2014 Volume 62 Issue 7 Pages 654-660
2014 Volume 62 Issue 7 Pages 654-660
The aim of this study was to obtain injectable high-drug-loading core–shell structure microspheres that release aripiprazole over 2 months. The microparticles were prepared by the oil-in-water emulsion solvent evaporation method and characterized. The microparticles were prepared with aripiprazole and 3 types of poly(lactic acid) (PLA) (DL-PLA: molecular weight (MW), ca. 20000; DL-PLA: MW, ca. 95000; or L-PLA: MW, ca. 110000), which were dissolved within the organic phase, and prepared in 3 temperature conditions for the external aqueous phase (two fixed temperature conditions and a gradually increased temperature condition). The theoretical drug loading in the particles was set to 80%. When prepared at fixed temperature conditions, all of the microparticles that were prepared with the 3 types of PLA were not spherical or smooth-surfaced. These microparticles released 100% of the drug within 1 week in the in vitro study. However, the microparticles that were prepared with DL-PLA (MW, 95000) in the gradually increased temperature condition were spherical with a smooth surface. The dissolution profile of the microparticles showed a long release over 7 weeks in vitro. The actual drug loading in the microspheres was 73–80%. A core–shell structure was observed in the inner structure of the microspheres. The core–shell microspheres were injected subcutaneously into rabbits. Aripiprazole was detected in the serum over 12 weeks. Production of high-drug-loading core–shell structure microspheres was successfully achieved by using high molecular weight of PLA and specific temperature condition at preparation. It showed long release profile in vitro and in vivo.
Schizophrenia is one of the most serious mental diseases. Globally, it affects approximately 1% of the population and is a major cause of disability.1,2) Antipsychotic medications are used to treat the symptoms of schizophrenia. Medication adherence is an important element in the treatment success of schizophrenia.3,4) However, a frequent cause of relapse among patients with schizophrenia is nonadherence of the antipsychotic medications. An estimated 40 to 50% of patients do not adhere to the prescribed medication regimens for a variety of reasons.5,6) The risk of hospitalization increases significantly with nonadherence, even if it is short-term. Thus, nonadherence patients with schizophrenia experience increased morbidity, decreased quality of life, and higher healthcare costs.7,8) Long-acting depot injections of antipsychotic medications can improve patient adherence in the effective management of the disease.9) Atypical antipsychotic medications are therapeutically more effective and produce fewer side effects compared to typical antipsychotic agents. Therefore, atypical antipsychotic drugs are suitable for long-acting depot injections. Especially, aripiprazole is well known for having a lower risk of hyperprolactinemia and a low potential for metabolic disturbances because of its unique pharmacology, partial agonism (agonism/antagonism) at dopamine D2 and serotonin 5-HT1A receptors, and antagonism at serotonin 5-HT2A receptors.10–15)
Presently, the long-acting depot formulation of aripiprazole, Abilify Maintena™, has been approved for use in the United States since February 2013, and it is commercially available.16,17) This formulation consists of an injectable aqueous suspension in which aripiprazole monohydrate is suspended without the use of any base for controlling release, and it is injected intramuscularly every 4 weeks. As injections are invasive to the patient, a longer sustained release injection that would reduce the frequency of drug administration would be preferable and would reduce their burden. We hypothesized that a technique involving a control release base with aripiprazole would permit a longer sustained release.
Poly(lactic acid) (PLA) and poly(lactic acid-co-glycolic acid) (PLGA) is extensively used as a polymeric control release base for the sustained release of microsphere formulations because of their biodegradability. Indeed, some types of microspheres have already been introduced to the medical field.18–21) Various conventionally known microspheres are of the matrix type in which a drug is uniformly distributed in a base matrix, such as PLA and PLGA.
However, it is commonly known that drug loading in microspheres for controlled release is about 30% or so at maximum.22) When such a matrix-type microsphere has a high drug-loading ratio, such as 50%, the drug constitutes a large part of the microsphere. Consequently, a large amount of the drug also exists on the surface of the microsphere. It has generally been considered that the presence of a large amount of the drug on the surface of the microsphere disables the control of the release by the base.23)
Abilify Maintena™, which has been approved by the Food and Drug Administration, is a formulation that requires injections every 4 weeks. The administration drug dose is 300 mg or 400 mg. If the typical matrix-type microsphere technique is applied to aripiprazole in order to achieve longer sustained release, 30% drug loading is not realistic for injections because of the need to increase the particle amount in the injection formulation.
For these reasons, this study aimed to develop high-drug-loading aripiprazole-biocompatible polymer microspheres that have a longer sustained release, such as over 2 months.
Aripiprazole was supplied by Otsuka Pharmaceutical Co., Ltd. (Tokyo, Japan). DL-PLA [molecular weight (MW), ca. 20000] was purchased from Wako Pure Chemical Industries, Ltd. (Osaka, Japan). DL-PLA (MW, ca. 95000) and L-PLA (MW, ca. 110000) were purchased from Sigma-Aldrich Chemie GmbH (Munich, Germany). Polyvinyl alcohol (PVA224) was supplied by Kuraray Co., Ltd. (Tokyo, Japan). Dichloromethane (DCM) and sodium hydrate were purchased from Wako Pure Chemical Industries, Ltd.
Preparation of Aripiprazole–PLA Complex MicroparticlesThe preparation formulations of the aripiprazole–PLA complex microparticles are shown in Table 1. The aripiprazole–PLA complex microparticles were prepared by an oil-in-water (O/W) emulsion solvent evaporation method that has been widely used in the preparation of microspheres.24,25)
| Sample ID | Optical isomerism of PLA | Molecular weight of PLA | External phase temperature at preparation | Procedure of micronization by homogenization | Theoretical drug loading (%) | Actual drug loading (%) | Encapsulation efficiency(%) | Mean particle size(μm) |
|---|---|---|---|---|---|---|---|---|
| A | DL | 20000 | Fixed 10°C | Not applied | 80 | 79 | 99 | 186 |
| B | DL | 20000 | Fixed 20°C | Not applied | 80 | 77 | 96 | 145 |
| C | DL | 20000 | From 10 to 20°C* | Not applied | 80 | 76 | 95 | 113 |
| D | DL | 95000 | Fixed 10°C | Not applied | 80 | 73 | 91 | 233 |
| E | DL | 95000 | Fixed 20°C | Not applied | 80 | 74 | 93 | 197 |
| F | DL | 95000 | From 10 to 20°C* | Not applied | 80 | 73 | 91 | 206 |
| G | L | 110000 | From 10 to 20°C* | Not applied | 80 | 77 | 96 | 216 |
| H | DL | 20000 | From 10 to 20°C* | Applied | 80 | 75 | 94 | 46 |
| I | DL | 95000 | From 10 to 20°C* | Applied | 80 | 80 | 100 | 65 |
| J | L | 110000 | From 10 to 20°C* | Applied | 80 | 77 | 96 | 48 |
* A temperature of 10°C was used at the time that the organic phase was added to the aqueous phase, and the temperature was then gradually increased to 20°C over 2 h and then fixed at 20°C over 12 h.
Briefly, 100 mg of aripiprazole and 25 mg of PLA were dissolved in 2 mL of DCM to form an organic phase, and then the organic phase was poured into 100 mL of an 1% polyvinyl alcohol (PVA) aqueous solution (pH about 11.5, adjusted by sodium hydrate) while stirring at 400 rpm with a propeller-type agitator (BL600, SHINTO Scientific Co., Ltd., Tokyo, Japan) with 4 blades over 12 h. Alternatively, the organic phase was poured into 20 mL of an 1% PVA aqueous solution (pH about 11.5) while shearing at 2000 rpm for 3 min with a high shearing homogenizer (Polytron Homogenizer PT3000, KINEMATICA AG, Lucerne, Switzerland), and, then, the resultant emulsion solution was poured into 80 mL of an 1% PVA aqueous solution (pH about 11.5) while stirring at 400 rpm with a propeller-type agitator with 4 blades. These preparations were conducted at the following 3 temperature conditions for the aqueous solution: 1) under a fixed ca. 10°C condition; 2) under a fixed 20°C condition; or 3) under a constantly increasing temperature condition (the organic phase was added in a drop-wise fashion to the aqueous phase at ca. 10°C in order to obtain an O/W emulsion, the temperature of the obtained O/W emulsion was increased gradually from ca. 10°C to ca. 20°C over 2 h, and, subsequently, the temperature was fixed at ca. 20°C over 12 h). After the aripiprazole–PLA complex microparticles were solidified, the entire dispersed suspension was centrifuged (1670×g for 5 min, Table Top Centrifuge 4000/4200, Kubota Corporation, Tokyo, Japan) and resuspended in distilled water. The resultant particles were collected by filtration with 10-µm-pore-size filter and dried at room temperature for 1 d.
Scanning Electron Microscope (SEM) AnalysisThe outer morphology and the inner structure of the resultant microparticles were observed by means of a SEM (VE-7800, KEYENCE Corporation, Osaka, Japan).
In order to observe the inner structure, the microspheres were embedded in paraffin and cut on a sliding microtome (SM2000R, Leica Microsystems GmbH, Wetzlar, Germany). Before their analysis, the microspheres were coated with platinum under vacuum conditions.
Particle Size AnalysisThe mean particle size of the resultant microparticles that were dispersed in distilled water was determined with a laser particle size analyzer (SALD-3000J, Shimadzu Corporation, Kyoto, Japan). The measurement conditions were as follows: Medium, water; Refractive index, 2.00 to 0.20 i.
Determination of the Aripiprazole in the Resultant MicroparticlesThe microparticles containing aripiprazole (ca. 50 mg) were dissolved in 5 mL of acetone. The solution was then diluted to 50 mL with a mobile phase (0.02 mM anhydrous sodium sulfate aqueous solution–acetonitrile–methanol–acetic acid; 56 : 33 : 11 : 1) of high-performance liquid chromatography (HPLC). The media was filtered (Acrodisc, Nihon Pall Ltd., Tokyo, Japan) and measured spectrophotometrically at 254 nm with a reverse-phase HPLC method [Pump, LC-20AD (Shimadzu Corporation); Detector, SPD-M20A (Shimadzu Corporation); Column, YMC-Pack ODS A-303 (4.6 mm×250 mm; pore size, 5 µm) (YMC Co., Ltd., Kyoto, Japan)]. The drug loading and entrapment efficiency were calculated from Eqs. 1 and 2, respectively.
 | (1) |
 | (2) |
In vitro release studies were conducted with the paddle method. Fifty milligrams of aripiprazole in the resulting microparticles were measured and added to 900 mL of 0.5% sodium dodecyl sulfate aqueous solution in order to conduct a release test (n=3 in each group) with the paddle method at 100 rpm at 37±1°C.
Crystallization Analysis of the Aripiprazole–PLA MicrospheresThe crystallization of aripiprazole in the microspheres was measured by powder X-ray diffraction (D8 ADVANCE, BRUKER AXS, Inc., Madison, WI, U.S.A.). The operating conditions were as follows: Range of measurement, 2θ 3–60°; Target, Cu, Voltage/Current, 40 kV/40 mA.
In Vivo Release Study of the Aripiprazole–PLA MicrospheresThe microspheres were dispersed in 1.5% aqueous sodium carboxymethylcellulose solution containing 0.75% sodium chloride such that the aripiprazole content became 10% (w/v). The resultant suspension was injected subcutaneously into the posterior cervical region of male rabbits (2.70±0.07 kg, n=4) at a dose of 25 mg aripiprazole/kg. Blood samples of the rabbits were collected from the auricular arteries at 1, 3, 7, 14, 21, 28, 35, 42, 49, 56, 70 and 84 d after microsphere injection. The serum was obtained by centrifugation (1700×g, 15 min, 4°C), and then the aripiprazole was extracted from the serum. The aripiprazole was measured with liquid chromatography-tandem mass spectrometry, and the aripiprazole concentration was calculated in the serum.
The aripiprazole–PLA complex microparticles were prepared with an O/W emulsion solvent evaporation method with DL-PLA of a MW of 20000 or a MW of 95000. The preparations were subjected to the following 3 temperature conditions for the external phase: Condition 1, under fixed ca. 10°C; Condition 2, under fixed 20°C; or Condition 3, under a constantly increasing temperature condition that went from ca. 10 to 20°C over 2 h after adding the organic phase to the aqueous phase and, after that, the temperature was fixed at ca. 20°C over 12 h. The morphologies of the resultant microparticles are shown in Fig. 1. The sample IDs are shown in Table 1 and in Fig. 1. When DL-PLA of a MW of 20000 was used, the morphologies of the microparticles that were prepared by each condition exhibited an awkward shape (particles A to C).
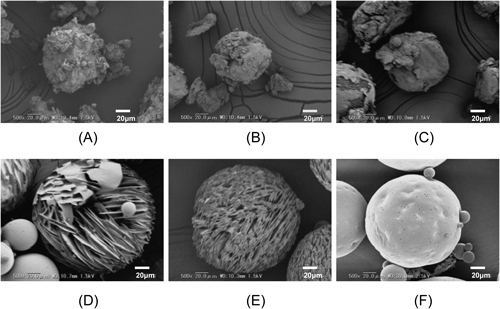
Particles prepared with DL-PLA with a MW of 20000 at (A) a fixed temperature of 10°C of the external phase, (B) a fixed temperature of 20°C of the external phase, or (C) at a gradually increasing temperature.* Particles prepared with DL-PLA with a MW of 95000 at (D) a fixed temperature of 10°C of the external phase, (E) a fixed temperature of 20°C of the external phase, or (F) at a gradually increasing temperature.* The scale bars indicate 20 µm. * A temperature of 10°C was used at the time that the organic phase was added to the external aqueous phase, and the temperature was then gradually increased in the external phase to 20°C over 2 h and then fixed at 20°C over 12 h.
When DL-PLA of a MW of 95000 was used, the microparticles that were prepared by the fixed temperatures of 10°C and 20°C for the external aqueous phase looked like a spicular agglomerate or a sponge-like structure (particles D and E, respectively). Meanwhile, when DL-PLA of a MW of 95000 was used and subjected to the increasing temperature condition, the morphology of the resultant microparticle (particle F) was spherical with a smooth surface. The crystalline form of particle F was amorphous (data not shown). Though aripiprazole has high crystalline property, this result suggested the aripiprazole might be interacted with tiny amounts of PLA in Core structure.
In order to examine the drug release from these microparticles, in vitro release studies were performed on particles A to F (Fig. 2). Particles A to E released all of the aripiprazole in the particles within 1 week in this evaluation system, which was the same as that of the aripiprazole monohydrate original powder (mean particle size, 216 µm). These findings indicated that these microparticles did not show release control. In contrast, particle F (microsphere) showed an extremely long release profile that lasted over 7 weeks. It has been showed that the erosion of microspheres did not occur until the polymers were degraded to MW of approximately 15000 and using higher MW of polymer delayed the collapse.26) Moreover, it is well known that the degradation rate of PLA is slower than poly(lactic-co-glycolic acid) (PLGA). Therefore, it is reasonable that Particle F which was used with DL-PLA of a MW of 95000 showed such a long release without the collapse of microspheres.
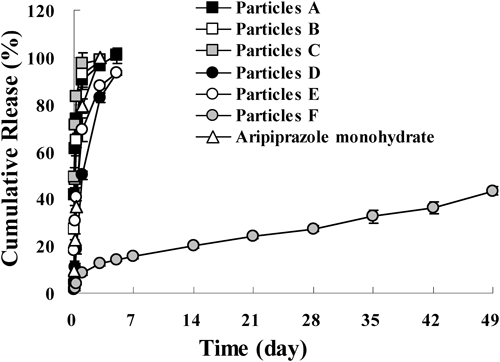
Mean±standard deviation (S.D.), n=3.
Particles C, F, G, H, I, and J were prepared by the gradually increasing temperature condition. The morphologies of the resultant microparticles are shown in Fig. 3. These microparticles were prepared with or without homogenization of the preparation of the O/W emulsion. Therefore, the mean particle size was different between particles C, F, and G (113 to 216 µm) and particles H, I, and J (46 to 65 µm).
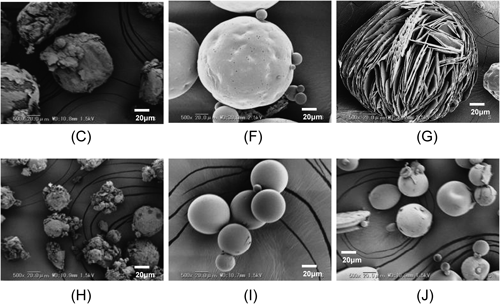
Particles prepared without homogenization with (C) DL-PLA with a MW of 20000, (F) DL-PLA with a MW of 95000, or (G) L-PLA with a MW of 110000. Particles prepared with homogenization with (H) DL-PLA with a MW of 20000, (I) DL-PLA with a MW of 95000, or (J) L-PLA with a MW of 110000. See the explanation for * in Fig. 1.
When PLA of a MW of 20000 was used, regardless if it was with or without homogenization, the obtained microparticles exhibited an awkward shape, as shown in Fig. 3 (particles C and H). When L-PLA was used, various shapes, such as a spicular agglomerate, a rhomboid-shaped crystal, and flattened microparticles, were observed in addition to the spherical microparticles (particles G and J). However, when DL-PLA of a MW of 95000 was used, the morphologies of all of the obtained microparticles were spherical with a smooth surface, regardless if it was with or without homogenization (particles F and I).
These differences may have been caused by the viscosity of DCM solution. The viscosity of DCM solution with smaller MW of DL-PLA (20000) was low and aripiprazole can be dissolved speedy in the solution. This property induces the equilibrium between the solidified aripiprazole in DCM emulsion and dissolved aripiprazole in the residual DCM, and the obtained microparticles formed an awkward shape as the result. On the other hands, that of DCM solution with larger MW of 95000 was high and it is difficult to dissolve aripiprazole in it. Therefore, it was considered that using MW of 95000 could prepare the Core–Shell structure microspheres with avoiding the equilibrium.
The release profiles of these microparticles in vitro are shown in Fig. 4. The profiles of the microparticles (microspheres) that were prepared with DL-PLA with a MW of 95000 showed very long releases. The microparticles that were prepared with homogenization at preparation (particle I) had a faster release profile than those prepared without homogenization (particle F). This was attributable to a smaller mean particle size. The microparticles prepared with L-PLA (particles G and J) showed a faster release profile than those prepared with DL-PLA with a MW of 95000. This was attributable to the incorporation of incomplete microspheres.

Mean±S.D., n=3.
In order to clarify the mechanisms of this long-release profile in vitro study, a microsphere (particle I) was cut with a microtome. The core–shell structure was observed in the inner area of the microsphere (Fig. 5i). The shell is indicated between the 2 triangles (▲) in the picture. For particle C, such a core–shell structure was not observed (data not shown). Furthermore, the cut microspheres (particle I) were immersed for 1 h in an acetic acid solution (20%) that does not dissolve PLA but dissolves only aripiprazole, washed with purified water, and then observed with SEM. The SEM image is shown in Fig. 5ii. Consequently, only the core was dissolved, and the shell was not dissolved. This revealed that the shell consisted essentially of PLA and the core consisted essentially of aripiprazole. These results suggested that PLA covering the core controlled the release of aripiprazole from the microsphere.

The shell structure is shown between the 2 triangles (▲).
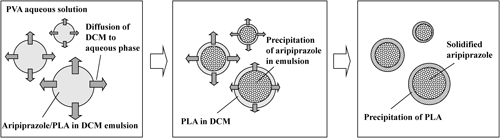
These findings suggested the mechanism described below for this core–shell structure preparation. The solubility of DCM in water is ca. 1 mL/100 mL at 20°C. When 2 mL of organic phase is poured into 100 mL of the aqueous phase, the DCM is not completely miscible at that time. The DCM forms an emulsion in the aqueous solution because of the saturated solubility. During mixing, aripiprazole which is dissolved with nearly saturated concentration is precipitated gradually in emulsion. Meanwhile, it is considered that the PLA is not precipitated at the time as the PLA is dissolved with lower concentration in the DCM. Subsequently, the DCM in the aqueous solution evaporates, and the residual DCM in the emulsion mixes into the aqueous solution. Finally, the aripiprazole is precipitated completely as core and the PLA is precipitated as shell. This consideration is supported by serial observation with a microscope. The spherical core structure forming in emulsion was observed and after that the shell was solidified with evaporation of DCM from the emulsion.
Indeed, when the microparticles were prepared with only aripiprazole in the gradually increasing temperature condition, the spherical aripiprazole microparticles that are shown in Fig. 6 were obtained. It is suggested that this spherical forming property of aripiprazole by this method enable to prepare the core–shell structure microspheres.

See the explanation for * in Fig. 1.
For the conditions of fixed 10°C and 20°C, spherical aripiprazole particles could not be obtained (data not shown). A possible reason is suggested below. Aripiprazole is precipitated slowly at 10°C during the diffusion of DCM into the aqueous solution, but, subsequently, the growth of a crystalline form may be caused by the equilibrium between the solidified aripiprazole and the dissolved aripiprazole in the residual DCM in the emulsion at 10°C. At 20°C, spherical aripiprazole microparticles may not be formed because of rapid evaporation of the DCM. However, in the gradually increasing temperature condition, spherical aripiprazole microparticles were obtained in the lower temperature, and, subsequently, equilibrium might not have occurred because of evaporation by the increasingly higher temperature. A schematic for obtaining aripiprazole–PLA core–shell microspheres is proposed (Fig. 7). That is, spherical aripiprazole was precipitated slowly in the emulsion at 10°C. By increasing the temperature of the aqueous solution after the precipitation, the DCM that was mixed with the aqueous solution evaporated, and it enabled the residual DCM in the emulsion to transfer into the aqueous solution. It therefore avoided an equilibrium between the solidified aripiprazole and the residual DCM, resulting in almost complete aripiprazole precipitation. Thereafter, further increases to the temperature to 20°C or the maintenance of 20°C resulted in more evaporation, and PLA was precipitated slowly on the outer surface of the core aripiprazole microparticles.
The aripiprazole release rate of the microspheres (particle I) was investigated in male rabbits. The microspheres (particle I) were dispersed in aqueous solution containing sodium carboxymethylcellulose and sodium chloride such that the aripiprazole content became 10% (w/v). The resultant suspension was administered to rabbits subcutaneously. Blood samples were collected for 84 d after administration, and the serum concentration of aripiprazole was measured (Fig. 8). In the in vivo release study, aripiprazole in the serum could be detected for 84 d after administration.

Dose: 25 mg aripiprazole/kg, mean±S.D., n=4.
In this study, high-drug-loading aripiprazole–PLA microspheres with a core–shell structure were prepared with an emulsion solvent evaporation method. The loaded drug levels in the microspheres were 73 to 80%. Many factors, such as MW, the optical isomerism of PLA, and the temperature condition of the preparation, affected the formulation of the microspheres. In addition, the release profile studies demonstrated that the microspheres released the drug over a long period of over 7 weeks in vitro and 12 weeks in vivo.
The authors thank Otsuka Pharmaceutical Co., Ltd. for providing infrastructure facilities and financial support for carrying out this work.