing effect, a formation of film and hydrogel. Additionally, it was recently discovered that sacran has an anti-inflammatory effect for atopic dermatitis model mice. In this study, in order to evaluate the feasibility of sacran-hydrogel as a novel sustained release system, we prepared a sacran-hydrogel containing 4-biphenyl acetic acid (BPAA, an acidic drug), prednisolone (PD, a neutral drug) or chlorpheniramine maleate (CPM, a basic drug), and performed the in vitro release studies. The sacran-hydrogel containing BPAA, PD or CPM provided a sustained release profile in accordance with a quasi-Fickian diffusion model. Furthermore, the release rate of drugs from sacran-hydrogels can be controlled by adjusting the concentration of aluminum chloride as a cross linker. These results suggest the potential use of sacran-hydrogel as a sustained release system for drugs.
Controlled delivery systems provide an alternative approach to regulate the bioavailability of therapeutic agents. In controlled drug delivery system (DDS), an active therapeutic is incorporated into a polymeric network structure, and is released from the material in a predefined manner.1) Hydrogels are three-dimensional, hydrophilic, polymeric networks capable of imbibing large amounts of water or biological fluids,2) and are favored in a broad range of pharmaceutical and biomedical applications.3–6) Both synthetic and natural polymers can be used for the formation of hydrogels. There are several excellent studies of intelligent hydrogel in DDS that utilize synthetic hydrophilic polymers,4,7–10) but most of them are either not biodegradable, e.g. [poly(N-isopropyl acrylamide), poly(2-hydroxyethyl methacrylate), polyvinyl alcohol] or suffer from other issues, such as local inflammation. Therefore, biocompatible and biodegradable hydrogels have been expected using natural polymers.
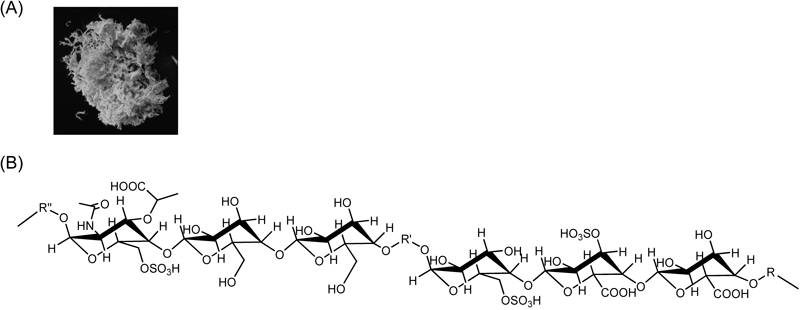
Recently, ampholytic polysaccharide sacran (Fig. 1) was extracted from the Japanese indigenous cyanobacterium Aphanothece sacrum, which is mass-aquacultured in rivers with a high ionic concentration, and which possesses an abundance of a jelly-like extracellular matrix (ECM) with a high water content (97.5%).11–14) Sacran is a heteropolysaccharide containing various sugar residues such as glucose, galactose, mannose, xylose, rhamnose, fucose, galacturonic acid and glucuronic acid, and traces of alanine, galactosamine and muramic acid; 11% of the monosaccharides contain a sulfate group and 22% of them contain a carboxyl group. Sacran also has an extremely high molecular weight (approximately 20 MDa) and is surprisingly long (more than 8 µm).11) Sacran is thought to be a safe biomaterial, because Aphanothece sacrum is a mass cultivated cyanobacterial species that has long been used as a functional food to ameliorate allergic tendency and gastroenteritis by inhabitants of the Kyushu region in Japan. Furthermore, sacran can retain a lot of water, compared to hyaluronic acid or xantangum, and forms a nanofilm on the platform. Recently, Okajima et al. revealed that sacran can form a hydrogel through an electrostatic interaction with cationic heavy metal ions and that it can be applied for the recovery of rare metals.11–13,15) However, until now there has been no report on the application of sacran-hydrogel for a transdermal drug carrier.
On the other hand, there is growing evidence on a novel bioactivity of sacran, which has attracted attention. Sacran is acknowledged to have an anti-inflammatory effect, because the chemical structure of sacran is similar to that of glucosaminoglycans which have various bioactivities including an anti-inflammatory effect.16–21) Actually, Ngatu et al. demonstrated that epicutaneous application of sacran to mice has significantly inhibited the development of allergic dermatitis skin lesions, and reduced the number of scratching behavior episodes by improving skin barrier and downregulating TH2 cytokine production, leading to inhibition of immunoglobulin E (IgE) and eosinophilic infiltration in mice.22)
Therefore, in the present study, in order to evaluate the feasibility of sacran-hydrogel as a novel transdermal drug carrier for dermatitis, we prepared sacran-hydrogel containing various drugs such as 4-biphenyl acetic acid (BPAA), prednisolone (PD) and chlorpheniramine maleate (CPM), and performed in vitro drug release study.
Sacran was kindly donated by Green Science Material (Kumamoto, Japan). BPAA, PD, CPM and aluminum chloride (AlCl3) were purchased from Nacalai Tesque (Kyoto, Japan). All other chemicals and solvents were of analytical reagent grade and deionized double distilled water was used throughout the study.
Sacran-Hydrogel FormationThe suspensions of sacran (450 µL, 1.0% (w/v)) and various drugs (BPAA; 10 mM, PD; 0.27 mM, CPM; 10 mM) were added to Tissue-tek® mold (1 cm×1 cm) from Sakura Finetek Japan (Tokyo, Japan). Then, 50 µL of aqueous solutions of AlCl3 (10, 50, 100 mM) were added to the sacran solutions containing the drugs. The mixtures were placed at 4°C for 5 h to form a sacran-hydrogel. The entrapment ratio was determined by following equation: Entrapment ratio (%)=[(the total amount of drug)−(the amount of drug in supernatant)]/(the total amount of drug)×100.
In Vitro Release of Drugs from Sacran-HydrogelThe in vitro release rate was measured by the modified dispersed amount method.23) A sacran-hydrogel containing BPAA, PD or CPM was diluted to a total volume of 10 mL with phosphate buffer (pH 7.4). The samples were incubated at 32°C with mild shaking (50 rpm) by a bioshaker (TAITEC, Saitama, Japan). At certain time intervals, an aliquot of the dissolution medium was withdrawn and replaced with the same volume of the fresh phosphate buffer (pH 7.4). The collected samples were centrifuged at 6300×g for 5 min. The concentrations of drugs in the supernatant were determined by an UV spectrophotometer V-630 (JASCO, Tokyo, Japan) at 253 nm, 294 nm and 261 nm for BPAA, PD and CPM, respectively.
Release KineticsData obtained from the in vitro release studies were fitted to the kinetics equations to reveal the mechanism of drug release from the formulations prepared. The kinetic models were used the Higuchi model24) and Korsmeyer–Peppas model.25) The rate constants were also calculated for the respective models.26)
The release data were analyzed according to the Higuchi equation
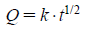 | (1) |
where Q is the percent of drug released at time t and k is the kinetic constant.
The Korsmeyer–Peppas model, describing drug release from polymeric system, takes into account that the drug release mechanism often deviates from the Fick’s law and follows anomalous behaviors described by the following equation:
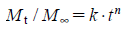 | (2) |
where Mt is the quantity of drug released at time t, M∞ is the quantity of the drug released at infinite time, k is the kinetic constant and n is the release exponent. The value of n determines the release mechanism. When n approximates to 0.5, a Fickian/diffusion controlled release is implied, where n<0.5; quasi-Fickian diffusion, 0.5< n<1.0; non-Fickian transport, and n=1; zero order (case II transport). When n approaches 1.0, phenomenologically one may conclude that the release is approaching zero order.
Statistical AnalysisData are given as the mean±S.E.M. Statistical significance of means for the studies was determined by ANOVA followed by Scheffe’s test. The p-values for significance were set at 0.05.
Recently, Okajima et al. reported that sacran can form a hydrogel through a chelate formation with cationic heavy metal ions such as Al3+, In3+ and Cr3+.11–13,15) In our preliminary study, we confirmed that the incubation with sacran solution at 4°C for 5 h after the addition of AlCl3 was necessary to obtain the homogenous sacran-hydrogel. Therefore, to examine whether sacran-hydrogels can be formed in the presence of various drugs, sacran-hydrogels were prepared by the addition of AlCl3 as a cross-linker, in BPAA, PD and CPM systems as model compounds of acidic, neutral and basic drugs, respectively (Fig. 2). Sacran alone formed a hydrogel by the addition of AlCl3, and maintained its shape in a mold at higher concentrations of AlCl3 (>50 mM). In the case of both BPAA and PD systems, the similar results were observed. On the other hand, sacran-hydrogel with CPM formed the shape in a mold at more than 10 mM AlCl3, suggesting that the sacran-hydrogel with a basic drug was more robust than those with acidic and neutral drugs. These results suggest that sacran-hydrogel can be formed even in the presence of various types of drugs.

The concentration of sacran was 1.0% (w/v). The concentrations of BPAA, PD and MCP were 10, 0.27, 10 mM, respectively.
Since the sacran-hydrogels were formed by the addition of AlCl3 as a cross-linker even in the presence of various drugs, we next examined the entrapment ratios of drugs in sacran-hydrogels. As shown in Fig. 3, the sacran-hydrogels showed high entrapment ratios of BPAA, PD and CPM. The entrapment ratios of BPAA and PD in the sacran-hydrogels decreased as the concentration of AlCl3 was increased, while that of CPM was almost 100% up to 100 mM AlCl3. This may be caused that BPAA and PD, but not CPM, were released with water outside of the sacran-hydrogels as the concentration of AlCl3 increased, probably due to the augmentation of cross-linking points between sacran and Al3+. Since sacran is highly negatively charged polysaccharide due to the high content of galacturonic acid and glucuronic acid,13,27) a charge of drug would be significantly involved in the entrapment efficacy in a sacran-hydrogel. Actually, the entrapment ratios of drugs were increased in the order of BPAA (an acidic drug)<PD (a neutral drug)<CPM (a basic drug), probably due to the electrostatic interaction with a negatively charged sacran molecule. These results suggest that sacran-hydrogel can retain the various types of drugs and the electrostatic interaction may involve in the entrapment ratios of drugs in sacran-hydrogels.

Each point represents the mean±S.E.M. of 3 experiments. * p<0.05 versus BPAA. † p<0.05 versus PD.
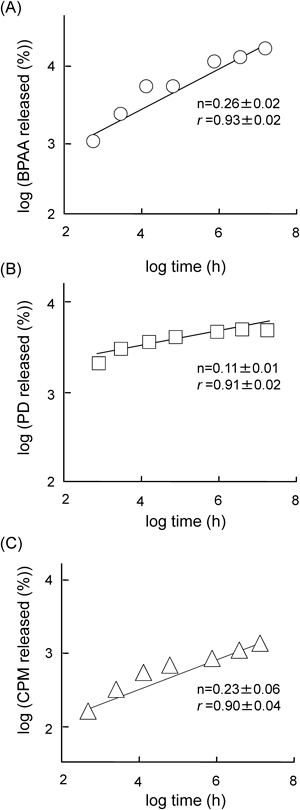
To reveal the drug release profiles from sacran-hydrogels, we examined in vitro release studies of BPAA, PD and CPM from sacran-hydrogels prepared by the addition of 100 mM AlCl3 in phosphate buffer (pH 7.4) at 32°C (Fig. 4). The sacran-hydrogels containing various drugs showed the sustained release profiles for at least 24 h. No obvious degradation of the sacran-hydrogel was observed up to 2 h, after that, the sacran-hydrogels were gradually collapsed during the release study. To evaluate the mechanism of drug release from the sacran-hydrogels, the data obtained from in vitro release studies were fitted to the kinetics equations. At first, we used a Higuchi model, which describes the drug release from an insoluble matrix as a square root of a time-dependent process based on Fickian diffusion, using the data obtained from the in vitro release studies within 2 h. Because during the in vitro release studies, the sacran-hydrogels containing various drugs did not collapse in phosphate buffer (pH 7.4) up to 2 h, and then gradually disintegrated after 2 h. As shown in Table 1, the slopes of BPAA, PD and CPM systems calculated from the Higuchi equation were 4.55, 4.52 and 2.20, respectively. The release rate of CPM was lower than that of BPAA or CPM in consistent with the results of the slope calculated by the Higuchi equation. The correlation coefficients (r) in BPAA, PD and CPM systems were 0.95, 0.88 and 0.96, respectively, suggesting that the release type of BPAA, PD and CPM from sacran-hydrogels up to 2 h was matrix-type based on a Fickian diffusion. Furthermore, to gain insight into the mechanism of drug release from sacran-hydrogel, the Korsmeyer–Peppas model which takes into consideration of erosion and dissolution of matrix was used. As shown in Fig. 5, all of the Korsmeyer–Peppas plots showed a good linearity (BPAA; r=0.93, PD; r=0.91 and CPM; r=0.90). Furthermore, the diffusion exponents (n) in the BPAA, PD and CPM systems were 0.26, 0.11 and 0.23, respectively, suggesting that the mechanism of drug release from sacran-hydrogel was determined as a quasi-Fickian diffusion, which may indicate a coupling of the diffusion and erosion mechanism.
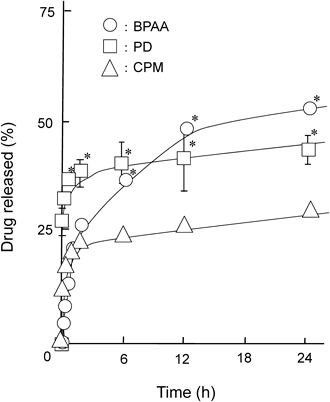
AlCl3 solution (100 mM) was used as a cross linker. The sacran-hydrogels containing drugs were shaken in phosphate buffer (pH 7.4) at 32°C. Each point represents the mean±S.E.M. of 3–6 experiments. * p<0.05 versus CPM.
| Parameter | BPAA | PD | CPM |
|---|---|---|---|
| Slope (%·min−0.5) | 4.55±0.37 | 4.52±0.22 | 2.20±0.37 |
| Correlation coefficient (r) | 0.95±0.06 | 0.88±0.02 | 0.96±0.04 |
Next, we examined the effects of AlCl3 concentration on the drug release profiles from sacran-hydrogels containing various drugs (Fig. 6). The release rates of BPAA and PD from sacran-hydrogels were augmented as the concentration of AlCl3 was decreased, probably due to the attenuation of magnitude of sacran-hydrogels. Meanwhile, the release rate of CPM from sacran-hydrogel was enhanced as the concentration of AlCl3 was increased. This may be caused that the electrostatic interaction of Al3+ with negatively charged sacran may attenuate the interaction between sacran and CPM (a cationic drug). The difference in the release rate of BPAA, PD and CPM from sacran-hydrogels may be ascribed to the different interaction mode between the drugs and sacran. Recently, Okajima et al. revealed that sacran is a heteropolysaccharide containing various sugar residues such as glucose, galactose, mannose, xylose, rhamnose, fucose, galacturonic acid and glucuronic acid, and traces of alanine, galactosamine and muramic acid; 11% of the monosaccharides contain a sulfate group and 22% of them contain a carboxyl group.13) Therefore, the potent negative charges of sacran derived from sulfate and carboxyl groups may affect drug release profiles from sacran-hydrogels. Actually, BPAA (an anionic drug) and CPM (a cationic drug) in the sacran-hydrogel showed rapid and slow release profiles, respectively, compared to PD (a neutral drug) (Fig. 4). The PD release from sacran-hydrogel cross-linked by 10 mM AlCl3 was much higher than that from sacran-hydrogels cross-linked by 50 and 100 mM AlCl3. These differences in the PD release rate might be caused by the differences of the magnitude of sacran-hydrogels. Further elaborate study on the effect of magnitude of sacran-hydrogel on PD release rate should be necessary. Furthermore, an initial burst release form sacran-hydrogel containing BPAA or PD, but not CPM, was observed at 0 mM of AlCl3, probably due to the electrostatic interaction of CPM with sacran molecules. In addition, the aqueous solubility of drug is one of the important factors to decide the drug release rate. The solubility of BPAA and CPM in water was much higher than that of PD. Therefore, in addition to electrostatic interaction between drugs and sacran-hydrogels, the solubilities of BPAA, PD and CPM may also affect the drug release profiles. Collectively, these results suggest that the release rates of BPAA, PD and CPM from sacran-hydrogels can be controlled by adjusting AlCl3 concentration, and the drug release profiles are involved in the charge of drugs and their aqueous solubility.
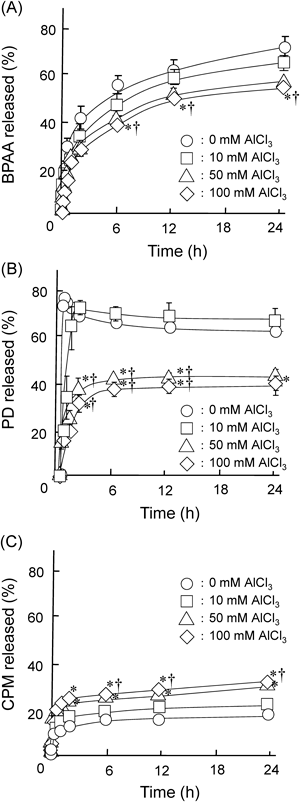
AlCl3 solution (0, 10, 50 or 100 mM) was added to the sacran/drug suspensions. The sacran-hydrogels containing various drugs were shaken in phosphate buffer (pH 7.4) at 32°C. Each point represents the mean±S.E.M. of 4–6 experiments. * p<0.05 versus 0 mM AlCl3. † p<0.05 versus 10 mM AlCl3.
AlCl3 is commonly used topically as the first-line therapy for primary hyperhidrosis, however, it causes skin irritancy or dermatitis at the high concentration. Therefore, further elaborate study on the safety of AlCl3 in sacran-hydrogel or an application of alternative cross-linkers is required.
Sacran is a novel biomaterial extracted from Aphanothece sacrum, an edible alga. Since sacran originally plays a role of extracellular matrix, it may be applicable for scaffolding domain of cells and regulating the cell function. In addition, we confirmed that the sacran-hydrogel can provide a sustained release of proteins (data not shown). Therefore, the application of sacran-hydrogel for regenerative therapy is expected as a function of not only an extracellular matrix but also a biomaterial with sustained release profiles for proteins such as growth factors.
The growing importance of hydrogels in drug delivery applications has led to the development of many novel and promising preparation strategies. For the encapsulation of bioactive substances, chemically crosslinked hydrogels are of great interest, especially the hydrogel can form under mild conditions in the absence of organic solvents. In this study, we revealed the formation of sacran-hydrogel containing various drugs based on the chemical crosslinking in the absence of organic solvents for the first time. The sacran-hydrogels containing various drugs provided sustained release profiles in accordance with quasi-Fickian diffusion model. Furthermore, the release rate of drugs from sacran-hydrogels can be controlled by adjusting the concentration of AlCl3. These results suggest the potential use of sacran-hydrogel as a novel sustained release system for drugs.
We acknowledge Dr. Maiko K. Okajima and Dr. Tatsuo Kaneko from Department of Material Science and Technology (JAIST) for their helps. We wish to thank for technical assistance of Mr. Kei Watanabe in Kumamoto University. This work was supported by Adaptable and Seamless Technology Transfer Program (A-STEP) from the Japan Science and Technology Agency.
This study was funded by Green Science Material Inc. (Kumamoto, Japan) and Daito Kasei Kogyo Co., Ltd. (Osaka, Japan). Mr. Shinichiro Kaneko is a president of Green Science Material, Inc.